Abstract
Iron overdose in its severest form is associated with fulminant hepatic failure and death. Indications for deferoxamine include a serum iron concentration greater than 500 mcg/dL. We present a case of an intravenous iron overdose to demonstrate the limitations of focusing solely on serum concentrations. A 50-year-old woman received a five-fold overdose of intravenous iron sucrose due to a medication error. Her only complaints were mild dizziness and abdominal pain. Her iron concentration 1 h after the iron infusion was 1301 mcg/dL. Due to the lack of vomiting, normal vital signs, and a normal anion gap, the treating team withheld deferoxamine and observed the patient. Her symptoms resolved spontaneously, her laboratory studies remained normal and deferoxamine was never administered. Iron sucrose is a large molecular weight compound (30,000–100,000 Daltons) designed to release iron slowly. We suspect the elevated iron concentration included iron that was complexed to carbohydrate and therefore not toxic. Clinicians need to know that intravenous iron complexed to carbohydrates may lead to serum concentrations that do not correlate with the degree of toxicity expected following iron salt ingestion. The decision to initiate chelation should depend on the patient’s clinical and laboratory findings.
Introduction
Iron overdose produces a well recognized constellation of signs and symptoms that include; nausea, vomiting, diarrhea, anion gap metabolic acidosis, hypotension, and in its most severe form fulminant hepatic failure and death [Citation1, Citation2]. In addition to gastrointestinal decontamination and supportive care, deferoxamine is typically administered when poisoning is severe. While the indications for deferoxamine are not established based on randomized controlled human trials, experts often use an iron concentration above 500 mcg/dL (89.5 micromol/L) as one criterion for therapy [Citation1, Citation3–5]. We present an uncommon case of intravenous iron overdose to illustrate one limitation of the assessment of iron concentrations in the setting of overdose.
Case report
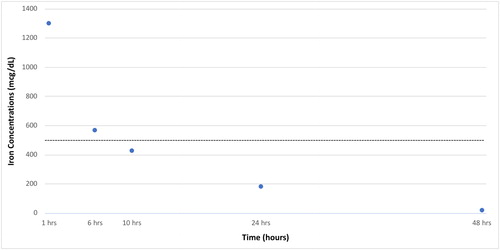
A 50-year-old (100 kg) woman with a history of iron deficiency anemia was ordered to receive 200 mg of intravenous iron as iron sucrose. A medication administration error resulted in her receiving 1 gram of intravenous iron sucrose. The error was discovered rapidly when the patient complained of mild dizziness and abdominal pain. Her vital signs at that time were: BP, 125/65 mmHg; HR, 71/min; RR, 18/min; temperature 96.6° F (35.9° C) with an oxygen saturation of 100% on room air. A rapid reagent glucose was 163 mg/dL (9.1 mmol/L). The patient had no vomiting, a normal anion gap, and a complete blood count that was unremarkable. Her iron concentration one hour after the iron infusion was 1301 mcg/dL (233 umol/L). Despite the high iron concentration, deferoxamine was not administered because of her lack of symptoms. Instead we observed the patient closely, monitoring her for any other signs and symptoms, provided supportive care, and trended her anion gap, liver function tests, and iron concentrations. The patient’s abdominal pain and dizziness resolved rapidly and she remained entirely asymptomatic and hemodynamically stable. Her anion gap and liver function tests remained normal. shows serial iron concentrations over time.
Discussion
Iron sucrose is a large molecular weight (30,000–100,000 Dalton) compound designed to release iron slowly over many hours to days [Citation6]. Unlike ingestion of iron salts, there are few reported cases of intravenous iron overdose. Wood and colleagues reported a 15 kg child who received 242 mg of iron sucrose and developed nausea, abdominal pain, and vomiting [Citation7]. A 1 h iron concentration was 2045 mcg/dL (366 umol/L). Although there was no mention of metabolic acidosis or hemodynamic instability, the child received deferoxamine and did well other than an asymptomatic rise of their aspartate aminotransferase to 1234 IU/L. In an abstract, Beuhler and Wallace reported three patients given a 10-fold dosing error of iron dextran (7 mg/kg instead of 0.7 mg/kg) [Citation8]. Despite iron concentrations as high as 1000 mcg/dL (179 umol/L) none developed signs or symptoms suggestive of iron toxicity. Finally, Yassin and colleagues reported a 27 year old woman complaining of abdominal pain after receiving 4000 mg of an elemental iron (60 mg/kg body weight) of an unspecified preparation intravenously over 20 days. Her iron concentration was 1117 mcg/dL (200 umol/L) and she received oral deferasirox without complication [Citation9].
In this and similar cases, we suspect that the serum iron concentrations were very elevated because the laboratory measured iron that was largely complexed to the carbohydrate and therefore not toxic. Although many practitioners rely on a specific iron concentration to initiate chelation even in the absence of signs and symptoms of toxicity, it is important to remember that these recommendations are based on ingestion of iron salts. In fact, the reported LD50 for iron sucrose in mice is 359 mg iron/kg, much higher than what physicians consider a toxic dose for oral preparations [Citation6]. We believe that until more data are obtained, the decision to chelate patients with intravenous iron overdoses should be based entirely on clinical findings. In the absence of multiple episodes of vomiting, an anion gap metabolic acidosis and signs of hemodynamic instability it is unclear whether iron concentrations have any prognostic value following intravenous administration of iron preparations.
References
- Bateman DN, Eagling V, Sandilands EA, et al. Iron overdose epidemiology, clinical features and iron concentration-effect relationships: the UK experience 2008–2017. Clin Toxicol (Phila). 2018;56:1098–1106.
- Tenenbein M. Hepatotoxicity in acute iron poisoning. J Toxicol Clin Toxicol. 2001;39:721–726.
- Anderson AC. Iron poisoning in children. Curr Opin Pediatr. 1994;6:289–294.
- Schauben JL, Augenstein WL, Cox J, et al. Iron poisoning: report of three cases and a review of therapeutic intervention. J Emerg Med. 1990;8:309–319.
- Tenenbein M. Benefits of parenteral deferoxamine for acute iron poisoning. J Toxicol Clin Toxicol. 1996;34:485–489.
- Geisser P, Burckhardt S. The pharmacokinetics and pharmacodynamics of iron preparations. Pharmaceutics. 2011;3:12–33.
- Wood DM, Thomson AH, Lawes M, et al. Hepatocellular damage following therapeutic intravenous iron sucrose infusion in a child. Ther. Drug Monit. 2005;27:405–408.
- Beuhler MC, Wallace KL. Benign outcome with toxic serum iron levels following IV iron dextran overdose in three patients. Clin Toxicol (Phila). 2003;41:738–739.
- Yassin M, Soliman AT, De Sanctis V, et al. A young adult with unintended acute intravenous iron intoxication treated with oral chelation: The use of liver ferriscan for diagnosing and monitoring tissue iron load. Mediterr J Hematol Infect Dis. 2017;9:e2017008.