ABSTRACT
A 42-year-old woman entered the hospital for treatment of intractable migraine. She received dihydroergotamine (DHE) 1 mg IV every 8 hours. Two days later, her blood pressure was unobtainable, but DHE treatment continued for two more doses. Her total dose of 10.5 mg DHE in 72 hours exceeded FDA approved doses. Her symptoms progressed to painful tingling and numbness in the hands and feet with cold and intermittently blue feet. CT angiogram demonstrated arterial constriction of the superficial femoral, popliteal, and tibial arteries. Oral nifedipine, IV heparin infusion, and IV nitroprusside were ineffective. Four days after symptom onset, she began receiving intra-arterial papaverine, which reduced her vasoconstriction. She had persistent disability more than 15 months later. Factitious hypotension is an early sign of vascular ergotism, which requires prompt recognition and treatment with a vasodilator.
Case report
A 42-year-old woman with a history of migraine headaches and depression entered the hospital on the neurology service for treatment of intractable migraine not controlled with topiramate 100 mg each evening, carbamazepine 200 mg twice daily, and rizatriptan 10 mg (as needed). Her other home medications included sertraline 100 mg daily and duloxetine 90 mg daily. Her hospital treatment included dihydroergotamine (DHE) 1 mg IV every 8 hours beginning an hour after a test dose of 0.5 mg. Each dose of DHE followed premedication with dexamethasone 4 mg IV and metoclopramide 10 mg IV.
On the evening of hospital day (HD) 2, she had leg pain and difficulty walking from the bed to the bathroom. At 17:00 on HD 3 (after the eighth dose of DHE), three nurses were unable to measure her blood pressure with either automatic or manual blood pressure cuff. They notified the neurology resident physician. Despite this, she continued to receive two additional doses of DHE for a total of 10.5 mg in 72 hours. The treating physicians then discontinued DHE and discharged her from the hospital on HD 4 without investigating or treating her vasospasm. Her vital signs at discharge included temperature 36.7 °C, pulse 60 beats/min, respirations 16 breaths/min, and unobtainable blood pressure.
Within hours of discharge, she returned to the Emergency Department with painful tingling and numbness in the hands and feet with cold and intermittently blue feet. Her vital signs in the ED included temperature 36.1 °C, pulse 60 beats/min, respirations 16 breaths/min, manual blood pressure of 104 mmHg/“palpable” and unobtainable pulse oximetry. The ED physician diagnosed vasospasm caused by ergotism and the neurology service readmitted her that evening for observation with no specific therapy that night.
Her signs and symptoms of vasospasm continued unabated into HD 5 (41 hours after the first observation of vasospasm and 24 hours after her last dose of DHE) before the neurology service requested vascular surgery consultation.
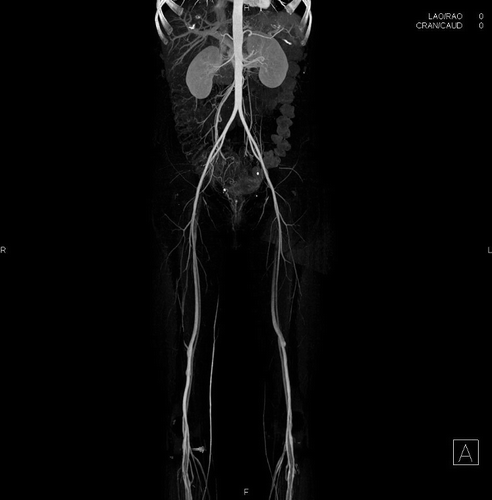
The vascular surgery service started oral nifedipine 20 mg every 8 hours at 48 hours after the vasospasm became apparent. Her symptoms failed to improve by the following morning (HD 6), and ankle-brachial indices were unmeasurable because her peripheral pulses remained difficult to detect by palpation or Doppler ultrasound. The vascular surgery service obtained a CT angiogram which demonstrated “marked vasoconstriction of the superficial femoral, popliteal, and tibial arteries” with “vasospasm involving the distal branches of the superior mesenteric artery” ().
Figure 1. CT angiography on hospital day 6 (four days after her first symptoms and three days after her first unobtainable blood pressure). Findings included “marked vasoconstriction of the superficial femoral, popliteal, and tibial arteries” with “vasospasm involving the distal branches of the superior mesenteric artery.”
After the CT angiogram, she was transferred to the Neurosurgical ICU, and continued receiving oral nifedipine 20 mg every 8 hours, with the addition of IV heparin infusion titrated to her partial thromboplastin time, and IV nitroprusside infusion at 0.3 mcg/kg/min.
On the morning of HD 7, the neurology service requested a medical toxicology consultation for ergotism and arterial line placement by interventional radiology. The toxicologist confirmed the diagnosis of “vascular ergotism.” The radiologist interpreted the angiogram was showing “diffuse vasospasm of the lower extremity arterial system bilaterally likely due to ergotamine toxicity.”
At 17:23 on HD 7 (fully 4 days after the first observations of vasospasm and ergot toxicity), she began intra-arterial infusion of papaverine 50 mg/h to dilate the severely constricted arteries. The IV nitroprusside infusion continued until HD 7, the intra-arterial papaverine infusion continued until HD 9, and oral nifedipine continued until HD 10.
On HD 8 (nearly 5 days after the first observations of vasospasm and ergot toxicity), a repeat angiogram found “significant improvement in the vasospasm of the lower extremity arterial system following intra-arterial papaverine administration.” This was the first objective evidence of improvement in her condition.
On HD 18, she “continued to be symptomatic and was unable to ambulate.” She was transferred to a skilled nursing facility for continued physical therapy and occupational therapy. The final discharge diagnosis was “ergotism” at the time of discharge.
Subsequent electromyogram (EMG) studies in two, three, and fifteen months later confirmed her permanent neuropathy caused by the ergotamine-induced vasospasm.
Discussion
Dihydroergotamine is available in 1 mL ampules containing 1 mg of DHE. A normal dose is 1 mg (1 mL) at a time, with the option to repeat the dose if needed in 1 hour [Citation1]. The FDA-approved package insert limits the total daily dose to 2 mg in any 24-hour period and a cumulative maximum of 6 mg within a week [Citation1]. The dosing limits are intended to avoid the toxic effects of DHE; the most significant toxic effect is severe spasm of peripheral blood vessels (vasospasm). Some authors have recommended an unapproved, off-label protocol that exceeds the dose limits recommended by the manufacturer and approved by the US Food and Drug Administration (FDA) [Citation2–4].
Factitious hypotension and inability to measure blood pressure are signs of ergotism [Citation5]. Vascular ergotism requires immediate cessation of the ergotamines and prompt treatment with a vasodilator, such as nifedipine, nitroprusside, or papaverine, although the optimal reversal agent remains unknown [Citation5–8].
In this case, the treating physicians failed to recognize the signs of ergotism, continued treatment after onset of toxicity, and then permitted a delay of two to three days before treating the vasospasm caused by DHE. The extent to which earlier recognition and treatment would have averted permanent disability is unclear.
Acknowledgments
Dr Mullins thanks the patient and her husband for their permission in sharing her remarkable case.
Disclosure statement
Michael E. Mullins MD is a consultant in concluded litigation.
References
- Package insert, D.H.E (dihydroergotamine). [ cited 31 Oct 2017]. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/label/2002/20148s7s8lbl.pdf
- Raskin NH. Repetitive intravenous dihydroergotamine as therapy for intractable migraine. Neurology. 1986;36:995–997.
- Silberstein SD, Young WB. Safety and efficiency of ergotamine tartrate and dihydroergotamine in the treatment of migraine and status migranosis. Neurology. 1995;45:577–584.
- Nagy AJ, Gandhi S, Bhola R, et al. Intravenous dihydroergotamine for inpatient management of refractory primary headaches. Neurology. 2011;77:1827–1832.
- Srisuma S, Lavonas EJ, Wananukul W. Ergotism and factitious hypotension associated with interaction of ergotamine with CYP3A4 inhibitors. Clin Toxicol. 2014;52:674–677.
- Schulman EA, Rosenberg SB. Claudication: an unusual side effect of DHE administration. Headache. 1991;31:237–239.
- Brancaccio G, Celoria GM, Falco E. Ergotism. J Vasc Surg. 2008;48:754.
- Baldwin ZK, Ceraldi CC. Ergotism associated with HIV antiviral protease inhibitor therapy. J Vasc Surg. 2003;38:676–678.
