Abstract
Clonazolam is a designer triazolobenzodiazepine first synthesized in the 1970s that has never been licensed for therapeutic use. Clonazolam first surfaced on the internet in 2014 as a drug of abuse. Cases of intoxication involving clonazolam have been identified since 2016 in Europe with a single case described in the US in 2017. It is typically found in tablet, capsule, pellet, blotter, or powder form. A 28-year-old man presented with somnolence to an emergency department after reportedly ingesting one-half of a 30 mL vial of liquid clonazolam he purchased from the internet. He had normal vital signs and had no distress on presentation. His laboratory tests were normal, and his mental status cleared during a 6-hour observation in the emergency department. Non-targeted analysis of the liquid using liquid chromatography-quadrupole time-of-flight mass spectrometry identified clonazolam (0.4 mg/mL) with no other pharmaceuticals. This is the second case of intentional clonazolam ingestion described in the United States, and is unique in that the substance was found in liquid form. Providers should be aware that clonazolam is available on the internet in liquid formulation, which may increase the risk of accidental overdose.
Introduction
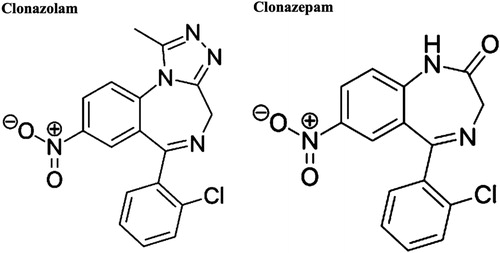
Clonazolam, or 6-(2-chlorophenyl)-1-methyl-8-nitro 4H-[Citation1,Citation2,Citation4]triazolo[4,3-α][Citation1,Citation4] benzodiazepine, is the triazolo-analogue of clonazepam and was first synthesized in 1971 () [Citation1]. It has never been licensed for therapeutic use in any part of the world or by the US Food and Drug Administration (US FDA) [Citation2–4]. It surfaced on the internet market in 2014 as a drug of abuse [Citation4]. Limited data regarding clonazolam prevalence and clinical effects has become available via the National Poison Data System (NPDS) and STRIDA project in Sweden [Citation2,Citation5]. Specific information regarding characteristics of intoxication is limited. We present a case of acute intoxication in a male patient who ingested a concentrated liquid solution of clonazolam.
Case
A bystander called emergency medical services (EMS) after discovering a somnolent male at an unclear location. EMS found a 28-year-old male patient who was drowsy but participatory with exam. The patient reported ingesting one-half the contents of a 30 mL vial of a designer drug purchased from the internet. The vial had a label stating “lethal if ingested” and EMS initiated contact with the poison center. The label listed the contents as “clonazolam” 0.5 mg/mL (total 15 mg) in ethyl alcohol and propylene glycol (). Initial vital signs of the patient included heart rate of 80 bpm, blood pressure 98/68 mmHg, respiratory rate 16, temperature 37˚C, and oxygen saturation 100% on room air. The emergency department (ED) staff described a somnolent male who participated minimally with exam but who was protecting his airway. There were no other significant physical exam abnormalities. A screening complete blood count and basic metabolic panel had unremarkable results without signs of acidosis. A serum ethanol concentration was undetectable. His blood pressure improved to 103/60 mmHg without intervention. He had no vital sign abnormalities during his monitoring period. He returned to his baseline mental status after six hours and was subsequently discharged. He did not provide a urine sample for immunoassay testing prior to his departure.
We evaluated the contents of the vial using liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-QTOF/MS) (Agilent LC1260-QTOF/MS 6550, Agilent technologies, Santa Clara, CA). We identified clonazolam at a concentration of 0.4 mg/mL without other pharmaceuticals (). LC-QTOF/MS does not measure ethyl alcohol nor propylene glycol. The 30 mL vial contained an estimated 12 mg of clonazolam – an equivalent of 30–60 (0.2–0.4 mg) doses.
Discussion
This case report describes a patient who ingested clonazolam and presented with a sedative-hypnotic toxidrome that resolved within a few hours without complication. The patient’s course appears typical compared to the cohort of 50 cases described by the NPDS review. The largest percentage of those cases exhibited minor effects (45%) and 68% experienced sedation/lethargy. Duration of effects was typically 8–24 hours (46%) or 2–8 hours (28%). The largest percentage of patients were treated and released from the ED (36%). There were zero deaths, however ten of the fifty patients were admitted to a critical care unit and three required intubation [Citation2].
Cases of intoxication with novel benzodiazepine agents are becoming more frequent in the US and Europe [Citation2,Citation5]. Clonazolam can be easily found and purchased on the surface internet utilizing “research chemical” websites. The majority of websites offering clonazolam are European and sell clonazolam in tablet, capsule, pellet or blotter form, typically €10–12 for a pack of ten. US-based research chemical websites sell clonazolam in larger quantities and are generally 10-fold more expensive with the exception of liquid clonazolam, which is available for $30 for a 30 mL vial.
Clonazolam belongs to the group triazolo-benzodiazepines that includes flubromazelam, flunitrazolam, and nitrazolam, among others. These analogues are accepted as having increased activity and higher potency than their structurally related parent compounds [Citation4,Citation6,Citation7]. There is no regulated dosing for clonazolam, but recommendations suggest 0.2–0.4 mg with warnings of profound sedation at 0.5 mg [Citation1,Citation4]. In keeping with the benzodiazepine class, clonazolam exerts its effects through binding of the GABA-A receptor. In vitro and in vivo data reveal that it undergoes hepatic metabolism via reduction and hydroxylation. The main metabolites are 7-aminoclonazolam, hydroxyclonazolam, and 7-acetamidoclonazolam [Citation8,Citation9]. Both metabolites and parent compound are eliminated in the urine. There is no current information available for volume of distribution, pKa, peak serum concentration associated with intoxication nor elimination half-life. Clonazolam has sufficient cross-reactivity to trigger a positive result on urine screening using the CEDIA, EMIT II Plus, HEIA and KIMS II assays [Citation10].
Clinical effects of clonazolam include sedation, muscle relaxation, amnesia, and respiratory depression [Citation7]. There are no specific reports for the effectiveness of flumazenil in confirmed cases. As clonazolam shares a mechanism of action with the benzodiazepine class, flumazenil is suggested for severe cases of intoxication, though safety is not established [Citation2,Citation4,Citation7]. Six patients exposed to flubromazolam, another triazolobenzodiazepine, had documented improvement following flumazenil administration, however two patients in this cohort still required intubation [Citation5]. There is currently no information on development of dependence on or withdrawal from clonazolam aside from a single case report described in the US in 2017 that was confounded by addiction to at least three other substances [Citation3].
Conclusion
Clonazolam is a potent, illicit benzodiazepine that is available in concentrated liquid form. The toxic effects are similar to those of other benzodiazepines.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Hester JB, Rudzik AD, Kamdar BV. 6-phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepines which have central nervous system depressant activity. J Med Chem. 1971;14(11):1078–1081.
- Carpenter JE, Murray BP, Dunkley C, et al. Designer benzodiazepines: a report of exposures recorded in the National Poison Data System, 2014-2017. Clin Toxicol (Phila). 2019;57(4):282–286.
- Ghazi M, Mohmand M. Co-occurring addiction of synthetic benzodiazepine clonazolam and propylhexedrine presenting as acute brief psychosis. J Addict Med Ther. 2017;5(2):1037.
- Moosmann B, Auwärter V. Designer benzodiazepines: another class of new psychoactive substances. In: Handbook of experimental pharmacology. 9th Ed. Vol. 252, 2018; pp. 383–410. Cham, Switzerland: Springer Nature.
- Bäckberg M, Pettersson Bergstrand M, Beck O, et al. Occurrence and time course of NPS benzodiazepines in Sweden - results from intoxication cases in the STRIDA project. Clin Toxicol (Phila). 2019;57(3):203–212.
- Huppertz LM, Bisel P, Westphal F, et al. Characterization of the four designer benzodiazepines clonazolam, deschloroetizolam, flubromazolam, and meclonazepam, and identification of their in vitro metabolites. Forensic Toxicol. 2015;33(2):388–389.
- Cornett EM, Novitch MB, Brunk AJ, et al. New benzodiazepines for sedation. Best Pract Res Clin Anaesthesiol. 2018;32(2):149–164.
- Meyer MR, Pettersson Bergstrand MP, Helander A, et al. Identification of main human urinary metabolites of the designer nitrobenzodiazepines clonazolam, meclonazepam, and nifoxipam by nano-liquid chromatography-high-resolution mass spectrometry for drug testing purposes. Anal Bioanal Chem. 2016;408(13):3571–3591.
- Pettersson Bergstrand M, Helander A, Beck O. Development and application of a multi-component LC-MS/MS method for determination of designer benzodiazepines in urine . J Chromatogr B Anal Technol Biomed Life Sci. 2016;1035:104–110.
- Pettersson Bergstrand M, Helander A, Hansson T, et al. Detectability of designer benzodiazepines in CEDIA, EMIT II Plus, HEIA, and KIMS II immunochemical screening assays. Drug Test Anal. 2017;9(4):640–645.


![Figure 3. Extracted mass spectrum of clonazolam’s parent ion showing the isotopic distributions of the [M + H]+ and [M + Na]+ ions. The top figure is obtained from the sample while the bottom figure is from an authentic reference standard of clonazolam.](/cms/asset/b1e4bf3f-7322-4778-b13b-64a15024af8a/ttxc_a_1661568_f0003_c.jpg)