Abstract
Anticholinergic activity is relevant for many medicinal and natural toxins. Delirium is a common consequence of toxicity. A direct antidote is available, but not often used. At one toxicology center, physostigmine continues to be employed in the emergency department and acute hospital setting. It is given 0.02 mg/kg IV at a rate of 0.5 mg/min with repeat doses q1-2h PRN. The following reports a 6-year retrospective review of the practice and a detailed prospective 1-year observational study of bedside use. One thousand one hundred and ninety seven patients were treated with physostigmine. The overall positive response rate was nearly 80%. The rate of arrhythmias was 0.17%; each event was self-limited. The rate of seizures was 0.75%; none resulted in significant sequelae. Cholinergic signs occurred in 6.4% of patients in prospective study; diaphoresis, nausea, emesis, and incontinence were all manageable. There were no cases of respiratory distress. The only documented adverse events in TCA cases were two episodes of diaphoresis out of 315 patients. Electrocardiographic abnormalities were not considered contraindications to physostigmine, and there were no serious adverse events regardless of QRS duration (72–168 msec) or QTc interval (341–662 msec). Physostigmine is a safe diagnostic and potentially therapeutic antidote for suspected toxic delirium.
Introduction
Delirium is a syndromic presentation of serious underlying medical conditions. It is a harbinger of increased risk of morbidity and mortality and complicates care because of behavioral disturbances and impediments to communication [Citation1]. Anticholinergic activity is a key factor in many delirious states. Pharmacologically, it has been identified in a host of natural sources and mediates the effects of a vast array of medications, including anti-depressants, antihistamines, antiparkinsonian drugs, antipsychotics, and muscle relaxants. The widespread availability of these medications has made them common intoxicants in both accidental and intentional overdoses [Citation2].
The anticholinergic ingestion often presents with a toxidrome, which includes tachycardia, mydriasis, dry skin and mucosae, urinary retention, ileus, and most importantly, neuropsychatric disturbance. However, the non-polar chemistry of many causative agents partitions them to fatty tissues including the central nervous system (CNS), thereby producing delirium without consistently yielding peripheral anticholinergic symptoms. Physostigmine reverses CNS effects of anticholinergic poisoning. It was widely used in psychiatry and anesthesiology in the 1960s and 1970s [Citation3]. It is a tertiary aminocarbamate that reversibly binds to and inhibits the action of acetyl cholinesterase. The result is an increase in acetylcholine at the muscarinic synapse and competitive reversal of neurotransmission blockade. The drug has been shown to be safe and effective in reversing delirium and associated neuropsychiatric unrest in multiple studies, with only minor adverse reactions such as emesis, salivation, and diaphoresis described in most patients [Citation4,Citation5]. More severe reactions, however, have been reported, including seizures and lethal arrhythmias [Citation6,Citation7].
The majority of isolated, severe adverse events associated with physostigmine administration reported in the literature occur in patients with tricyclic antidepressant (TCA) overdoses. These patients suffer other toxic effects of the ingested drug in addition to its anticholinergic activity, including γ-aminobutyric acid (GABA) inhibition, adrenergic volatility, and both sodium and potassium channel blockade. Electrocardiographic changes are commonly seen in severe TCA overdoses and have often been cited as a contraindication for physostigmine use in TCA toxic patients [Citation6,Citation7]. However, other authors have suggested that the severe adverse events, such as asystole and seizures are the result of TCA toxicity, itself, and not physostigmine therapy [Citation8]. Others have proposed that fast drug administrations may have contributed to the severity of the reactions observed in some of the most often cited reports of harm [Citation9].
While the safety of physostigmine has been debated in the literature for over 25 years, many physicians err on the side of caution, and as a result, physostigmine continues to be an underutilized antidote in even the most obvious cases of antimuscarinic poisoning [Citation10]. With this study combining both retrospective review and prospective observation, we present seven years of physostigmine experience in a diverse patient population. Our work supports the safety and efficacy of physostigmine use as both a diagnostic and therapeutic modality in the delirious patient.
Methods
Clinical use of physostigmine
Clinical use of physostigmine based upon its observed safety and efficacy in routine acute toxicologic care serves as the foundation for this study. The setting is the highest volume acute care toxicology practice in North America, whose senior medical toxicologists (including JWD) have over three decades of experience with the antidote. PinnacleHealth Hospitals serve a diverse urban, suburban, and rural population. The toxicology service at Harrisburg Hospital is a regional center in the downtown city, which cares for patients throughout central and eastern Pennsylvania by direct acute presentation and by referral from hospitals across the state. Its sister campus, Community General Hospital, is located on the suburban outskirts of Harrisburg, Pennsylvania, where the catchment is more locally limited. Patients are either referred to the toxicology service through the emergency departments of PinnacleHealth Hospitals or transferred directly to the intensive care of the service from one of over 50 referring hospitals in central and northern Pennsylvania. Initial history is gathered from first responders, other providers, family members, and the patient to the extent that it is possible. Direct bedside care is provided by rotating emergency medicine and internal medicine residents, medical toxicology fellows, and/or toxicology attending physicians.
A general outline for the use of physostigmine by this service in this setting over the course of the study period is presented in . Physostigmine is considered potentially beneficial for symptoms of delirium or coma. There is typically a suspicion of access to xenobiotics with anticholinergic properties, though the ubiquity of such compounds trumps any demand for confirmatory history. Rapid assessment including physical examination with attention to autonomic and neurologic status is performed. Typically, but not always, patients who represent good candidates for a diagnostic and therapeutic trial of physostigmine have normal to exaggerated deep tendon reflexes and an increase in heart rate with mild stimulation. The antidote is not given to patients who are profusely diaphoretic—a sign of cholinergic excess that suggests mental status abnormalities are unlikely to be antimuscarinic in nature. Mild sweating does not rule out central anticholinergic toxicity.
Figure 1. Clinical use of physostigmine. The algorithm summarizes the clinical circumstances and procedure for the use of physostigmine in the emergency department and acute inpatient areas of the study hospital setting.

Continuous cardiac monitoring is attempted during antidote delivery, but sometimes is rendered impractical in patients with delirious agitation prior to treatment. Electrocardiography is performed during initial assessment when it is feasible, as well. A widened QRS complex, elevation of the terminal R-wave in lead aVR, and/or deep, widened S-waves in limb leads are deemed potential signs of sodium channel blockade [Citation11,Citation12]. As these cardiac finding serve as a marker of analogous activity in the CNS with accompanying risk of seizures [Citation11,Citation13], benzodiazepines are given as a prophylactic pretreatment to the use of physostigmine in such cases. Sodium bicarbonate is typically administered, as well, if the QRS duration exceeds 115 msec, but this intervention is not required prior to physostigmine.
The physostigmine is then given by slow intravenous infusion of 0.5 mg/min at a dose of 2 mg in adults, weight-based in younger patients of smaller size. Slow infusion permits the tertiary amino compound to partition to its site of preferential effect in the brain, while minimizing peripheral impact on the cardiopulmonary system. As the effects of this indirect-acting agent are delayed until cholinesterase inhibition is achieved, assessment for effect is performed approximately 15 minutes after infusion. A positive response produces improved wakefulness, cleared cognition, and/or decreased agitation. The Riker Sedation-Agitation Scale is typically used to simply and effectively describe the psychobehavioral status of patients, though they are not routinely recorded in the medical record, and were not for this study [Citation14]. In terms of this scale, patients with a positive response to antidote would be described as having a Riker score that moves from their pretreatment state nearer to 4 (the score associated with calm, cooperative wakefulness). In the event of such a response, repeat doses of physostigmine are given every 1–2 hours as needed to treat delirium and allow patients to participate in their own care so they do not require other sedative agents and accompanying interventions such as urinary catheterization and endotracheal intubation. In the event of cholinergic side effects like profuse diaphoresis, nausea, emesis, and incontinence of urine or feces, physostigmine are discontinued. Patients are treated and monitored with the torso elevated whenever possible to minimize sequelae of emesis, which will abate in most cases within minutes. The routine use of physostigmine in this general fashion underlies the systematic study of its safety and efficacy as outlined below.
Retrospective study
For the retrospective portion of this work, the authors reviewed the electronic medical records in the health system, first identifying all patients under the care of the toxicology service attending physicians from June 2003 to June 2009. The patients included in the study were seen as emergency department consultations, inpatient consultations and/or cared for primarily by the toxicology service in the medical toxicology or intensive care units. Within this group, patients whose treatment included physostigmine were identified through a keyword search of the electronic medication reconciliation database. Patient demographic information, comorbidities, and toxicologic diagnoses were obtained by a review of the medical records associated with each case. In addition, for each identified patient, a separate, written database record, kept by toxicology service attending physicians during patient care, was consulted for supporting information about the patient’s presentation, treatment course, treatment outcome, and adverse events. In accordance with clinical practice as outlined above, treatment outcome was recorded as positive on the basis of increased responsiveness, cleared cognition, and/or decreased agitation. A negative response to antidote implies no change in a patient’s level of consciousness or symptomology. Adverse events related to the use of physostigmine that were routinely recorded in the electronic medical record and in the written service record included seizures, arrhythmias, and respiratory distress. The primary author compared accuracy of the data sources and reconciled discrepancies with the assistance of and clarification by the senior author when necessary.
Prospective study
For the prospective leg of the study, beginning in July 2009, an intentional gathering of data on all patients treated with physostigmine was undertaken, concluding in July 2010. This period corresponded to the first year experience of two fellows in training (JJR and KKS) who, together, saw every patient cared for by PinnacleHealth Toxicology in that year. As described above, patients were treated directly by the toxicology service in multiple settings throughout the health system. In addition to the greater detail and accuracy of data gathering, one particular advantage of this prospective year of study over the previous retrospective years was to identify cases of antidote usage that were confined only to the emergency department, as the electronic medical record does not capture medication orders until after the time of admission. Patient demographic information, co-morbidities, toxicologic diagnoses, laboratory findings, treatment outcomes, and adverse events were thoroughly gathered and carefully recorded. Diagnoses were made, as in previous years, on the basis of clinical presentation, occasionally but not routinely supported by formal toxicologic testing. Criteria for response were consistent with the retrospective study methodology (v.s.), but assessed directly by one of the toxicology fellows on the service (JJR and KKS) in addition to corroborating input from the toxicology attending. Taking the time to add other measures of improved clinical status was not deemed feasible due to the acute nature of clinical demands in the emergency care of patients. Fellows and/or attendings directly read and evaluated electrocardiography that was available prior to administration of antidote. QRS duration, QTc measurement, and morphologic signs of sodium channel blockade were recorded.
In addition to seizures, arrhythmias, and respiratory distress—the adverse events related to use of physostigmine that were routinely recorded in the electronic medical record and in the written service record—other effects were ascertained, including signs of cholinergic excess. No specific laboratory or diagnostic criteria were used for identifying patients to receive physostigmine. The decision to use the drug was based upon the judgment of the treating physician evaluating the clinical data as outlined (). In adults and adolescents 50 kg or greater 2 mg IV of physostigmine was administered at a rate of 0.5 mg/min. In children and adolescents 50 kg or less 1 mg IV of physostigmine was administered at the same rate as the adult dose. Toddlers under 20 kg received 0.5 mg IV of physostigmine over 1 minute. These parameters were the same as those used to guide therapy during the retrospective years of the study.
Logistics and statistics
Both phases of the study were approved by the Institutional Review Board at PinnacleHealth. Results were tabulated using Microsoft Office Excel. All statistical analyses were completed using the chi-square test for results involving dichotomous categorical variables and logistical regression for results involving the continuous independent variables of age and electrocardiographic intervals. In addition, results were analyzed with respect to electrocardiographic intervals by sorting cases into clinically meaningful subgroups and performing chi-square analysis. Specifically, because 100 msec is the top limit of normal for QRS duration, and thresholds of 130 and 160 msec have previously been identified as risk predictors for seizures and arrhythmias [Citation13], four groups were created for analysis based on ventricular conduction. And, because 450 msec is commonly taken to be the top limit of normal for QTc duration (an average of accepted limits for the two genders), this threshold, along with the round numbers of 500, 550, and 600 msec that sometimes guide clinical practice management of repolarization delay, was used to define five groups for analysis of this variable.
Results
Retrospective review - demographic information and overall response rates
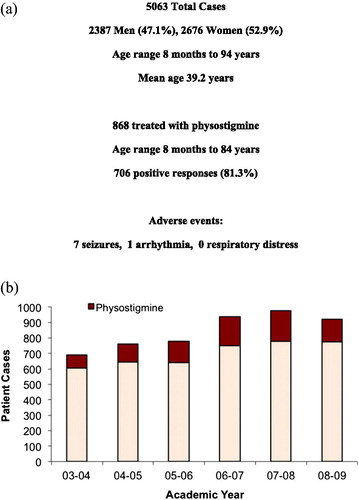
Across the health system, a total of 5063 patients were treated by the Toxicology Service between June 2003 and July 2009 (). Of these cases, 868 patients were identified who received physostigmine. Patients ranged from 8 months to 84 years of age. 706 (81.3%) of these patients demonstrated a positive response to physostigmine therapy. There were just eight documented adverse events attributable to the antidote. demonstrates the annual use of physostigmine. There was a general trend toward increased use over time during the study period.
Figure 2. Retrospective study overview. (a) Summarizes the medical toxicology practice cases for the retrospective study, the number treated with physostigmine, the overall response rate, and reported side effects. (b) Reports the number of cases year-by-year over the retrospective study period, and highlights the increasing proportion treated with physostigmine each year.
Retrospective review – physostigmine use, response rates, and adverse events by comorbid diagnoses
TCA overdoses, seizure disorders, and electrocardiogram (EKG) abnormalities are often cited as contraindications to physostigmine administration. demonstrates the number of patients treated with multiple toxicologic diagnoses, TCA toxicity, sedative abuse, alcohol abuse, and comorbidities such as heart and lung disease and seizure disorders between 2003 and 2009. The category of Parkinsonism includes patients with Parkinson’s Disease along with individuals who had Parkinsonian symptoms from medications and other causes. Retrospective review of the record was not sufficient to distinguish the specific etiologies. However, patients with Parkinsonism were more likely to receive the antidote and have a positive response to it than patients without the movement condition; and they were no more likely to have a documented adverse event.
Table 1. Retrospective review of response rates and adverse events with physostigmine.
The number of patients treated with physostigmine in each sub-population is reported in . Individuals with multiple toxicologic diagnoses were statistically more likely to receive the antidote—a finding consistent with the described clinical practice of using physostigmine diagnostically and therapeutically in cases of undifferentiated poisoning. Specific diagnoses with phenomenologic overlap with anticholinergic toxicity also predicted a higher rate of antidote use. These include serotonin syndrome, stimulant toxicity, and lithium toxicity. A large percentage of patients with neuroleptic toxicity received the antidote, as well. And the overwhelming majority of patients with TCA poisoning were treated with physostigmine.
A positive response rate to physostigmine ranging from 64% to 86% was observed across the various demographic and comorbidity subgroups. Male patients were somewhat more likely to have a documented positive response to the antidote; this significant difference disappeared when controlling for toxicologic diagnosis, as male patients were also more likely to have been exposed to an anticholinergic agent. There was a trend toward patients with an alcohol abuse or dependence history being slightly less likely to receive physostigmine, but discounting those whose toxicologic diagnosis was alcohol withdrawal, those with an alcohol history were more likely to receive the antidote.
Positive response rates to physostigmine on the basis of toxicologic diagnosis varied from 38% to 96%, with patients having multiple diagnoses responding at rate of 83.9%—similar to the overall response rate of 81.3%. Patients with TCA toxicity were statistically more likely to respond favorably to therapy. Response rates were lower in patients with serotonin syndrome, opioid toxicity, anticonvulsant toxicity, and lithium toxicity, but adverse events were still very uncommon regardless of diagnosis.
Eight adverse events were documented in the retrospective analysis—one arrhythmia and seven seizures. The rhythm disturbance involved a 25-year-old female patient with diphenhydramine toxicity and a history of mitral valve prolapse who developed premature ventricular contractions for less than 30 seconds, approximately 25 minutes after physostigmine administration (). The event was captured on cardiac monitoring, but documentation indicates that the patient remained asymptomatic for its duration, and no further arrhythmias were recorded; subsequent doses of physostigmine were given during her hospital course. All of the cases of seizures were described as generalized tonic-clonic. None resulted in further complications or morbidity. In each instance, the ictal events were self-limited and non-recurrent; both medical and pharmacy records corroborate that seizures stopped on their own before benzodiazepines were given. In five of the seben cases, the patients also had a positive response to physostigmine, and in four cases the antidote was administered again, after lorazepam treatment, with no further seizures. Intoxicants in these cases included stimulants, SSRI, and SNRI antidepressants, antipsychotics, lithium, and tramadol. None of the recorded medical comorbidities or toxicologic conditions (including TCA toxicity or having multiple toxicologic diagnoses) were associated with statistically greater likelihood of adverse events. There were no documented cases of respiratory distress or extra pyramidal reactions to physostigmine.
Table 2. Response rates and adverse events with prospective use of physostigmine.
Prospective study – physostigmine use, response rates, and adverse events
A total of 1026 patients between the ages of 2 and 89 years were treated by the Toxicology Service between July 2009 and July 2010, and each case was included in the prospective arm of this study. Of these cases, 329 received physostigmine. Patients given the antidote ranged in age from 3 to 89 years, with 154 males and 175 females. 243 (73.9%) of these patients demonstrated a clinically meaningful positive response. reports the number of patients treated for multiple toxicologic diagnoses, TCA toxicity, sedative abuse, alcohol abuse, and comorbidities such as heart and lung disease and seizure disorders between July 2009 and July 2010. More details were captured with prospective study, and some differences in patient characteristics are reported as a result.
The prospective study identified a significantly greater proportion of patients with seizure disorders, alcohol abuse, and abuse of sedative medications and illicit substances than the retrospective design. The category of Parkinsonism, again, includes patients with Parkinson’s Disease along with individuals who had Parkinsonian symptoms from medications and other causes. The prospective study methodology made it possible to distinguish the specific etiologies for each case—nine with medication induced Parkinsonism and two with Parkinson’s disease. As in the retrospective review, patients with Parkinsonism were again more likely to receive the antidote than patients without the movement condition; the positive response rate was high in these patients, and they were no more likely to have a documented adverse event. None had an exacerbation of movement disorder symptoms.
Fifteen patients with cardiac disease were given physostigmine, including four with documented rhythm disorders. Six patients presenting with TCA toxicity also had a history of coronary artery disease—three with prior myocardial infarction. None of these cardiac patients had an arrhythmogenic event with antidote. The one adverse event reported in this group was a case of mild nausea and diaphoresis. Pulmonary conditions represented in the cohort exposed to physostigmine include asthma, chronic obstructive pulmonary disease, sarcoidosis, recent pulmonary embolism, and lung cancer. Most patients with seizure disorders were prescribed medications to control the condition; adherence to treatment was generally low. Abuse disorders involved a variety of substances, including alcohol, heroin, cocaine, cannabis, phencyclidine, opioid analgesics, benzodiazepines, synthetic cannabinoids, amphetamines, synthetic cathinones, and hydrocarbon inhalants. Greater than 25% of patients had nicotine dependence, as well. None of these conditions conferred an increased risk of adverse events with physostigmine.
The number of patients treated prospectively with physostigmine in each toxicologic diagnostic group is demonstrated in . As in the retrospective study, individuals with multiple toxicologic diagnoses were statistically more likely to receive the antidote—a finding consistent with the described clinical practice of using physostigmine diagnostically and therapeutically in cases of undifferentiated poisoning. Serotonin syndrome and stimulant toxicity again predicted a higher rate of antidote use, as well as a lower rate of positive response. Patients with neuroleptic toxicity were more likely to receive the antidote as well, and have a positive response. Opioid toxicity and sedative toxicity predicted a lower likelihood of administration and response, though nearly half of these patients still did show clinically positive responses. The majority of patients with TCA poisoning were treated with physostigmine, and all but one had a positive response. The only patient with a negative response coingested oxycodone and presented late, with evidence of damage to multiple organs due to shock. None of the TCA patients suffered serious adverse effects from physostigmine; two manifested diaphoresis late in their hospital course after multiple doses of antidote had been administered previously with positive responses.
In 329 patients given physostigmine during the prospective year, twenty-four total adverse events were observed, the majority of which were minor cholinergic signs (). Thirteen patients experienced diaphoresis and eight patients had gastrointestinal effects in the form of nausea, vomiting or stool incontinence. None of these effects led to clinically significant sequelae. The most common adverse event was transient diaphoresis. No adverse effects involving extra pyramidal reactions or respiratory distress (secondary to bronchospasm or pulmonary congestion) were observed during the prospective year. Three adverse events were noted similar to those documented from the retrospective review.
Two patients developed seizures approximately 12 minutes after antidote administration. One occurred in a 62-year-old patient with intellectual disability and epilepsy whose laboratory studies later confirmed sub-therapeutic serum concentrations of both of his two antiepileptic medications. He presented with quetiapine toxicity, and the associated delirium responded well to physostigmine, despite the isolated 20-second ictus. So, he was treated with lorazepam prior to subsequent doses of the antidote and had no further seizures. The other seizure arose in a 39-year-old man with schizophrenia who had taken a purposeful overdose of clozapine and trifluphenazine. He also had a positive response to physostigmine first, and then a tonic-clonic seizure began just shortly after delirium cleared and lasted approximately 25 seconds. Lorazepam had not been given despite recommendation for it by standard protocol for epileptogenic toxins, but it was given prior to future doses of antidote, which yielded positive responses and no further adverse events. The same patient also accounts for one of the cases of physostigmine-induced seizure activity in the retrospective study period.
Physostigmine administration was associated with one arrhythmia during the prospective period, as well. A 45-year-old man presented with quetiapine toxicity and alcohol intoxication in a state of delirium. In violation of standard protocol, the patient was given two doses of 2 mg physostigmine 14 minutes apart, the second via rapid IV push. His heart rate dropped to 40 bpm, and he felt subjectively-light headed. Within minutes, his rhythm converted spontaneously to atrial fibrillation. The patient felt subjectively well again, and the new electrocardiographic pattern converted spontaneously back to sinus rhythm within 75 minutes. Subsequent doses of physostigmine were delivered in accordance with the established dosing protocol (), and the patient suffered no further side effects. None of the patients with these reactions encountered complications or lasting sequelae that could be attributed to the dosing of antidote.
Prospective study – electrocardiographic characteristics of physostigmine treated patients
The majority of acute toxicology patients in our practice have an EKG performed early in their assessment. Of the 329 patients treated with physostigmine during the prospective year, 312 had an EKG before receiving the first dose of antidote, and 245 of those were performed within 30 minutes of physostigmine infusion. describes the EKG characteristics of patients given physostigmine during the prospective study year. Over half of patients had at least one abnormal interval measurement, with the most common being mild QTc prolongation. More than 20% of patients, however, had more significant repolarization delay, and over one third of patients had impaired ventricular conduction as evidenced by QRS duration greater than 100 msec.
Table 3. Electrocardiographic characteristics of patients treated with physostigmine.
Those patients with an abnormally wide QRS complex were statistically more likely to have a positive response to physostigmine than those with normal QRS duration. Logistic regression indicated a roughly 0.6% greater probability of positive response for each 1 msec above 100 in QRS measurement. This trend correlated with a larger proportion of patients with a diagnosis of TCA toxicity. A QTc interval between 450 and 550 msec was associated with greater likelihood of positive response to antidote than measurements outside this range. As noted above, the number of serious side effects was very low; none of the EKG findings predicted physostigmine-related adverse events. The patient who experienced arrhythmia secondary to physostigmine overdosing had a QRS duration of 96 msec and a QTc interval of 484 msec. Patients with seizures both had QRS measurements of 92 msec; the QTc for one was 477 msec and the other 536 msec. All patients with TCA toxicity had an EKG within 30 minutes of receiving physostigmine, and none had a seizure or arrhythmia.
Discussion
Anticholinergic toxicity is a relatively common, but often unrecognized direct precipitant of delirium. As delirium, regardless of cause, confers an increased risk of morbidity and mortality, its prompt identification and treatment is a central concern in acute medical practice. Although debate continues regarding the most effective management strategies for the symptoms of the syndrome, it has been well established that the most important intervention for any delirious state is treatment of the underlying cause. For anticholinergic delirium, there is a direct antidote available that addresses the neurochemical etiology, but it has been vastly underutilized for decades due to concerns about adverse effects. The clinical data outlined above should largely dispel any myth that physostigmine is an unsafe antidote for suspected central anticholinergic toxicity.
Combining both the retrospective review and prospective study we present 7 years of experience with physostigmine at PinnacleHealth, where bedside use of the antidote increased over that time period on the basis of its observed utility and safety profile. 1197 patients treated with physostigmine for suspected anticholinergic toxicity showed a positive response rate of nearly 80% and experienced a total of just 11 significant adverse reactions, none of which resulted in complications or lasting sequelae. Mild peripheral cholinergic signs were observed during the detailed prospective study year, but each occurrence was self-limited with the dosing algorithm we have adopted. The antidote is therefore used frequently for diagnostic purposes in cases of undifferentiated poisoning with delirium and then continued as targeted treatment when anticholinergic toxicity is identified by the favorable response.
Based upon the treatment practice outlined above (), the only patients who would receive a diagnosis of anticholinergic toxicity and not receive physostigmine would be those with very mild central effects or just peripheral symptoms. Routine practice has been to assign a diagnosis of anticholinergic toxicity to all patients who have a demonstrable positive response to physostigmine. One could contend with this practice on the basis of the notion that physostigmine might function as an analeptic even in cases in which compounds with anticholinergic activity are not present and responsible for altered mentation and behavior. There have been cases reporting positive response to physostigmine in reversing general anesthesia and in cases of toxicity involving other agents like opioids and benzodiazepines [Citation15–17]; though one could also argue that impaired cholinergic neurotransmission is an accompanying effect of such agents [Citation18–20]. Nevertheless, we do appreciate that the assignment of the diagnosis of anticholinergic toxicity in this clinical sample may be viewed as dependent upon a degree of circular logic, so the report of all the positive responses in this diagnostic subgroup may not be statistically meaningful. However, the safety (and, in our estimation, utility) of the antidote cannot be denied, especially noting that 38% of the patients studied had multiple toxicologic diagnoses, and before confirmation of those toxicities, physostigmine was used in a significantly higher proportion of that complex subgroup with a response rate exceeding 80%. Therefore, the oft quoted physostigmine contraindication of “undifferentiated poisoning” is not supported by bedside experience [Citation9,Citation10]. In addition, virtually every patient with TCA toxicity will have anticholinergic delirium, and none of those patients in this large study suffered a significant adverse reaction to physostigmine, while there was a very high response rate.
Although we appreciate that a medication with cholinergic activity has the potential to impact cardiac function, there has never been a solid physiochemical rationale for fearing the use of physostigmine in TCA patients. The mechanisms involved in cardiotoxicity from TCAs are based upon altered cation flow—both sodium channel blockade and potassium efflux inhibition increase the risk of a lethal arrhythmia. The former also markedly impairs cardiac inotropic function with the potential for hypotension and shock. Physostigmine, however, reduces chronotropy via enhanced vagal activity, and does not impact myocardial conduction or the flow of ions that modulate it. The major documented risk factor in cases of morbid and mortal outcomes of TCA patients given physostigmine that have been propagated through the medical literature is the presence of TCA toxicity itself [Citation8,Citation10]. Severe, progressive cardiotoxicity with sodium channel blockade devolves to shock and arrest on its own, as evidenced by many cases of overdose death involving these compounds. It has merely been post hoc ergo propter hoc reasoning that lays blame with the cholinesterase inhibitor antidote. As long as it is delivered by slow infusion, thereby allowing the lipophilic tertiary aminocarbamate to partition to the CNS without being circulated quickly and undiluted to the pulmonary and coronary vasculature, concerns about toxicity are unfounded.
This assertion is supported by the electrocardiographic data presented in . Patients with a wide variety of conduction variability, both in width of the QRS complex and duration of the QT segment were given physostigmine without suffering arrhythmias. Longer QRS duration was actually associated with a statistically greater likelihood of positive response to the antidote. This effect appears to be driven by the fact that TCAs almost invariably produce an anticholinergic delirium in toxic exposure that will respond to physostigmine, and they frequently cause sodium channel blockade that widens the QRS complex, as well. Most TCA patients in our study had QTc intervals ranging between 450 and 550 msec—also the range that predicted a somewhat greater likelihood of positive response to physostigmine. Many patients with delirium from other anticholinergic agents also had this degree of QT prolongation. Otherwise, the EKG abnormalities caused by scores of different toxins, including TCAs, had no significant impact on safety or response rates. The single case of arrhythmia in our prospective study involved a dosing error, in which an adult patient with anticholinergic delirium from quetiapine toxicity was given 4 mg of physostigmine within 14 minutes. Absent such misuse, even the most severely TCA poisoned patients in our critical care toxicology practice received physostigmine without cardiac side effects.
The question of seizures does require some discussion. Many anticholinergic toxins, including TCAs, have other pharmacologic properties that increase the risk of seizures [Citation2]. Physostigmine is an analeptic, and as a stimulating antidote, can increase neural activity in such a way that augments this risk and precipitates an ictus. Seizures and the resulting acidosis can complicate the care of toxicologic patients, so individuals whose suspected ingestion would yield a significant seizure risk should receive a dose of benzodiazepines prior to intervention with physostigmine. This is obviously the case for TCA patients with any significant toxicity, since the noradrenergic surge and GABA inhibition have robust synergistic epileptogenicity; attention to this key aspect of TCA poisoning management explains the absence of physostigmine induced seizures in our clinical study. As a result, we find the antidote a safe and useful treatment for anticholinergic delirium, especially in TCA patients, reducing the need for restraint, oversedation, mechanical ventilation, and bladder catheterization with a very high response rate with toxins that produce anticholinergic delirium. Clearing of delirium may reduce the need for further workup and expensive testing, including computed tomography scanning of the head.
The overall rate of positive response to physostigmine was higher for men than for women in both the retrospective and prospective arms of this study. However, the significant difference between the genders does not persist when controlling for toxicologic diagnosis; more men in our practice had exposure to anticholinergic agents, including TCAs. Although there may be neuropsychiatric differences in the cholinergic system between men and women [Citation21], we know of no physiologic reason for there to be differential response to antidote. Therefore we do not favor any modification of the use of the antidote on the basis of gender.
We suspect that the majority of differences in patient characteristics between the prospective and retrospective arms of the study relate to our ability to gather more complete clinical datasets during the prospective year. It is unlikely, for instance, that rates of alcohol use and seizure disorders would have increased significantly in one year’s time without changes in practice or referral patterns, which were not observed. The only notable exception was a rise in the use of synthetic cannabinoids and cathinones whose clinical presentations prompted the use of physostigmine in many cases without positive response; this trend may explain some of both the greater use of and lower response rate to physostigmine in substance using patients in the prospective year.
In accordance with the study design, we also suspect that overall use of physostigmine was not markedly higher in the prospective year as compared to the final year of the retrospective study as it appears. Rather, the prospective study captured cases in which the antidote was used in the emergency department but then not used again after admission. This interpretation is consistent with the overall lower rate of positive response in the prospective year, as a lack of improvement with a single dose of physostigmine in the emergency setting would be documented as a negative response, and repeat doses would not be given later in the hospital course after admission. The antidote would have served a diagnostic purpose to rule out central anticholinergic toxicity, but would not be of further therapeutic value to warrant subsequent use. Such was likely the case in our practice from 2003 to 2009, as well, but simply not captured by the retrospective methodology. Single use cases also occurred with positive results, as well though, in exposures involving short acting toxins like doxylamine and diphenhydramine. The use of physostigmine in these cases would have been captured in the prospective year, but not the retrospective review.
With respect to side effects, in the prospective study year the majority of those reported pertain to the first dose of antidote given to any particular patient. In the event of an adverse reaction in the absence of positive response, no further doses of physostigmine would be administered in accordance with clinical practice (). The two patients who both responded favorably to the antidote and developed seizures, however, were treated with benzodiazepines and then responded to subsequent doses of physostigmine for management of their delirium during the remainder of hospitalization. Occasionally patients had positive responses to a series of doses and then, with clearance of toxins, would develop cholinergic signs when given physostigmine later in their course. Even these adverse events are reported in the data (), and yet the total incidence of side effects using the dosing regimen for physostigmine as outlined remained low. Only major adverse reactions were documented in the medical record for the years reviewed prior to prospective study; all eight correspond to the first dose of physostigmine in a given episode of care, at the point of diagnostic assessment.
Serotonin syndrome is the most common toxicologic differential diagnosis in patients with anticholinergic syndrome. Although textbook descriptions of the two would suggest being able to differentiate on the basis of reflexes, lower extremity tone, bowel motility, and/or level of secretions and sweating, the bedside reality is more complicated [Citation22]. Furthermore, as many of the compounds involved in such cases are highly lipophilic and preferentially partition to the central compartment, effects of serotonergic agents and anticholinergic agents often will cause similar deliria while yielding non-distinct peripheral manifestations. This biochemical partitioning with corresponding clinical predominance of CNS effects supports the use of the centrally acting physostigmine without concern for peripheral signs such as pupil size, bowel sounds, or heart rate. Only profuse sweating is a contraindication with our clinical protocol (), therefore many patients with serotonin syndrome receive a dose of physostigmine in our practice to differentiate the two conditions and potentially provide benefit in treating toxic delirium. A negative response in such circumstances lends more support for a diagnosis of serotonin syndrome with corresponding abandonment of physostigmine in favor of benzodiazepines and/or other sedative treatments.
Comorbid alcohol intoxication was a common finding in our patient population. No more adverse events attributable to physostigmine were observed in this subgroup, either retrospectively or prospectively, and response rates were similarly robust. Positive response rates were lower in patients with a diagnosis of anticonvulsant toxicity. However, carbamazepine has significant anticholinergic activity, so the response rate in cases involving this particular agent was actually higher than average—over 90%. Similarly, a number of antipsychotic medications are anticholinergic. Thus a larger proportion of patients with toxic exposures to neuroleptics was given physostigmine, and correspondingly, displayed a significantly higher response rate. A fraction of the patients listed in this category had initial presentations involving dystonic reactions or other extra pyramidal manifestations without delirium, and were therefore obviously not given physostigmine, but treated with anticholinergic medications, instead.
Although rates of positive response were lower in patients with opioid and lithium toxicity diagnoses, major adverse events were no more likely. There were no adverse events in patients with toxicity from cardiovascular medications apart from one case of nausea, and the likelihood of positive response to physostigmine (due to coingestion of anticholinergic compounds) was comparable to the overall response rate. We reported data for this subgroup of patients to further highlight the cardiac safety profile of the antidote. In general, the clinical experience underlying these studies indicates that physostigmine can be used safely and effectively in patients with multiple toxicities to diagnose and treat the neurobehavioral impairments associated with anticholinergic burden, even if that is only one of several manifestations of acute poisoning. A list of substances with anticholinergic activity (representing a variety of chemical and medicinal classes) for which we have found physostigmine efficacious is listed in .
Table 4. Antimuscarinic compounds for which physostigmine is antidotal.
Attempts to be thorough and unbiased were pursued throughout the course of this research, but the limitations of the work must be noted. The retrospective design of the first leg of this study resulted in a cohort of patients with an occasionally-incomplete medical record and reliance on a secondary written record. Some adverse events may have been missed, because they may not have been recorded in the medical record. Such is not the case, however, for the prospective year of study. As already noted, the lack of an electronic medical record for the emergency department limited full evaluation of some cases and inevitably resulted in undercounting of cases of antidote use during the retrospective period, some of which potentially involved adverse reactions, as well.
The possibility of this research, however, depended upon the invaluable resource of the toxicology practice database—a systematic documentation routine established by the medical director of the service (JWD) at its inception in June 2003 and consistently maintained for the study period. Although potentially biased by the practitioner’s perspective on the course and care of patients, the detailed notes represent extensive bedside experience in toxicology and the use of antidotes based not upon offsite case consultative input, but upon direct physician assessment and intervention. This system served as the model and starting point for designing the prospective arm of the study. During that study year, both junior fellows (JJR and KKS) gathered data for the study, and these records were reconciled with each other and with the attending’s notes (per JWD’s established routine). Most patients were seen by both fellows during their care by the toxicology service—discrepancies were rare, and reconciled by the primary author with the documentation and guidance of the senior author. We acknowledge the potential for biased reporting in this process. Furthermore, not every administered dose of antidote was observed directly by the authors (or even a rotating trainee physician). The use of physostigmine as outlined () is so routine in our practice, that nursing staff follow PRN orders as long as patients show improvements in mental status; a physician is contacted if cholinergic signs are observed, so although these reactions were recorded by study physicians, personnel may have gone to the bedside after they occurred, and would obviously not administer physostigmine again merely to reproduce the side effect for confirmation.
The subjective nature of the clinical assessment of outcomes may have also contributed to recorder bias with respect to antidotal response. And although responses to antidote were recorded as simply positive or negative, the reality of bedside treatment is that some patients experienced much more complete restoration of neuropsychiatric function than others. The design of this study did not account for that variability, but instead ascribed a positive response to all patients who displayed at least some clinically relevant improvement in wakefulness, cognition, or behavior. A portion of patients still required adjunctive pharmacologic and non-pharmacologic interventions for management of neurobehavioral manifestations of toxicity, even when physostigmine was deemed effective.
With respect to efficacy, this study does not directly address that question. Despite its size and the report of regular clinical use based on this extensive experience, the present study does not definitively indicate whether physostigmine use in toxicologic practice is responsible for better clinical outcomes. Such a claim would require a placebo-controlled trial of the antidote with the clinical methodology suggested (). Our service has previously reported shortening the length of stay in Datura poisoned patients who received physostigmine rather than placebo as part of their care [Citation23]. Since that time, the clinical experience documented for a wide variety of toxic patients in the retrospective portion of this study has convinced our service and numerous trainees rotating through it that physostigmine is safe and effective. Scores of patients who would have been intubated and sedated, thereby increasing their risk of nosocomial infection and other complications from greater instrumentation and increased lengths of stay serve as testament to this perspective. In routine clinical practice after this study, positive antidotal response has also reduced utilization of computed tomography and allowed more thorough assessment of patients through productive interviewing and detailed physical examination that revealed different acute care needs that might otherwise have been missed. It is experience with those patients that convinced the authors the prospective year would be more ethically conducted as an observational study instead of a randomized trial that would deny a large number of patients an efficacious treatment. We appreciate, however, that a placebo-controlled experiment has not been conducted to measure lengths of stay, complication rates, and long-term outcomes to support this claim; our report of positive results in this study, despite its size, lacks a comparison group.
It is worth noting, however, that clinical science does already support use of physostigmine in the manner described. As noted above, the best treatment for any delirial state is the one aimed at the underlying cause. Physostigmine has the potential to address a toxic etiology directly and diminish or even reverse CNS dysfunction—a more targeted and definitive treatment for the syndrome than more commonly employed agents for managing agitation that typically produce sedation but leave patients impaired. Such impairments demand a higher rate of catheterization, intubation, and restraint. And those instruments, in turn, come with increased risk of complications. So although we do not have direct data on the ability of physostigmine to decrease complication rates and lengths of stay, logic and circumstantial evidence point in that direction. More definitive data, however, speak to implications that last long after patients first arrive in the emergency department with an overdose or toxic misadventure.
Some physicians choose not to treat delirial symptoms unless they appear to be a threat to life and limb as a result of agitation, or at the very least, pose challenges to nursing care. However, recent studies indicate that any untreated delirium increases the risk of long-term poor health. Post-traumatic stress disorder (PTSD) as a consequence of medical trauma is much more likely in patients who suffer delirium regardless of the cause, and the more severe and longer lasting the delirium, the greater that risk [Citation24]. Furthermore, other independent predictors of PTSD one and two years after discharge include amnesia for the early portion of hospitalization, youth, female gender, low education level, trait anxiety, and lack of social support [Citation24]—all characteristics that are more common in the acute toxicology patient than those hospitalized for other reasons.
The risk of functionally meaningful depression during the years following hospitalization also increases greatly as a function of delirium and its severity. Depression prevalence following a hospital stay with delirium is 31% [Citation25], an estimated two-fold increase in mood disorder diagnosis as compared to those whose hospitalizations proceed without similar CNS insult. As with PTSD, common characteristics of toxicology patients—female gender and lack of social support—increase this depression risk [Citation26]. Additionally, the cumulative dose of benzodiazepines during an intensive care unit stay is positively correlated with depression rates in the years following discharge [Citation27], and this is the class of medications most commonly employed to manage agitation and maintain sedation when the direct therapy, physostigmine, could reduce their use in many of cases [Citation5]. So although not routinely employed in most emergency settings, physostigmine may be useful not only as a short term diagnostic tool and treatment for agitation in toxicology patients, but also helpful in taking the long view of patients’ physical and mental resilience against the threat of delirium.
As supported by the largest study to date, physostigmine is a safe and potentially effective medication in the undifferentiated patient with delirium and a differential diagnostic list that includes toxic etiologies for the alterations in mental status. Even in the setting of TCA and mixed drug ingestions and in patients with a variety of medical comorbidities, the antidote produces few side effects when properly dosed. The key to its safe use is clinical assessment of vital signs, cognition, neuromuscular activity, and secretory status followed, where indicated, by slow weight-based infusion and reassessment of neurobehavioral status after 15 minutes. With attention to the other demands of toxicologic care, the incidence of seizures is low, and cardiotoxic sequelae are essentially absent. Cholinergic adverse effects are generally mild and self-limited; maintaining an erect posture of the torso prevents complications from emesis. Based on the ubiquity of anticholinergic activity in widely used medicinal and abusable substances, the potential to diagnose toxicity, clear cognition rapidly, and allow more patients to participate in their own care is great. On the basis of this study, we advocate for the expanded use of physostigmine in cases of altered mental status in emergency and hospital medicine.
Acknowledgments
The authors thank Erica E. Smolcic, M.D. and Amanda Cresswell R.N., M.S.N., C.M.S.R.N. for assistance with chart review. We are also grateful to Kara Gemberling and Patti Metherell for arranging data extraction from the electronic medical and pharmacy records of PinnacleHealth. In addition, Jeremiah Escajeda, M.D. provided invaluable assistance with background research for the preparation of this manuscript. JJR and JWD conceived the study and designed the trial. JJR, KKS, and JWD supervised the conduct of the trial and collected data. JJR managed and analyzed the data and assured quality control. JJR drafted the manuscript, and all authors contributed substantially to its revision. JJR takes responsibility for the paper as a whole.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Clary GL, Krishnan KR. Delirium: diagnosis, neuropathogenesis, and treatment. J Psychiatr Pract. 2001;7:310–323.
- Smith SW. Drugs and pharmaceuticals: management of intoxication and antidotes. EXS. 2010;100:397–460.
- Heiser JF, Gillin JC. The reversal of anticholinergic drug-induced delirium and coma with physostigmine. Am J Psychiatry. 1971;127:1050–1054.
- Schneir AB, Offerman SR, Ly BT, et al. Complications of diagnostic physostigmine administration to emergency department patients. Ann Emerg Med. 2003;42:14–19.
- Burns MJ, Linden CH, Graudins A, et al. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35:374–381.
- Knudsen K, Heath A. Effects of self poisoning with maprotiline. Br Med J (Clin Res Ed). 1984;288:601–603.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9:588–590.
- Kulig K, Rumack BH. Physostigmine and asystole. Ann Emerg Med. 1981;10:228–229.
- Shannon M. Toxicology reviews: physostigmine. Pediatr Emerg Care. 1998;14:224–226.
- Suchard JR. Assessing physostigmine's contraindication in cyclic antidepressant ingestions. J Emerg Med. 2003;25:185–191.
- Liebelt EL, Francis PD, Woolf AD. ECG lead aVR versus QRS interval in predicting seizures and arrhythmias in acute tricyclic antidepressant toxicity. Ann Emerg Med. 1995;26:195–201.
- Liebelt EL, Ulrich A, Francis PD, et al. Serial electrocardiogram changes in acute tricyclic antidepressant overdoses. Crit Care Med. 1997;25:1721–1726.
- Boehnert MT, Lovejoy FH. Jr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313:474–479.
- Brandl KM, Langley KA, Riker RR, et al. Confirming the reliability of the sedation-agitation scale administered by ICU nurses without experience in its use. Pharmacotherapy. 2001;21:431–436.
- Shulman MS, Sandler A, Brebner J. The reversal of epidural morphine induced somnolence with physostigmine. Can Anaesth Soc J. 1984;31:678–680.
- Rupreht J. Physostigmine reversal of diazepam. Anesthesiology. 1980;53:180–181.
- Ongini E, Parravicini L, Bamonte F. Effects of physostigmine on benzodiazepine toxicity. Arch Int Pharmacodyn Ther. 1981;253:164–176.
- Eisendrath SJ, Goldman B, Douglas J, et al. Meperidine-induced delirium. Am J Psychiatry. 1987;144:1062–1065.
- Tune LE. Serum anticholinergic activity levels and delirium in the elderly. Semin Clin Neuropsychiatry. 2000;5:149–153.
- Tune LE. Anticholinergic effects of medication in elderly patients. J Clin Psychiatry. 2001;62 Suppl 21:11–14.
- Furey ML, Khanna A, Hoffman EM, et al. Scopolamine produces larger antidepressant and antianxiety effects in women than in men. Neuropsychopharmacol.. 2010;35:2479–2488.
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120.
- Burkhart KK, Magalski AE, Donovan JW. A retrospective review of the use of activated charcoal and physostigmine in the treatment of jimson weed poisoning. J Toxicol Clin Toxicol. 1999;37:389.
- Granja C, Gomes E, Amaro A, JMIP Study Group, et al. Understanding posttraumatic stress disorder-related symptoms after critical care: the early illness amnesia hypothesis. Crit Care Med. 2008;36:2801–2809.
- Davydow DS. Symptoms of depression and anxiety after delirium. Psychosomatics. 2009;50:309–316.
- Myhren H, Ekeberg O, Tøien K, et al. Posttraumatic stress, anxiety and depression symptoms in patients during the first year post intensive care unit discharge. Crit Care. 2010;14:R14.
- Davydow DS, Gifford JM, Desai SV, et al. Posttraumatic stress disorder in general intensive care unit survivors: a systematic review. Gen Hosp Psychiatry. 2008;30:421–434.
