Abstract
Envenoming by hump-nosed pit vipers (genus Hypnale) causes local effects, mild coagulopathy, and rarely, acute kidney injury. Neuromuscular dysfunction has not been clinically reported but, in vitro studies show a weak, reversible, neurotoxic effect on the neuromuscular junction. We investigated the neuromuscular dysfunction in H. hypnale envenoming. Eighteen patients with authenticated H. hypnale envenoming were included. All 18 had serial clinical and neurological examinations. We evaluated neuromuscular dysfunction using stimulated concentric needle single-fibre electromyography (sfEMG) of orbicularis oculi in the patients within two days of the bite and compared to 29 normal subjects. Patients with abnormal jitter were reviewed six weeks later. All 18 patients had local effects and one-third had non-specific systemic symptoms. No patient had clinical features of neuromuscular paralysis. Median jitter values of patients were statistically significantly higher than normal subjects (19.6 ± 13.0 µs compared with 15.6 ± 7.4 µs.). None of the patients or normal subjects had neuromuscular blocks. Three patients had median jitter values higher than the maximum in normal subjects, which returned to normal by six weeks. H. hypnale envenoming leads to sub-clinical neuromuscular dysfunction, despite patients not showing any clinically detectable neurotoxicity.
Introduction
Snakebite is a neglected tropical disease and causes significant morbidity and mortality in many parts of the world [Citation1]. There are 398 snakebites for 100,000 population per year in Sri Lanka, which is one of the highest snakebite incidence rates in the world [Citation2]. Merrem’s hump-nosed viper (Hypnale hypnale) envenoming is common and a significant public health issue in Sri Lanka and South India [Citation3,Citation4]. H. hypnale has been estimated to account for 35 to 45% of the snakebites in Sri Lanka [Citation5]. Envenoming by H. hypnale causes significant local effects that include pain, swelling, blistering, haemorrhage, and necrosis at the bite site. In severe cases, the local effects may result in the loss of a digit or even a partial limb amputation [Citation6]. The systemic effects of H. hypnale include mild venom-induced consumption coagulopathy and acute kidney injury [Citation7,Citation8]. Electroencephalographic changes without clinically detectable neurological effects have been reported in five patients with envenoming by H. hypnale [Citation9].
Neurotoxic snake envenoming most commonly results in flaccid paralysis, due to the pre- and post-synaptic neurotoxins that disrupt the neurotransmission across the neuromuscular junction [Citation10]. Clinically detectable neuromuscular dysfunction in H. hypnale envenoming has not been reported in previous clinical studies with case-authentication [Citation6,Citation8]. However, weak neurotoxic and myotoxic effects of H. hypnale venom have previously been shown in in vitro nerve-muscle preparations [Citation11]. Mice envenomed by Hypnale showed reduced motor activity, hypotonia accompanied by muscarinic effects [Citation12]. As seen in most viper venoms [Citation13], H. hypnale venom is rich in phospholipase A2 toxins and lacks three-finger toxins [Citation14]. The former toxin group includes pre-synaptic neurotoxins while the latter group includes post-synaptic neurotoxins. No neurotoxins have been isolated from H. hypnale venom to date.
Using a series of concentric needle jitter estimations after envenoming, we have previously demonstrated the evolution and resolution of neuromuscular dysfunction in Indian krait (Bungarus caeruleus) [Citation15] and Russell’s viper (Daboia russelii) [Citation16] envenoming. Furthermore, we have shown the usefulness of concentric needle jitter estimation to detect sub-clinical neuromuscular dysfunction in patients with Russell’s viper envenoming, who did not develop clinically detectable paralysis [Citation16].
Jitter estimation is a measure of the variation in time for the nerve impulse to cross the neuromuscular junction and excite the muscle fibre. An increase in jitter demonstrates dysfunction of the neuromuscular junction before failure of impulse transmission from nerve to muscle, and therefore before clinical muscle weakness develops. It is formally measured as the mean consecutive difference (MCD) between one impulse and the next. Neuromuscular transmission is secure and never normally fails, even in fatigue. If the neuromuscular junction is compromised, the jitter or MCD becomes prolonged and some impulses may fail to cross to the muscle fibre. This is neuromuscular blocking and when about 20% of impulses are blocked, muscle weakness becomes clinically apparent [Citation17].
The lack of clinically detectable paralysis in envenomed patients despite the venom showing neurotoxicity in vitro, prompted us to investigate a possible sub-clinical neuromuscular dysfunction in H. hypnale envenoming using concentric needle jitter estimation. We used portable equipment at the bedside in tropical conditions. This differs from the usual temperature controlled laboratory conditions used by others, so we compared the jitter values of patients with H. hypnale envenoming with a group of healthy volunteers in tropical conditions.
Methods
Ethics
This study was approved by the human ethics committees of the Rajarata University and University of Peradeniya in Sri Lanka. All patients and normal subjects gave their written informed consent.
Patients with H. hypnale envenoming
We recruited 18 patients with H. hypnale envenoming treated in the professorial medical unit of the Anuradhapura Teaching Hospital between August 2013 and October 2014. The recruited patients had the offending snake specimen available for identification by an experienced herpetologist (AS) and had symptomatic bites with at minimum local pain and swelling. In all cases, the offending snake was identified as H. hypnale by AS who is a herpetologist, by examining the dead specimen brought in with the patient.
The patients had a detailed neurological examination on admission and at 4, 8, 12, 24 hours post-envenoming and daily thereafter. A clinical examination (other than neurological examination) was done on admission, 12 and 24 hours post-envenoming and daily thereafter. A 20-min whole blood clotting test (WBCT20) was done in all patients on admission. Clot formation at the end of 20 min was considered a negative test. All routine investigations of the patients carried out in the hospital were recorded. Patients had stimulated “concentric needle jitter electromyography” undertaken within 3 to 47 hours of envenoming. The treating clinicians determined patient treatment, which included analgesics, intravenous or oral cloxacillin 500 mg every six hours, and no antivenom. The Indian polyvalent antivenoms available in Sri Lanka are not raised against H. hypnale and are ineffective [Citation8,Citation18–20]. Patients who had abnormal jitter were reviewed for repeat jitter estimation six weeks after the snakebite.
Normal subjects
We recruited the normal volunteers from the Faculty of Medicine of the Rajarata University. There were 21 males and 8 females, median age 36 years (18–59 years). All were healthy, and neither had a history of any neuromuscular related disease, nor were they on any medication.
Concentric needle single-fibre electromyography (sfEMG)
We undertook jitter recordings of orbicularis oculi muscle as per the previously described sfEMG method, [Citation21–23] with minor modifications. The orbicularis oculi muscle was used because it is one of the earliest muscles to become affected in neurotoxic snake envenoming. We used monopolar needle electrodes to stimulate the supra-zygomatic branch of the facial nerve. Disposable monopolar EMG needles 37 mm long, 26G (TECA, Natus neurology, Ireland) were used as the cathode and 10 mm diameter EEG scalp electrodes as the stimulating anodes applied to the cheek.
We recorded from the orbicularis oculi muscle using a facial concentric needle EMG electrode (TECA elite, Natus neurology, Ireland, 25 mm X 30G) and Medelec Synergy equipment VIASYS healthcare, USA). A ground electrode was placed on the forehead. The amplifier bandwidth was set from either 1 kHz or 2 kHz to 10 kHz using recursive filters and the sampling rate was 100 kHz. Stimulation duration was 0.05–0.2 ms and at 10/sec at just supra-threshold strength (0.8–2.0 mA.) We recorded jitter measurements using time gated amplitude threshold detection of the fibres. We recorded 20 to 50 separate jitter measurements in each subject or patient. A Toshiba laptop ran the software. The investigators then checked the analysis of the results after recording and only included all apparent single fibres meeting the inclusion criteria (see below) for the jitter measurements.
In the process of undertaking jitter measurements, there is a risk of recording a spike-like potential, which is a composite of several overlapping single fibres. To minimise this risk, we adjusted the stimulus strength to just supra-threshold to excite a minimum number of motor units. Records were scrutinised off-line before MCD measurements using an amplitude-crossing algorithm. We examined traces at a sweep speed of 0.2 ms per division and potentials less than 100 µV amplitude and any positive going potentials were excluded. Potentials with a rise time of greater than 0.3 ms and those with notches or shoulders on the rising phase or which had an inconsistent shape were deemed to be composites of more than one fibre, and were rejected from the analysis. All others were included. Normal data have been reported and are used here for comparison [Citation21].
Data analysis
Analysis of jitter data was done using PRISM, version 7.02 (GraphPad Software, Inc., La Jolla, CA). Continuous variables were reported as medians, range, and interquartile range (IQR). Jitter values of patients and normal subjects were analysed using the Mann Whitney test.
Results
Characteristics of the snakebites and the clinical features of envenoming
All the 18 patients had fang marks at the bite site and local features of envenoming. Six patients had non-specific systemic symptoms (). None of the patients developed any sign of neuromuscular paralysis, including ptosis, ophthalmoplegia, diplopia, facial and bulbar palsy, or respiratory paralysis. No patient had any evidence of venom-induced consumption coagulopathy based on them all having negative WBCT20 and normal INR. One patient had clinical and biochemical evidence of acute kidney injury.
Table 1. Demographic characteristics of 18 patients with H. hypnale envenoming.
sfEMG jitter estimates of envenomed patients versus normal subjects
We recorded jitter measurements in all 18 patients, 3 to 47 hours (median: 15 hours; IQR: 9 to 30 hours) after H. hypnale envenoming. We compared these with the median jitter estimates of 29 normal subjects. Neither the patients nor normal subjects developed neuromuscular blocks. Patients with H. hypnale envenoming had higher median jitter values compared to the normal subjects, indicating neuromuscular dysfunction (). Three patients had median jitter values greater than the upper limit of the normal subjects ( and Citation2). Six weeks after the snakebite, the MCD of all three patients had reduced significantly, with the median jitter values lying within the range of median jitter values of the normal subjects, indicating a recovery of the neuromuscular function (). There was no association between the percentage of abnormal fibres in patients and the bite-to-jitter time gap (R2 = 0.0194). Measurements from the pooled fibres in patients with H. hypnale envenoming and normal subjects are shown in .
Figure 1. Comparison on median jitter of 18 patients with H. hypnale envenoming (HNV) and 29 normal subjects. Envenomed patients have higher mean and median jitter values compared to normal subjects (*p < 0.05, Mann Whitney test).
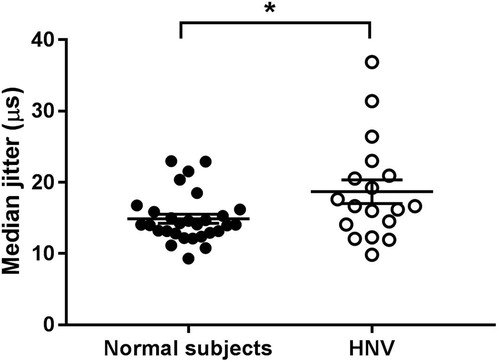
Figure 2. Stimulated single-fibre electromyography recordings of the orbicularis oculi muscle of a patient with H. hypnale envenoming. (a) superimposed and (b) non-superimposed recordings of a fibre with a MCD of 56.01 μs in a patient who had a mean jitter of 41.04 μs, recorded 29 hours after the bite. (c) Superimposed and (d) non-superimposed recordings of a fibre with a MCD of 6.36 μs in the same patient who had a mean jitter of 15.12 μs, recorded six weeks after the bite.
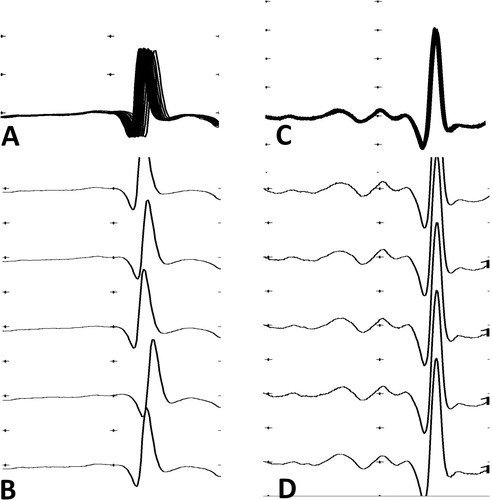
Figure 3. Comparison of the MCD of the sampled fibres during the hospital admission following the snake bite and six weeks after the snakebite of three patients (Patients A, B, and C) who had higher jitter values compared to the normal subjects, following the snakebite (*p < 0.05, Mann Whitney test).
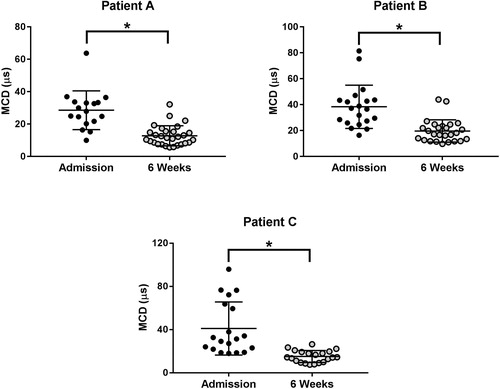
Table 2. The pooled jitter values recorded by stimulated concentric needle EMG of 18 patients with H. hypnale envenoming and 29 normal subjects.
Discussion
Our study demonstrates sub-clinical neuromuscular dysfunction in patients envenomed by H. hypnale based on increased jitter measured by concentric needle sfEMG. The electromyographic abnormalities were limited to slightly prolonged jitter, without any neuromuscular blocks, and the patients had no evidence of clinical neuromuscular weakness. The abnormalities were detected within the first two days of the snakebite and had resolved by 6 weeks after the snakebite. This is similar to the previously reported high jitter observed in Russell’s viper (Daboia russelii) envenoming in patients without clinically detectable paralysis [Citation16].
Neurotoxin are yet to be isolated from H. hypnale venom. Recent venom proteomic studies of H. hypnale venom suggested the absence of post-synaptic neurotoxins [Citation14]. However, the venom contained large amounts of phospholipase A2 toxin, and it is possible that there is a weak pre-synaptic neurotoxin in the venom responsible for this sub-clinical neurotoxicity. The jitter values in themselves do not distinguish a pre- or post-synaptic action, so functional characterisation of the venom is required to confirm this.
H. hypnale bites generally only cause local effects while systemic manifestations such as venom induced consumption coagulopathy and acute kidney injury are rare [Citation6]. Venom induced consumption coagulopathy in patients with H. hypnale bites is incomplete and mild, so is often not detectable with a WBCT20 [Citation7]. H. hypnale is a small bodied pit viper species rarely exceeding 40 cm in length [Citation24]. The mild coagulopathy and sub-clinical neuromuscular dysfunction observed in patients with H. hypnale envenoming could therefore be partly due to the smaller venom doses likely to be injected during the bite.
Single fibre EMG was developed as a sensitive test for the detection of neuromuscular dysfunction in myasthenia gravis because it detects abnormalities in motor end plate function when there is no detectable weakness [Citation25]. We have successfully modified this technique of concentric needle jitter analysis to investigate neuromuscular paralysis in snake envenoming [Citation15,Citation16]. The traditional method of repetitive nerve stimulation demonstrates the failure of nerve-muscle transmission in some of the fibres. However, jitter values increase before there is failure of nerve-muscle transmission and hence is useful to detect sub-clinical neuromuscular paralysis or impending neuromuscular paralysis in snake envenoming [Citation15,Citation16]. Stimulated concentric needle jitter measurement is based on the original sfEMG technique, but is quicker and cheaper to use [Citation26]. Cooperation of the patient is not required and the test can be easily applied to the facial muscles and is therefore more appropriate in studying snakebite patients who are often critically ill.
Conducting serial sfEMG tests of patients during the hospital stay was not possible and the timing from the bite to sfEMG varied among the patients. It is therefore possible to miss any transient sfEMG abnormalities in some of the study patients.
The normal ranges of jitter values for fibres in individuals has been established based on studies conducted in electromyography laboratories with controlled environments. This is not the case for snakebite patients, who are mostly treated in uncontrolled environments in resource poor settings in the tropics. We established normal ranges for comparison, with a group of normal subjects under tropical laboratory conditions. The mean jitter of our normal subjects (15.62 ± 7.39 µs) compares well with the means obtained from a multicentre study [Citation26].
Our results in patients with H. hypnale envenoming, and the in vitro studies of the venom [Citation11] suggest the presence of a neurotoxic component in H. hypnale venom.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Chippaux J-P. Snakebite envenomation turns again into a neglected tropical disease! J Venom Anim Toxins Incl Trop Dis. 2017;23:38.
- Ediriweera DS, Kasturiratne A, Pathmeswaran A, et al. Mapping the risk of snakebite in Sri Lanka - a national survey with geospatial analysis. PLoS Negl Trop Dis. 2016;10:e0004813.
- Silva A. Dangerous snakes, deadly snakes and medically important snakes. J Venom Anim Toxins Incl Trop Dis. 2013;19:26.
- Simpson ID, Norris RL. Snakes of medical importance in India: is the concept of the “big 4” still relevant and useful? Wilderness Environ Med. 2007;9:2–9.
- Kasturiratne A, Pathmeswaran A, Fonseka MMD, et al. Estimates of disease burden due to land-snake bite in Sri Lankan hospitals. Southeast Asian J Trop Med Public Health. 2005;36:733–740.
- Maduwage K, Isbister GK, Silva A, et al. Epidemiology and clinical effects of hump-nosed pit viper (Genus: Hypnale) envenoming in Sri Lanka. Toxicon. 2013;61:11–15.
- Maduwage K, Scorgie FE, Silva A, et al. Hump-nosed pit viper (Hypnale hypnale) envenoming causes mild coagulopathy with incomplete clotting factor consumption. Clin Toxicol. 2013;51:527–531.
- Ariaratnam CA, Thuraisingam V, Kularatne SAM, et al. Frequent and potentially fatal envenoming by hump-nosed pit vipers (Hypnale hypnale and H. nepa) in Sri Lanka: lack of effective antivenom. Trans R Soc Trop Med Hyg. 2008;102:1120–1126.
- Ramachandran S, Ganaikabahu B, Pushparajan K, Wijesekara J. Electroencephalographic abnormalities in patient with snake bites. Am J Trop Med Hyg. 1995;52:25–28.
- Silva A, Armstrong BC, Rash LD, et al. Defining the role of post-synaptic α-neurotoxins in paralysis due to snake envenoming in humans. Cell Mol Life Sci. 2018;75:4465–4478.
- Maduwage K, Hodgson WC, Konstantakopoulos N, et al. The in vitro toxicity of venoms from South Asian hump-nosed pit vipers (Viperidae: Hypnale). J Venom Res. 2011;2:17–23.
- Silva A, Weilgama D, Gawarammana I, et al. Venoms of South Asian hump-nosed pit vipers (Genus: Hypnale) cause muscarinic effects in BALB/c mice. Anuradhapura Med J. 2014;8:13–15.
- Tasoulis T, Isbister G. A review and database of snake venom proteomes. Toxins. 2017;9:290.
- Tan CH, Tan NH, Sim SM, et al. Proteomic investigation of Sri Lankan hump-nosed pit viper (Hypnale hypnale) venom. Toxicon. 2015;93:164–170.
- Silva A, Maduwage K, Sedgwick M, et al. Neuromuscular effects of common krait (Bungarus caeruleus) envenoming in Sri Lanka. PLoS Negl Trop Dis. 2016;10:e0004368.
- Silva A, Maduwage K, Sedgwick M, et al. Neurotoxicity in Russell's viper (Daboia russelii) envenoming in Sri Lanka: a clinical and neurophysiological study. Clin Toxicol. 2016;54:411–419.
- Stalberg E, Trontelj J. Single fiber electromyography: studies in healthy and diseased muscle. Lippincott Williams and Wilkins, New York; 1994.
- Sellahewa K, Kumararatne M. Envenomation by the hump-nosed viper (Hypnale hypnale). Am J Trop Med Hyg. 1994;51:823–825.
- Kularatne SA, Ratnatunga N. Severe systemic effects of Merrem's hump-nosed viper bite. Ceylon Med J. 1999;44:169–170.
- Joseph JK, Simpson ID, Menon NCS, et al. First authenticated cases of life-threatening envenoming by the hump-nosed pit viper (Hypnale hypnale) in India. Trans R Soc Trop Med Hyg. 2007;101:85–90.
- Kouyoumadjian JA, Stalberg EV, Kouyoumdjian JA, et al. Concentric needle jitter on stimulated orbicularis oculi in 50 healthy subjects. Clin Neurophysiol. 2011;122:617–622.
- Trontelj JV, Zidar J, Denigli M, et al. Facioscapulohumeral dystrophy: jitter in facial muscles. 1988;51:950–955.
- Stålberg EV, Sanders DB. Jitter recordings with concentric needle electrodes. Muscle Nerve. 2009;40:331–339.
- Maduwage K, Silva A, Manamendia-Arachchi K, et al. A taxonomic revision of the South Asian hump nosed pit viper (Squamata: Viperidae: Hypnale). Zootaxa. 2009;2232:1–28.
- Padua L, Caliandro P, Di Iasi G, et al. SFEMG: a piece in the diagnostic puzzle of myasthenia. Clin Neurophysiol. 2014;125:2318–2319.
- Stålberg E, Sanders DB, Ali S, et al. Reference values for jitter recorded by concentric needle electrodes in healthy controls: a multicenter study. Muscle Nerve. 2016;53:351–362.
