Abstract
Barium poisoning is uncommon. Barium salts are used in a number of industries, including mining, ceramics, plastics, and adhesives and as a green coloring in fireworks. It is also a rodenticide. In poisoning, it blocks passive efflux potassium channels without affecting the Na/K-ATPase pump resulting in an increase in intracellular potassium and extracellular hypokalemia. A 44-year-old man self-presented to hospital six hours following deliberately ingesting 10 g of barium carbonate with suicidal intention. On presentation, he had profuse vomiting with decreased power globally and hyporeflexia in his upper and lower limbs. His ECG was in sinus rhythm with first-degree heart block and diffuse T wave flattening and U wave formation. He had severe hypokalemia with a potassium concentration of 2.4 mmol/L. He was managed supportively with antiemetics and supplemental potassium, receiving 80 mmol of intravenous potassium chloride in total. He was discharged home 24 hours post-ingestion. Soluble barium salts are highly toxic and clinical effects include hypokalemia, flaccid paralysis, cardiac arrest and death. Management of barium toxicity is largely supportive, focusing on the correction of hypokalemia. Oral sulfate salts may prevent absorption through precipitating insoluble barium sulfate in the gastrointestinal tract. Hemodialysis increases elimination and can be considered in severe poisoning.
Keywords:
Introduction
Barium is an alkaline metal. Its salts are used in a variety of industries including mining, ceramics, plastics, adhesives, and as a green coloring in fireworks. It is also used as a rodenticide. In the 18th and 19th centuries it was used medicinally as a tonic and in the treatment of a wide variety of conditions including asthma, tuberculosis, and heart failure. With its rise in popularity as a therapeutic agent, came its first report of toxicity in overdose in 1794 [Citation1].
Barium poisoning is uncommon, but potentially life-threatening. It blocks passive efflux potassium channels without affecting the Na/K-ATPase pump resulting in an increase in intracellular potassium and extracellular hypokalemia [Citation2]. When severe, the hypokalemia leads to weakness which may progress to respiratory paralysis and cardiac arrhythmia.
Case
A 44-year-old man self-presented to hospital six hours following deliberately ingesting 10 g of barium carbonate with suicidal intention. He had purchased the powder from the internet for this purpose after researching methods of suicide. To improve palatability, he mixed it into his coffee. Two hours following ingestion he began vomiting and shortly after developed diarrhea. His vomiting and diarrhea worsened, prompting him to call an ambulance. On their arrival six hours post ingestion, he was complaining of generalized weakness and tingling to his face. His heart rate was 92 bpm and blood pressure was 182/119 mmHg.
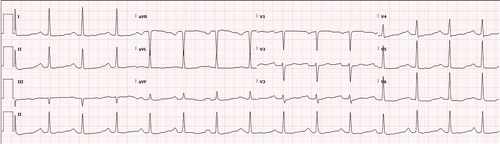
On arrival to hospital half an hour later, it was noted he had global weakness with 4/5 power in upper and lower limbs with diminished reflexes. His ECG () was in sinus rhythm with first degree AV block, diffuse T-wave flattening, and U-wave formation consistent with hypokalemia. His potassium was found to be critically low at 2.4 mmol/L (reference range 3.5–5.2 mmol/L). The rest of his blood investigations were unremarkable apart from a mildly elevated lactate at 3.5 mmol/L (reference range 0.5–2.2 mmol/L).
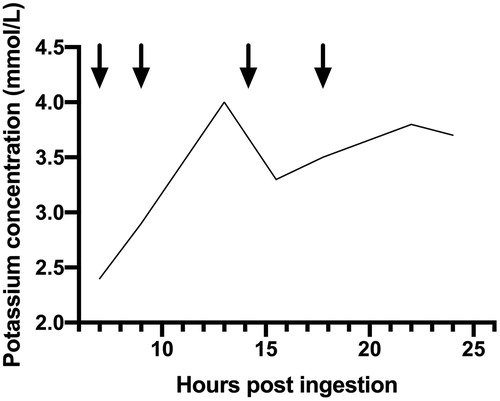
He received intravenous ondansetron, potassium, and magnesium supplementation and was observed overnight. Over the subsequent 16 h he received a total of 80 mmol potassium chloride (). By the next day, he was asymptomatic, and his potassium had stabilized at 3.7 mmol/L. He was reviewed by the mental health team and discharged home.
Discussion
Barium toxicity has been reported with ingestions as small as 200 mg [Citation3]. The toxicity of individual barium salts depends on their solubility. The more soluble salts such as chloride, hydroxide and nitrate are more toxic while insoluble salts such as arsenate, fluoride, and sulfate are rarely associated with toxicity. Some, like barium carbonate, while poorly soluble, are converted to the more soluble chloride by stomach acids when ingested.
The clinical features of barium toxicity are well described. In 1945, Dr William Morton reported three stages of toxicity following two outbreaks caused by barium carbonate contaminated flour in 85 British troops [Citation4]. Stage 1 occurred within ninety minutes and consisted of tingling around the mouth and neck along with vomiting followed by diarrhea. The pupils were initially dilated and there was a slow pulse. Stage 2 occurred within two to three hours. The face and neck tingling was replaced by tingling of the limbs. Gastrointestinal effects continued for up to twenty-four hours. Varying degrees of motor weakness and hyporeflexia occurred. Improvement occurred within hours post ingestion and resolution expected by 36 h. A minority went on to develop stage 3, defined as the progression to more significant paralysis including respiratory compromise. In this group, recovery occurred by day 4. There were no deaths. Further epidemics have been described with contaminated flour in Bangladesh, potato flour in Israel [Citation5], and salt in China [Citation6]. Individual exposures have been reported with industrial exposure to barium salts [Citation7], ingestion of barium containing shaving powder [Citation8], and fireworks [Citation9]. Additional clinical features that have been described with barium poisoning include hypertension, metabolic acidosis, elevated lactate, hypophosphatemia, and rhabdomyolysis.
The management of barium toxicity is largely supportive with a focus on potassium replacement. Activated charcoal is ineffective. The use of oral sulfate salts such as sodium or magnesium sulfate may be considered early to decrease absorption by converting the barium into the nontoxic barium sulfate in the gastrointestinal tract [Citation10]. Intravenous sulfates should be avoided to prevent the theoretical risk of intravascular precipitation and subsequent acute kidney injury. Hemodialysis has been shown to rapidly increase barium clearance while stabilizing potassium levels and may be considered in cases of severe toxicity not responding to potassium supplementation [Citation11].
Barium poisoning is an uncommon but potentially life-threatening poisoning that results in gastrointestinal toxicity and severe hypokalemia. Management is supportive with intravenous potassium supplementation. There may be a role for oral sulfate salts to decontaminate early, and in severe poisoning hemodialysis to aid elimination.
Ethical consideration
This case report details a presentation from the Princess Alexandra Hospital Clinical Toxicology Unit database. The Clinical Toxicology Unit has ethical approval from the Metro South Human Research Ethics Committee to publish observational research involving database presentations HREC/14/QPAH/308.
Disclosure statement
Neither author has any conflicts of interest to declare.
References
- Mather A. An account of the effects of an over-dose of the Terra Ponderosa Muriata. Med Comment. 1795;9:265–270.
- Bhoelan BH, Stevering CH, van der Boog ATJ, et al. Barium toxicity and the role of the potassium inward rectifier current. Clin Toxicol. 2014;52(6):584–593.
- Dawson AH. Barium. In: Nelson LS, Howland MA, Lewin NA, et al., editors. Goldfrank’s toxicologic emergencies. 11th ed. McGraw-Hill Education; 2019. p. 1454–1456.
- Morton W. Poisoning by barium carbonate. Lancet 1945;246(6380):738–739.
- Ghose A, Sayeed AA, Hossain A, et al. Mass barium carbonate poisoning with fatal outcome, lessons learned: a case series. Cases J. 2009;2:9327.
- Deng J, Jan IS, Cheng HS. The essential role of a poison center in handling an outbreak of barium carbonate poisoning. Vet Hum Toxicol. 1991;33(2):173–175.
- Jacobs IA, Taddeo J, Kelly K, et al. Poisoning as a result of barium styphnate explosion. Am J Ind Med. 2002;41(4):285–288.
- Downs JC, Milling D, Nichols CA. Suicidal ingestion of barium-sulfide-containing shaving powder. Am J Forensic Med Pathol. 1995;16(1):56–61.
- Rhyee SH, Heard K. Acute barium toxicity from ingestion of “snake” fireworks. J Med Toxicol. 2009;5(4):209–213.
- Payen C, Dellinger A, Pulce C, et al. Intoxication by large amounts of barium nitrite overcome by early massive K supplementation and oral administration of magnesium sulphate. Hum Exp Toxicol. 2011;30(1):34–37.
- Wells JA, Wood KE. Acute barium poisoning treated with haemodialysis. Am J Emerg Med. 2001;19(2):175–177.