Abstract
A 35-year-old woman presented to the emergency department in cardiac arrest after ingesting large quantities of powdered cocaine. With an initial rhythm of ventricular fibrillation, emergency medical providers were able to achieve return of spontaneous circulation prior to hospital transport. Shortly after emergency department arrival the patient experienced pulseless electric activity arrest. Despite routine advanced cardiac life support, she remained critically ill and intermittently achieved palpable pulses before returning to pulseless ventricular tachycardia. She received intravenous lipid emulsion and dual defibrillation therapies without clinical improvement. After a lidocaine bolus and intravenous infusion, she had a sustained return of spontaneous circulation. The patient was taken to the intensive care unit before withdrawal of care two days later due to a poor prognosis for recovery.
Introduction
In this case report we describe a patient experiencing pulseless ventricular tachycardia secondary to massive cocaine ingestion, and expand on the use of intravenous (IV) lidocaine in cocaine overdose.
A 35-year-old woman with a history of bipolar disorder and polysubstance abuse presented to the emergency department (ED) in cardiac arrest. Per emergency medical services (EMS), law enforcement officers caught the patient buying illicit substances and then witnessed her both ingesting and inserting cocaine packages intravaginally. Shortly thereafter, the patient experienced a seizure with rapid progression to cardiac arrest.
Upon EMS arrival she was in ventricular fibrillation. Advanced cardiac life support was performed resulting in the patient receiving epinephrine 1 mg boluses (5 mg total) and 150 mEq of sodium bicarbonate during transport. After nearly 30 minutes of cardiopulmonary resuscitation, she had return of spontaneous circulation (ROSC). Once arriving to the ED the patient lost pulses again with an initial rhythm of pulseless electrical activity (PEA).
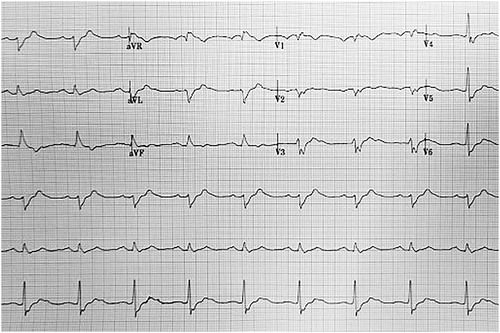
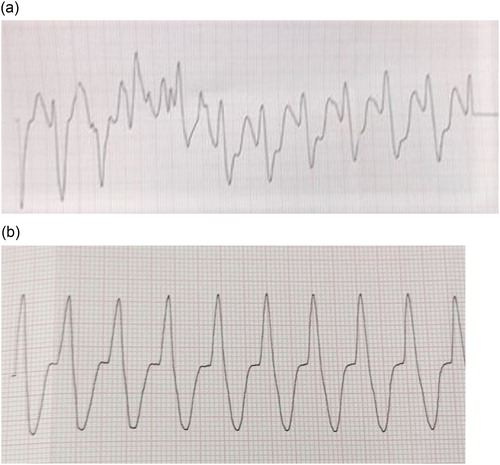
Emergency management began with 1 mg epinephrine boluses (4 mg total), 100 mEq of sodium bicarbonate, 2 gm of magnesium sulfate, and 1 gm of calcium chloride. This resulted in rhythm conversion from PEA, to polymorphic ventricular tachycardia (VT), and ultimately to monomorphic VT. Initial point of care venous blood gas provided a pH of 6.81. Follow up arterial blood gas and basic laboratory measurements were significant for a pH of 7.08, pO2 of 95 mmHg, pCO2 of 72 mmHg, bicarbonate of 21 mmol/L, lactic acid of 13.2 mmol/L, potassium of 2.5 mEq/L, glucose of 177 mg/dL, sodium of 153mEq/L, and chloride of 112 mEq/L. Preliminary monomorphic ventricular tachycardia rhythm strips may be found in . Twelve minutes into cardiac arrest management the patient underwent failed defibrillation, prompting use of additional magnesium, sodium bicarbonate, and a 1.5 mg/kg IV lidocaine bolus. The patient experienced intermittent pulses throughout, but repeatedly converted back into polymorphic VT. She underwent an attempted dual sequential defibrillation along with a 1.5 mL/kg (60 mL) bolus of ILE, with no changes in cardiac rhythm seen. Eighteen minutes into the arrest, she received a second IV lidocaine bolus (0.75 mg/kg) followed by a lidocaine infusion, resulting in sustained ROSC after 25 minutes of ACLS care. Electrocardiogram obtained following ROSC appears in . The patient was then admitted to the intensive care unit for further care.
Figure 1. (a) Initial pulseless ventricular tachycardia rhythm strip. (b) Pulseless ventricular tachycardia rhtythm strip after 10 minutes of care.
Once admitted to the intensive care unit, she remained unresponsive and critically ill. Medications continued during her stay included a lidocaine infusion ranging between 2 and 4 mg/min, an amiodarone infusion running at 1 mg/min, and a norepinephrine infusion last measured at 0.06 mcg/kg/min. Due to persistent unresponsiveness, she underwent magnetic resonance imaging (MRI) of the brain. MRI demonstrated symmetric areas of restricted diffusion within the cortex, basal ganglia, and caudate nuclei, concerning for hypoxic ischemic injury. After two days of care the decision was made to withdraw life-sustaining measures due to the poor MRI prognosis and persistent critical illness.
Discussion
Cocaine abuse continues to be a significant health-related burden in the United States, with nearly 1.5 million Americans aged 12 years or older reporting use in a 2014 survey [Citation1]. The pharmacologic mechanisms attributed to cocaine’s physiologic effects include inhibition of catecholamine reuptake, increase in excitatory amino acid concentrations within the central nervous system, and blockade of neuronal and cardiac sodium channels [Citation2,Citation3]. Not surprisingly, a sympathomimetic toxidrome generally results with the propensity for the development of seizures and ventricular tachydysrhythmias.
The pharmacodynamic interaction of cocaine and its metabolites with cardiac sodium receptors is the major causative factor in the development of cardiac dysrhythmias [Citation3]. Cocaine is a class IC anti-arrhythmic due to its slow binding kinetics with the cardiac myocyte sodium channels, placing it within the same family of anti-arrhythmics as flecainide and propafenone [Citation3,Citation4]. Ingestion, especially in large quantities, may lead to a prolongation of the QRS duration and a reduction in myocyte conduction velocity [Citation5,Citation6]. The binding kinetics of cocaine and its physiochemical properties may concomitantly worsen a patient’s clinical status by exacerbating QRS prolongation. Cocaine shows “use-dependent” kinetics with higher affinity for more active sodium channels. Furthermore, due to the pKa of cocaine (8.8), acidosis may increase the proportion of ionized drug present leading to greater interaction between cocaine and sodium channel receptors [Citation3]. Tachycardia and acidosis both accentuate the sodium channel blockade from cocaine, with simultaneous presentation worsening cocaine toxicity.
Management of cocaine toxicity is generally supportive, with benzodiazepines serving the largest therapeutic role [Citation2]. Benzodiazepines aid in sedating patients, decreasing the likelihood of rhabdomyolysis, self-harm, and the propagation of cardiac arrhythmias. Clinicians treating the acutely toxic cocaine patient presenting with an arrhythmia should take into consideration the rhythm that is present, and the potential effects of the medications used to treat these arrhythmias.
Although recently questioned, beta blockers are considered contraindicated in toxic cocaine patients due to the “unopposed alpha” agonism that results with their use [Citation2,Citation7–9]. Instead, patients presenting with either a narrow-complex arrhythmia or atrial fibrillation should receive a calcium channel blocker like diltiazem. Wide-complex arrhythmias pose a critical issue for practitioners, and may be refractory in severe ingestions. Amiodarone lacks data for its use in the setting of cocaine toxicity and may acutely worsen the clinical picture due to its off-target beta adrenergic blockade [Citation2]. Hypertonic sodium bicarbonate may be useful as an initial therapy for wide-complex arrhythmias. Sodium bicarbonate reduces QRS duration by increasing extracellular sodium load, and by reducing ionized cocaine concentrations through serum alkalinization [Citation2,Citation3]. Due to cocaine’s reported Log P value of 2.3 [Citation10], IV fat emulsion might provide benefit in acutely toxic cocaine patients, with a prior case report documenting its successful use [Citation11]. Although potentially beneficial, this therapeutic modality has been disputed in an animal model [Citation12]. Lastly, “double” or “dual-sequential” defibrillation has been used in obese patients and those with refractory ventricular arrhythmias with some success [Citation13].
Lidocaine is a therapeutic alternative for acutely toxic cocaine patients presenting with ventricular arrhythmias. As a class IB anti-arrhythmic with similar affinity to both neuronal and cardiac sodium channels, lidocaine possesses the ability to antagonize cocaine’s effects at the sodium receptor due to its fast on/off binding kinetics [Citation3,Citation14]. Animal models generally corroborate this theoretical benefit, with one guinea pig heart model showing significant QRS reduction with lidocaine use [Citation15,Citation16]. Human case reports are lacking; however, lidocaine is safe in the setting of cocaine-induced myocardial infarction [Citation17].
Lidocaine dosing consists of an initial 1–1.5 mg/kg IV bolus for pulseless rhythms [Citation18]. Subsequent boluses may range from 0.5 to 0.75 mg/kg, but should not exceed a total cumulative dose of 3 mg/kg. A lidocaine infusion may follow bolus dosing, starting at a rate of 1 mg/min and titrated up to 4 mg/min based on response [Citation2]. When used, patients should be continually monitored for the development of additional dysrhythmias, and measurement of serum lidocaine concentrations considered if new arrhythmias or toxicity develop.
Although return of spontaneous circulation in this case could have been multifactorial, lidocaine administration may have provided benefit. While evidence behind its use is sparse, lidocaine is an anti-arrhythmic with cardiac stabilizing properties and may be a viable option for refractory toxicity associated with cocaine overdoses.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- NIDA. Cocaine. National Institute on drug abuse website; 2016 May 6 [cited 2018 Aug 28]. Available from: https://www.drugabuse.gov/publications/research-reports/cocaine
- Prosser JM, Hoffman RS. Cocaine, In: RS Hoffman, M Howland, NA Lewin, et al., editors. Goldfrank’s toxicologic emergencies, 10e. New York, NY: McGraw-Hill Education; 2015.
- Wood DM, Dargan PI, Hoffman RS. Management of cocaine-induced cardiac arrhythmias due to cardiac ion channel dysfunction. Clin Toxicol (Phila). 2009;47:14–23.
- Katz JL, Terry P, Witkin JM. Comparative behavioral pharmacology and toxicology of cocaine and its ethanol-derived metabolite, cocaine ethyl-ester (cocaethylene). Life Sci. 1992;50:1351–1361.
- Isner JM, Estes NA, Thompson PD, et al. Acute cardiac events temporally related to cocaine abuse. N Engl J Med. 1986;315:1438–1443.
- Schrem SS, Belsky P, Schwartzman D, et al. Cocaine-induced torsades de pointes in a patient with the idiopathic long QT syndrome. Am Heart J. 1990;120:980–984.
- Catravas JD, Waters IW. Acute cocaine intoxication in the conscious dog: studies on the mechanism of lethality. J Pharmacol Exp Ther. 1981;217:350–356.
- Guinn MM, Bedford JA, Wilson MC. Antagonism of intravenous cocaine lethality in nonhuman primates. Clin Toxicol. 1980;16:499–508.
- Sand IC, Brody SL, Wrenn KD, et al. Experience with esmolol for the treatment of cocaine-associated cardiovascular complications. Am J Emerg Med. 1991;9:161–163.
- National Center for Biotechnology Information. PubChem Compound Database; CID = 446220 [cited 2018 Nov 21]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/446220
- Arora NP, Berk WA, Aaron CK, et al. Usefulness of intravenous lipid emulsion for cardiac toxicity from cocaine overdose. Am J Cardiol. 2013;111:445–447.
- Chai PR, Hack JB. Intravenous lipid emulsion in the resuscitation of cocaine-induced cardiovascular arrest in a rat model. Am J Emerg Med. 2016;34:1452–1454.
- Hajjar K, Berbari I, El Tawil C, et al. Dual defibrillation in patients with refractory ventricular fibrillation. Am J Emerg Med. 2018;36:1474–1479.
- Richards JR, Garber D, Laurin EG, et al. Treatment of cocaine cardiovascular toxicity: a systematic review. Clin Toxicol (Phila). 2016;54:345–364.
- Grawe JJ, Hariman RJ, Winecoff AP, et al. Reversal of the electrocardiographic effects of cocaine by lidocaine. Part 2. Concentration-effect relationships. Pharmacotherapy. 1994;14:704–711.
- Hondeghem LM, Katzung BG. Antiarrhythmic agents: the modulated receptor mechanism of action of sodium and calcium channel-blocking drugs. Annu Rev Pharmacol Toxicol. 1984;24:387–423.
- Shih RD, Hollander JE, Burstein JL, et al. Clinical safety of lidocaine in patients with cocaine-associated myocardial infarction. Ann Emerg Med. 1995;26:702–706.
- Al-Khatib SM, et al. 2017 AHA/ACC/HRS Guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Circulation. 2018;138:e272–e391.