abstract
We describe a case of mixed propranolol and tizanidine overdose and response to treatment with intravenous lipid emulsion (ILE) in conjunction with glucagon therapy. A 35-year-old male was unresponsive after intentional overdose of propanolol and tizanidine. He was bradycardic, hypertensive, and obtunded on the first medical contact. He received glucagon with moderate improvement in heart rate, followed by a bolus of ILE. Minutes after receiving ILE he became alert and responsive, and had further improvement in heart rate. This case suggests that ILE may reverse the effects of propanolol and tizanidine toxicity in overdose.
Background
At present, intravenous lipid emulsion (ILE) therapy is only approved for parenteral nutrition. ILE is also used off label as a rescue treatment for local anesthetic systemic toxicity (LAST) [Citation1]. However, ILE’s use is only supported by the American Society of Regional Anesthesia (ASRA) and Pain Medicine guidelines in the setting of bupivacaine toxicity after initiating advanced cardiac life support (ACLS), and the timing of administration remains controversial [Citation2]. A growing collection of case reports suggests potential benefit of ILE for overdoses with other lipophilic drugs, such as beta blockers, calcium channel blockers, and some antipsychotics/antidepressants [Citation1,Citation3,Citation4]. These cases often describe improvement in both cardiovascular function and neurologic status when conventional therapy has failed [Citation5]. Both tizanidine and propranolol are lipophilic and centrally acting drugs (LogP 1.4 and 3.48, respectively) suggesting ILE may improve symptoms of toxicity in overdosed patients [Citation6].
Case description
A 35-year-old male was found by a family member unresponsive, with empty propranolol and tizanidine bottles in his lap, and an intact 15 mcg/h buprenorphine patch hanging from his mouth. The patient appeared normal to a family member 2 h before discovery. He used propranolol (immediate release) 40 mg tab twice per day for anxiety, plus 20 mg every 6 h as needed; and tizanidine 4 mg tab every 6 h as needed for muscle spasm and chronic pain. He had filled his propranolol prescription earlier that morning, and the suspected total ingestion was approximately 2.4 g. The maximum possible ingestion of tizanidine was 480 mg based on a prescription filled several weeks prior.
When paramedics arrived, they found him lying on the floor unconscious but spontaneously breathing. He did not respond to 2 mg of IV naloxone. He was transported to a local health center where intubation was attempted without paralytics or sedation, but was unsuccessful due to “patient resisting.” An oral pharyngeal airway was inserted and bilevel positive airway pressure (BIPAP) initiated.
Approximately 3.5 h after the patient was last seen normal (LSN), his vital signs included heart rate (HR) 48 beats/min, blood pressure (BP) 151/90 mmHg, spontaneously breathing 30 times per minute on BIPAP, pupils 4mm and reactive bilaterally, and finger stick glucose of 309 mg/dL. He sat up once and said something about “a cigarette,” then immediately returned to an unresponsive state. The aeromedical transport team successfully intubated the patient prior to transport to a tertiary hospital with etomidate 20 mg and rocuronium 80 mg. He remained unresponsive during air transport with HR 48–64 beats/min and systolic blood pressure (SBP) 149–177 mmHg. Aeromedical transport team gave him lorazepam 4 mg and morphine 5 mg in flight due to hypertension and concern for patient discomfort with ongoing paralysis.
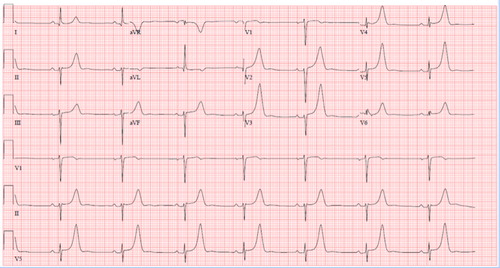
Patient arrived to the tertiary referral hospital 5.5 h after LSN, and vital signs on arrival were HR 44 beats/min, BP 119/99 mmHg, and respiratory rate (RR) 13 breaths/min on the ventilator. The patient was unresponsive to voice or painful stimuli. His ECG showed marked sinus bradycardia (44 beats/min), peaked T-waves on electrocardiogram (ECG), 1mm of ST elevation in V5/6, QRS of 88 ms, and QTc 434 ms (). He received 1 g of calcium chloride. Initial laboratory data demonstrated arterial blood gas (pH 7.37, pCO2 40 mmHg, pO2 241 mmHg, and HCO3 22 meq/L); sodium 138 mEq/L, potassium 3.3 mEq/L, magnesium 1.6 mg/dL, CO2 19 mEq/L, calcium 6.4 mg/dL, anion gap 3, creatinine 0.57 mg/dL, CK 108 units/L, normal liver enzymes and bilirubin, and normal carboxyhemoglobin. Acetaminophen and salicylate concentrations were below the limit of detection.
He received 5 mg of glucagon about 5.7 h after LSN with no improvement. Twenty minutes later, an additional 10 mg of glucagon given, and HR increased from 39 to 58 beats/min with a blood pressure of 152/95 mmHg. He then received a continuous infusion of glucagon at 10mg/h, and HR gradually increased to 65 beats/min. Approximately, 8.5 h after LSN, he had few non-purposeful movements in the extremities, but remained unresponsive to stimuli. He received 100 mcg fentanyl and 2 mg midazolam 8.75 h after LSN to ensure no movement during head CT. Non-contrast head CT was negative for acute pathology. Approximately, 9.5 h after LSN, a 100 mL bolus of 20% lipid emulsion was given, followed by 400 mL over 15 min. About 10 min after initiation of the lipid bolus the patient sat up in bed and looked around, with HR up to 80 s beats/min on the monitor. He was transferred to the intensive care unit (ICU) with downward titration of his glucagon infusion over the next 9 h. Heart rate remained in the 70–90 s beats/min, and the patient remained awake and following commands intermittently. He was started on a propofol infusion at 20 mg/h. He failed first spontaneous breathing trial due to agitation and tachypnea. The ICU team continued glucagon therapy until 19.5 h after LSN despite sustained improved HR and mental status. He was alert and oriented after extubation 21 hours after LSN with HR 96 beats/min, RR 18 breaths/min, BP 155/71 mmHg. After extubation, he confirmed the intentional overdose of an unknown quantity of tizanidine and propranolol. He remained hemodynamically stable for the remainder of the hospitalization.
Discussion
A recent systematic review of non-local anesthetic drug toxicity treated with ILE reported several cases of ILE use in propranolol overdose with mixed response but no cases of tizanidine toxicity managed with ILE [Citation3,Citation7–12]. Our case is distinct from the existing literature in that he did not have cardiac arrest or pressor requirement prior to receiving ILE. Both tizanidine and propranolol cause central nervous system (CNS) depression and bradycardia, and our patient had apparent neurologic and heart rate improvement minutes after receiving ILE.
It is difficult to differentiate the degree of toxicity of propranolol versus tizanidine in this case, as we do not have blood concentrations, and we can only speculate on quantities of either drug taken. Though he was bradycardic prior to receiving glucagon, and severely neurologically depressed until ILE given 9.5 h after LSN, he never became unstable with hemodynamic collapse despite presumed large quantity ingestion of both medications. Furthermore, his duration of symptoms exceeded expected elimination of either drug. Despite the acknowledged change in typical pharmacokinetics in overdoses, the elimination half-life are 2 h for tizanidine and 3–6 h for immediate release propranolol [Citation13,Citation14]. Prior cases of propranolol toxicity do show sustained high blood concentrations and toxic effects >11 h in massive overdose, though these cases had much more severe hemodynamic collapse than our patient had [Citation9,Citation15]. Prior tizanidine toxicity cases with estimated doses of 120–360 mg showed normalizing mental status and vital signs in patients by approximately 5–6 h post ingestion [Citation16]. These prior cases may suggest that propranolol was more likely contributing to the prolonged CNS depression and bradycardia in our patient. Given expected pharmacokinetics, the patient’s improved neurologic status might correspond to natural elimination of propranolol and tizanidine rather than administration of ILE. However, his dramatic change in mental status from unresponsive to sitting up and looking around, along with an increase in the HR from 60s to 80s, correlated precisely with ILE infusion. He also received midazolam and fentanyl 2 h prior to his neurologic improvement, which further confounds his response to ILE. However, given his limited response to midazolam for agitation later in his course, we doubt that a 2 mg dose would have significantly masked neurologic recovery.
Although his heart rate improved with glucagon, and he was hemodynamically stable, we gave ILE due to a limited glucagon supply and due to his ongoing obtundation with unclear expected clinical course. We gave an initial 100 mL bolus of ILE followed by 400 mL infusion over 15 min based on a 70 kg weight. This dosing was consistent with recommendations from ASRA and Pain Medicine and the American College of Medical Toxicology (ACMT) for 1.5 mL/kg initial bolus of 20% lipid emulsion followed by 0.25 mL/kg/min continuous infusion for treating LAST [Citation2].
Propranolol is the most lipophilic of the beta-blockers, which is why ILE may reverse toxicity. Propranolol, unlike other beta-blockers, can cause sodium channel blockade, ultimately contributing to fatal dysrhythmias [Citation17]. Despite the very large dose of propranolol presumably ingested by the patient, he had neither QRS widening nor hypotension. Despite having peaked T waves on the initial ECG, he had marked hypokalemia, hypophosphatemia, and hypomagnesemia. The reason for this is unclear, however, his outpatient pharmacy records did reveal that he was taking furosemide, magnesium, and potassium, so these electrolyte disturbances may have been chronic. He was also markedly hyperglycemic for unclear reasons. He had no history of diabetes, and neither propranolol nor tizanidine causes hyperglycemia.
Tizanidine is an imidazoline derivative muscle relaxant with a mechanism of action and toxicity profile similar to clonidine and other centrally acting alpha-2 agonists [Citation16]. Hypotension, bradycardia, and coma have been seen in adults with doses as low as 60 mg [Citation16]. Tizanidine may in part explain his initial hypertension despite bradycardia due to peripheral alpha-2 agonism, a phenomenon known to occur in overdoses of clonidine and other imidazolines [Citation18].
Our suspicion for significant buprenorphine ingestion was low given the lack of response to a robust dose of naloxone and the lack of miosis or respiratory depression.
Conclusion
ILE infusion appeared to improve CNS depression and bradycardia in a case of mixed propranolol and tizanidine overdose.
Disclosure statement
The authors have no potential conflicts of interest.
References
- Hoegberg L, Bania T, Lavergne V, et al. Systematic review of the effect of intravenous lipid emulsion therapy for local anesthetic toxicity. Clin Toxicol. 2016;53(3):167–193.
- American College of Medical Toxicology. ACMT position statement: interim guidance for the use of lipid resususcitation therapy. J Med Toxicol. 2011;7:81–82.
- Levine M, Hoffman R, Lavergne V, et al. Systematic review of the effect of intravenous lipid emulsion therapy for non-local anesthetics toxicity. Clin Toxicol. 2016; 54(3):194–221.
- Cave G, Harvey M. Intravenous lipid emulsion as antidote beyond local anesthetic toxicity: a systematic review. Acad Emerg Med. 2009;16(9):815–824 .
- Gosselin S, Hoegberg L, Hoffman R, et al . Evidence-based recommendations on the use of intravenous lipid emulsion therapy in poisoning. Clin Toxicol. 2016;54(10):899–923.
- Wishart D, Feunang Y, Guo A, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2017;46(D1):1074–1082.
- Le Fevre P, Gosling M, Acharya K, et al. Dramatic resuscitation with Intralipid in an epinephrine unresponsive cardiac arrest following overdose of amitriptyline and propranolol. BMJ Case Rep. Published online March 2017.
- Thompson A, Franco Palacios C, Henriksen M. Intravenous lipid emulsion and high-dose insulin as adjunctive therapy for propranolol toxicity in a pediatric patient. Am J Health – Syst Pharm. 2016;73(12):880–885.
- Jovic-Stosic J, Gligic B, Putic V, et al. Severe propranolol and ethanol overdose with wide complex tachycardia treated with intravenous lipid emulsion: a case report. Clin Toxicol. 2011;49(5):426–430.
- Hopkins L, Sunkersing J, Jacques A. Too many pills to swallow: a case of a mixed overdose. J Intensive Care Soc. 2017;18(3):247–250.
- Harvey M, Cave G, Lahner D, et al. Insulin versus lipid emulsion in a rabbit model of severe propranolol toxicity: a pilot study. Crit Care Res Pract. 2011;2011 Article ID 361737, 1–7.
- Harvey M, Cave G. Intralipid infusion ameliorates propranolol-induced hypotension in rabbits. J Med Toxicol. 2008;4(2):71–76.
- Product Information: HEMANGEOL(TM) oral solution, propranolol HCl oral solution. Parsippany, NJ: Pierre Fabre Pharmaceuticals, Inc. (per FDA); 2014.
- Product Information: ZANAFLEX(R) oral tablets, tizanidine HCl oral tablets. Ardsley, NY: Acorda Therapeutics Inc. (per FDA); 2013.
- Halloran TJ, Phillips CE. Propranolol intoxication. A severe case responding to norepinephrine therapy. Arch Intern Med. 1981;141:810–811.
- Spiller H, Bosse G, Adamson L. Retrospective review of tizanidine overdose. Clin Toxicol 2004;42(5):593–596.
- Love J, Litovitz T, Howell J, et al. Characterization of fatal betal blocker ingestion: a review of the American Association of Poison Control Centers data from 1985 to 1995. Clin Toxicol. 1997;35(4):353–359.
- Talke P, Caldwell J, Richardson C, et al. The effects of clonidine on human digital vasculature. Anesth Analg. 2000;91(4):793–797.