Abstract
Hospitals often perform urine drug screens (UDS) upon inpatient admission to confirm self-reported psychoactive substance use for patients with opioid use disorder (OUD). We sought to evaluate the agreement between UDS and patient self-report for psychoactive substances detected with UDS for adults with OUD admitted to hospital. For 11 substance categories, we evaluated agreement between the UDS and the documented history over a 5-year period for consecutive adults admitted to one academic center with a history of OUD. Among the 153 patients, overall agreement across the 1683 different history/UDS pairs (i.e. either history+/UDS + or history-/UDS-) was high (81.3%) but varied (from lowest to highest) by substance [opiates (56.9%), benzodiazepines (66.0%), 6-acetylmorphine (67.3%), cocaine (81.0%), cannabinoids (81.0%), methadone (83.7%), buprenorphine (85.0%), amphetamine (94.8%), barbiturates (95.4%), and phencyclidine (98.7%)]. History+/UDS- pair mismatches were most frequent for 6-acetylmorphine (32.7%), methadone (14.3%) and oxycodone (12.4%); history-/UDS + pair mismatches were most frequent for opiates (43.1%), benzodiazepines (24.8%) and cannabinoids (18.3%). The change in agreement over time of self-reported heroin use may reflect an increasing number of patients unknowingly using illicit fentanyl products. Among hospitalized patients with OUD, agreement between reported psychoactive substance use history and UDS results is strong with the exception of opiates, heroin, and benzodiazepines.
Introduction
More than 2 million Americans currently have opioid use disorder (OUD) and deaths from opioid overdose have quintupled since 1999 [Citation1]. The nation’s overdose crisis is characterized in three waves; the first beginning with increased opioid analgesic prescribing in the 1990s, the second from a rapid rise in heroin-related overdose deaths starting in 2010, and a third from an exponential rise in overdose deaths associated with illicit fentanyl products beginning in 2013 [Citation2]. Alarmingly, illicit fentanyl products are increasingly identified in products sold as heroin [Citation3]. As more people are affected by this crisis, many eventually interact with the healthcare system, often for acute care needs [Citation4, Citation5].
Hospitalizations among patients with OUD more than doubled over the past decade [Citation6]. Additional substance use and psychiatric comorbidities are common in individuals with OUD who often self-administer both prescribed and non-prescribed psychoactive substances [Citation7]. Patients with OUD require a thorough review of medication and substance use at the time of admission. This helps ensure individuals receive adequate OUD treatment while hospitalized, risk factor(s) for a withdrawal syndrome(s) are recognized, other substance-related medical issues are identified, and the scope of current substance use is accurately characterized.
While some suggest urine toxicology screening should require informed consent, urine drug screens (UDS) often occur at the time of admission to confirm self-report or when patients cannot communicate their use [Citation8–10]. For hospitalized patients with OUD, agreement between self-reported use and the admission UDS remains unknown. We therefore sought to measure the agreement between self-reported psychoactive substance use and UDS results among hospitalized patients with OUD.
Methods
We undertook an IRB-approved, retrospective, secondary analysis of a cohort of patients admitted for OUD [Citation11]. We used the Partners Research Patient Data Registry (RPDR) to electronically identify consecutive adults admitted to one academic medical center in Boston, MA between October 1, 2011 and September 30, 2016 with either an ICD-9-CM or ICD-10 diagnostic code or problem list item suggestive of OUD (e.g. ICD-9-CM: 304 opioid dependence, 965.09 poisoning by other opiates and related narcotics; ICD-10: F11.10 opioid abuse-uncomplicated, F11.23 opioid dependence with withdrawal, etc.) [Citation12, Citation13].
We included patients with a UDS conducted within 24 h of hospital admission that reported on all of the following substances: 6-acetylmorphine (6-AM; heroin metabolite), amphetamines, barbiturates, benzodiazepines, buprenorphine, cocaine, methadone, opiates, oxycodone, phencyclidine, and cannabinoids. We excluded patients with a UDS completed ≥ 24 h after admission to avoid potential detection of in-hospital psychoactive medication administration. We identified self-reported substance use through documented medication and substance use histories and excluded patients if both were not documented.
Over the 5-year study period, the hospital utilized the Cobas C501 analyzer (Roche Diagnostics; Indianapolis, IN) for all urine drug screen immunoassays. summarizes target substances and test characteristics as specified by each assay manufacturer [Citation14–24]. In this analysis, “opiates” refers to the UDS immunoassay panel of multiple opioids. For the purposes of this analysis, we did not seek confirmatory test results to establish agreement with patient self-report. Rather, we determined agreement between patient self-report and UDS using presumptive positive results of the screening immunoassays.
Table 1. Summary of urine drug screen immunoassay specifications.
Trained data extractors collected data from the electronic medical record system [from October 1, 2011 to March 31, 2016 using the Longitudinal Medical Record (LMR) system (an in-house system developed at Partners Healthcare System) and thereafter using Partners eCare [developed in conjunction with Epic (Verona, WI)]. We used Research Electronic Data Capture (REDCap), a secure, web-based application for validated data entry, transmission, and storage to manage all extracted data.
We analyzed data on an individual level rather than in aggregate to avoid ecological fallacy. We cross-referenced each UDS result with the patient’s self-reported history. We recorded agreement for each UDS result-history pair when the agent was present in the UDS and the patient reported taking it or when the agent was not present in the UDS and the patient denied taking it. We calculated descriptive and comparative statistics using SAS software version 9.4 for MS Windows (SAS, Cary, NC).
Results
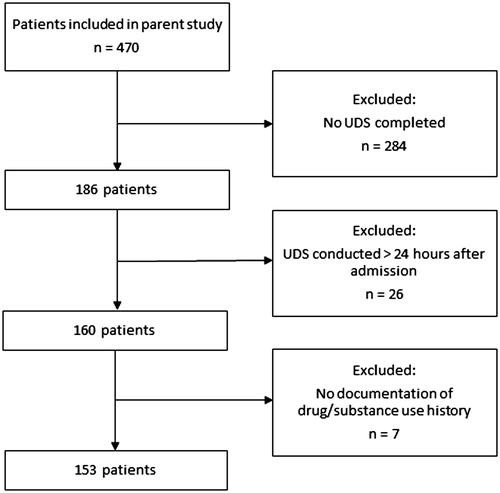
Among the 470 patients in the parent cohort, 160 (34.0%) had a UDS within 24 h of hospital admission, 26 (5.5%) had a UDS completed over 24 h after admission, and 284 (60.4%) never had a UDS completed. Among the 160 patients with a UDS within 24 h of admission, 7 (4.4%) did not have a medication/substance use history documented in their medical records and thus we included 153 patients in the final analysis (). presents the patient characteristics.
Table 2. Study cohort patient demographics.
The 153 patients represented a total of 1683 different UDS/history pairs. illustrates the frequencies of agreement and disagreement between UDS results and histories. Overall paired agreement was high (1369/1683 = 81.3%). Agreement was highest for phencyclidine (151/153 = 98.7%), barbiturates (146/153 = 95.4%), and amphetamines (145/153 = 94.8%) and lowest for 6-AM (103/153 = 67.3%), opiates (87/153 = 56.9%), and benzodiazepines (101/153 = 66.0%).
Table 3. Paired comparison between history and urine drug screen for each substance across the study cohort (n = 153).
Disagreements (positive UDS/negative history and negative UDS/positive history) were common (). There were three potential false-positive UDS results (amphetamine + due to trazodone; benzodiazepine + due to sertraline; and methadone + due to quetiapine) [Citation25]. Of note, 36 of the 50 UDS-negative 6-AM cases occurred after 2012, aligning with the timing of increasing prevalence of illicitly manufactured fentanyl product distribution [Citation26].
Discussion
Previous studies of agreement between UDS results and self-reported substance use history focused on patients receiving care in outpatient or emergency department (ED) settings. In the present study of hospitalized patients, agreement between patient self-report and UDS results was common for all substances (>80%), with the lowest level of agreement for opiates, benzodiazepines, and 6-AM (57–67%). The level of overall agreement between self-report and UDS was comparable to a prospective analysis of patients receiving psychiatric consultation in the ED [Citation8]. These investigators reported overall agreement between self-report and UDS of 85.3%. Disagreement was most common in cases when patients reported cannabis or alcohol use but these substances were not detected via UDS. In a prospective, cross-sectional study, Rashidian et al. [Citation10] evaluated the sensitivity of self-report and UDS to detect opioid use in healthy individuals and hospitalized patients. Sensitivity of self-report was comparably high for hospitalized patients (77.5%) and occurrence of positive UDS results when patients denied use, was similarly rare (7.9%). The frequent agreement between self-report and UDS across multiple studies demonstrates that UDS does not usually appear to provide more information than what a patient is already willing to acknowledge.
The higher rates of positive UDS for opiates and benzodiazepines in our study (when patients did not report use of these substances) may indicate non-prescribed use of these agents in individuals with OUD. This may occur when patients do not feel comfortable disclosing their use out of fear of stigmatizing or punitive approaches taken by clinicians treating them. Health care workers in various treatment settings are identified as a common source of stigma towards patients with OUD [Citation27]. It is possible patients would be more open to disclosing their use if clinicians were trained on compassionate approaches when treating this patient population.
Patients frequently reported heroin use, but had negative results for opiate and 6-AM screens. One explanation may be the short time window for detection of 6-AM [Citation28]. Alternatively, patients may have thought they used heroin and thus reported doing so, when instead, they unknowingly used something else. This scenario is increasingly likely since 2013, when many regions of the United States, including New England, started seeing a dramatic rise in distribution of illicitly manufactured fentanyl (IMF) [Citation29]. In our study, 72% of the UDS that were negative for 6-AM when a patient reported using heroin occurred after 2012, coinciding with the rising rates of IMF distribution. Fentanyl was not included as an agent analyzed in the UDS panels utilized at our center, therefore we were unable to confirm its presence in this subset of samples.
However, 83% of patients presenting to a community ED in Baltimore, MD for treatment of OUD, overdose, or withdrawal tested positive for fentanyl [Citation30]. Another recent investigation of non-hospitalized volunteers with self-reported use of heroin or IMF from Dayton, OH compared self-reported use of these substances to results of UDS [Citation31]. These researchers found that individuals who reported use of heroin, but denied use of IMF, frequently had UDS positive for IMF products, suggesting these individuals were unaware of the contents of their supplies. The addition of fentanyl to standard UDS testing may be warranted, as suggested in a study showing over 96% of patients presenting to a New England ED following suspected heroin overdose tested positive for nonpharmaceutical fentanyl [Citation32, Citation33]. False-positive interference is also a known issue with UDS immunoassays [Citation34].
Our analysis has limitations. First, due to the retrospective nature of our study, we were unable to characterize the quality of the medication and substance use histories conducted, thus their reliability remains uncertain. Second, patients with OUD admitted to our center might represent a different demographic from those at other centers and thus our results may limit external validity. Additionally, only one-third of patients from the initial cohort met inclusion criteria for this study. Third, our use of results from screening immunoassays, rather than confirmatory testing, introduces potential for inaccuracies. Future prospective analyses could utilize confirmatory testing to compare with self-report to minimize false-positive rates. Finally, it is possible that a positive UDS with negative history could occur in a patient who received a therapeutic dose of medication before the urine collection occurred. Due to the retrospective nature of our study and limitations of electronic medical record documentation in the earlier years of our data collection window, we could not confirm that each specimen was collected prior to administration of any of the screened substances.
Given the inherent limitations of UDS along with evidence that patients with OUD are mostly accurate in their reporting of substance use, the utility of UDS in this patient population may have a narrower scope than is often employed at healthcare centers. One option is to rely on patient reports of substance use and reserve UDS for patients who are unable to communicate or are unsure of what they may have used. With this arrangement, clinicians might be better able to build rapport by including patients as members of the care team and demonstrating their trust towards them (rather than skepticism). In cases when patients can communicate, it is reasonable to obtain informed consent prior to conducting any toxicology testing.
Conclusions
Agreement between patient self-report and UDS among hospitalized patients with OUD was high. Frequencies of agreement were lower for opiates, 6-acetylmorphine, and benzodiazepines than for other substance tested. The increasing frequency of reported heroin use with negative UDS in later years likely reflects the transition to illicit fentanyl use. In cases when UDS is considered warranted, adding a screen for fentanyl may increase agreement.
Disclosure statement
The authors declare that they have no conflict of interest
References
- Centers for Disease Control and Prevention. CDC Wonder; 2017 [accessed 2019 Jul 11]. Available from: https://wonder.cdc.gov
- Dasgupta N, Beletsky L, Ciccarone D. Opioid crisis: no easy fix to its social and economic determinants. Am J Public Health. 2018;108(2):182–186.
- Drug Enforcement Administration, US. DEA Intelligence Report. Washington DC; 2016. (The Heroin Signature Program and Heroin Domestic Monitor Reports 2014).
- Owens PL, Barrett ML, Weiss AJ, et al. Hospital inpatient utilization related to opioid overuse among adults, 1993–2012: HCUP Statistical Brief #177. Agency for Healthcare Research and Quality, Rockville (MD); August 2014 [Accessed 2019 Jul 11 14]. Available from: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb177-Hospitalizations-for-Opioid-Overuse.pdf
- Kirson NY, Scarpati LM, Enloe EJ, et al. The economic burden of opioid abuse: updated findings. J Manag Care Spec Pharm. 2017;23:427–445.
- HCUP Fast Stats. Healthcare Cost and Utilization Project (HCUP). Agency for Healthcare Research and Quality, Rockville (MD); October 2018 [accessed 2019 Jul 11]. Available from: http://www.hcup-us.ahrq.gov/faststats/opioid/opioiduse.jsp
- NIDA. Common comorbidities with substance use disorders. Last modified 2018 February 27 [accessed 2019 Jul 11]. Available from: https://www.drugabuse.gov/publications/research-reports/common-comorbidities-substance-use-disorders
- Perrone J, de Roos F, Jayaraman S, et al. Drug screening versus history in detection of substance use in ED psychiatric patients. Am J Emerg Med. 2001;19(1):49–51.
- Warner EA, Walker RM, Friedman PD. Should informed consent be required for laboratory testing for drugs of abuse in medical settings? Am J Med. 2003;115(1):54–58.
- Rashidian H, Hadji M, Marzban M, et al. Sensitivity of self-reported opioid use in case-control studies: healthy individuals versus hospitalized patients. PLoS One. 2017;12(8):e0183017.
- Moreno JL, Wakeman SE, Duprey MS, et al. Predictors for 30-day and 90-day hospital readmission among patients with opioid use disorder. J Addict Med 2019;13(4):306–313.
- Moore BJ, Barrett ML. Case study: exploring how opioid-related diagnosis codes translate from ICD-9-CM to ICD-10-CM. U.S. Agency for Healthcare Research and Quality. Last modified 2017 April 24 [accessed 2019 Jul 11]. Available from: https://www.hcupus.ahrq.gov/datainnovations/icd10_resources.jsp
- Heslin KC, Owens PL, Karaca Z, et al. Trends in opioid-related inpatient stays shifted after the US transitioned to ICD-10-CM diagnosis coding in 2015. Med Care. 2017;55(11):918–923.
- CEDIA® Heroin Metabolite (6-AM) Assay [package insert]. Fremont (CA): Microgenics Corporation; 2003.
- Amphetamines II (AMPS2) [package insert]. Indianapolis (IN): Roche Diagnostics; 2010.
- Barbiturates Plus (BARB) [package insert]. Indianapolis (IN): Roche Diagnostics; 2006.
- Benzodiazepines Plus (BENZ) [package insert]. Indianapolis (IN): Roche Diagnostics; 2006.
- CEDIA® Buprenorphine Assay [package insert]. Fremont (CA): Microgenics Corporation; 2004.
- Cannabinoids II (THC2) [package insert]. Indianapolis (IN): Roche Diagnostics; 2006.
- Cocaine II (COC2) [package insert]. Indianapolis (IN): Roche Diagnostics; 2006.
- Methadone II (MDN2) [package insert]. Indianapolis (IN): Roche Diagnostics; 2006.
- Opiates II (OPI2) [package insert]. Indianapolis (IN): Roche Diagnostics; 2006.
- DRI® Oxycodone Assay [package insert]. Fremont (CA): Microgenics Corporation; 2004.
- Phencyclidine Plus (PCP) [package insert]. Indianapolis (IN): Roche Diagnostics; 2006.
- Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and false-positive urine drug screens. Am J Health Syst Pharm. 2010;67(16):1344–1350.
- Rudd RA, Aleshire N, Zibbell JE, et al. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378–1382.
- Woo J, Bhalerao A, Bawor M, et al. “Don’t judge a book by its cover”: a qualitative study of methadone patients’ experiences and stigma. Subst Abuse. 2017;11:1178–1187.
- Goldberger BA, Darwin WD, Grant TM, et al. Measurement of heroin and its metabolites by isotope dilution electron-impact mass spectrometry. Clin Chem. 1993;39(4):670–675.
- Scholl L, Seth P, Kariisa M, et al. Drug and opioid-involved overdose deaths–United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2019;67:1419–1427.
- Dezman Z, Felemban W, Bontempo LJ, et al. Evidence of fentanyl use is common and frequently missed in a cross-sectional study of emergency department patients in Baltimore, Maryland. Clin Tox. 2019;1–3. Epub ahead of print.
- Daniulaityte R, Carlson RR, Juhascik MP, et al. Street fentanyl use: experiences, preferences, and concordance between self-reports and urine toxicology. Int J Drug Policy. 2019;71:3–9.
- Center for Disease Control and Prevention Health Alert Network. Rising numbers of deaths involving fentanyl and fentanyl analogs, including carfentanil, and increased usage and mixing with non-opioids. Last modified 2018 July 11 [accessed 2019 Jul 11]. Available from: https://emergency.cdc.gov/han/han00413.asp
- Griswold MK, Chai PR, Krotulski AJ, et al. Self-identification of nonpharmaceutical fentanyl exposure following heroin overdose. Clin Toxicol. 2018;56(1):37–42.
- Saitman A, Park HD, Fitzgerald RL. False-positive interferences of common urine drug screen immunoassays: a review. J Anal Toxicol. 2014;38(7):387–396.