Abstract
Here we report a rare case of reversible neurological symptoms due to chronic ethanol vapor and mist exposure in a 50-year-old machinist who intentionally used undiluted 200 proof ethanol as a cutting fluid while turning metal machine parts on a toolroom lathe for a period of 3 years. Shortly after switching to ethanol as a cutting fluid, the worker began to experience central nervous system symptoms including headaches, fatigue, ataxia, and concentration and memory problems. Clinical neuropsychological assessment revealed mild deficits on tests of attention, executive function, and memory. The worker was subsequently advised to stop using ethanol as a cutting fluid. At follow-up after cessation of exposure the worker reported that his symptoms were remarkably improved.
Introduction
Here we report on the rare case of a machinist who developed reversible neurological symptoms of fatigue, ataxia, and, concentration and memory deficits in association with a 3 years history of chronic exposure to undiluted 200 proof ethanol vapor and mist when he substituted this solvent for conventional cutting fluids used in a machining process.
Case report
A 47-year-old male machinist with a history of anxiety controlled with escitalopram began using undiluted 200 proof ethanol as a coolant and cutting fluid for the work he routinely performed on a precision toolroom metal lathe (see ). He made the decision to switch to using ethanol as a coolant when the shop he worked in was relocated to the basement of a building where there was insufficient air movement to remove the smoke generated from using conventional cutting oils.
Figure 1. Exposure circumstances. (A) Lathe. (B) Gallon bottle of 200 proof ethanol. (C) Type of squirt bottle used to spray ethanol directly onto hot cutting surfaces; (D) Relationship between worker’s breathing zone and source of exposure to 100% ethanol vapors.
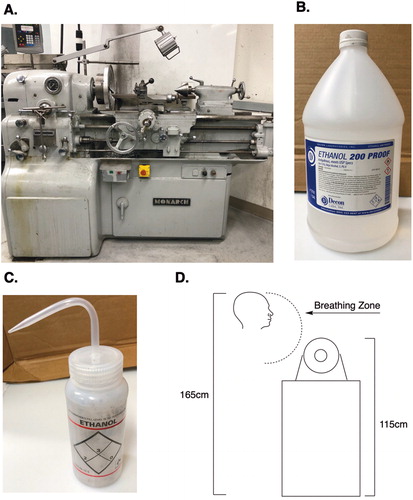
In order to cool the metal (e.g. aluminum) parts being machined, the worker would squirt liquid ethanol directly onto the hot cutting tip and surface of the part using a 1-quart squirt bottle (see ). Depending on how the stream of liquid ethanol hit the chuck and/or part being machined, he could at times feel liquid ethanol droplets hit his face indicating the turning chuck and machine parts were capable of throwing the liquid ethanol back into his breathing zone as a mist in addition to being vaporized by contact with hot cutting surface. When he first started using ethanol as a cutting fluid he was able to smell it (the odor threshold for ethanol is 80 ppm) but he eventually no longer noticed the odor [Citation1]. He did not experience any symptoms of lachrymation, mucous membrane irritation or coughing typically associated with exposure to very high concentrations of ethanol vapor [Citation2].
Shortly after switching to using ethanol, the worker began to experience fatigue, headaches, balance problems, muscle pain, and what he called “brain fog” characterized by concentration and memory impairments. These symptoms progressed during the first few months of exposure but then plateaued. These symptoms were also observed by his wife. The worker noticed that his symptoms improved when he was away from work leading him to suspect these might be related to his exposure to metals including manganese since he also did some welding at work.
The worker contacted an occupational medicine specialist to ascertain if his symptoms were work-related. Because the worker also did some welding as part of his job, a heavy metal screen was ordered. This revealed slightly elevated blood concentrations of aluminum (13/μg/L; ref range <7 μg/L, manganese (1.9 μg/L; ref range <1.2 μg/L) consistent with his history of occupational exposure to these two metals while machining and welding; his blood lead as in the normal range (1 μg/dL; ref range <5 μg/dL). In addition, analysis of his hair for these two metals which, reflects prior exposure, was also within normal limits (Aluminum 11 μg/g: ref range <19.0 μg/g; Manganese 0.39 μg/g: ref range <1.3 μg/g) indicating that his past exposures to these two metals was also low. The concentrations of metals such as manganese in hair have advantages over blood and urine concentrations of these metals because this biomarker of exposure is influenced less by short-term variability in exposure circumstances [Citation3].
An MRI of his brain was also interpreted as normal with no evidence of hyperintensities on T1 images of the globus pallidus typically associated with occupational manganese exposure as occurs among welders [Citation4, Citation5]. Formal psychiatric evaluation found no evidence of somatoform disorder nor psychosis the latter of which, can be associated with manganese exposure [Citation6,Citation7]. A clinical neuropsychological assessment performed during the workweek without allowing for washout revealed mild deficits on tests of learning and memory function. On part A of the Trail Making Test which, requires the subject to connect numbers in sequential order his performed in the high average range. By contrast, his performance on the more difficult Trails Part B which, requires the subject to alternate between sequentially connecting a set of numbers and letters, was in the low average range. His impaired performance on Trails B but not Trails A is consistent with findings showing that performance on Trails B is more sensitive to increasing blood ethanol concentrations based on measurements of breath concentrations [Citation8]. His performance on tests of language and communication skills was within expectation. There were no indications of exaggeration or malingering.
The worker subsequently underwent post-chelation challenge urinary metal testing. Urinalysis for aluminum and manganese following chelation challenge were both within the normal range (Aluminum 2.3 μg/g creatinine: ref range <25.0 μg/g creatinine; Manganese 0.002 μg/mg creatinine: ref range 0.0003–0.005 μg/mg creatinine). This controversial test was performed despite the concentrations of metals in his hair not being elevated and only slightly elevated blood concentrations of aluminum and manganese. The American College of Medical Toxicology [Citation9] has issued a position statement indicating that “post-challenge urinary metal testing has not been scientifically validated, has no demonstrated benefit, and may be harmful”. He took time off from work during this chelation challenge test and he did not return to work for four months after this during which time he felt much better. He attributed this improvement to the chelation therapy. However, upon returning to work his symptoms reemerged. Thus, his clinical improvement was not actually due to chelation challenge but rather to his not working with ethanol.
A neurological exam by a movement disorder specialist at that time did not reveal any signs or symptoms of parkinsonism as would be expected with manganese exposure but, the Romberg test revealed problems as with balance. The worker was advised to seek a consultation with a neurotoxicologist (MHR).
The neurotoxicology consult which was with one of the authors who is a clinically trained behavioral neuroscientist and board certified toxicologist (MHR) included a review of the worker’s medical records and, his answers to questions on an occupational and environmental exposure history questionnaire [Citation10]. The full record review indicated that in addition to the worker’s previously recognized occupational exposures to aluminum and manganese from machining and welding, that he also used undiluted 200 proof ethanol as a cutting fluid while machining metal parts on the toolroom lathe. His use of ethanol for this purpose was not mentioned in any of his medical records indicating that it had previously been overlooked. Based on this new information, the worker was advised to stop using ethanol as a cutting fluid and to follow-up with the neurotoxicologist after 1 month. He was also advised to return to using conventional cutting oils and, to wear a disposal N95 type respirator when working with this to reduce his risk for respiratory exposure to cutting oils. At follow-up 1 month after cessation of exposure to ethanol vapor and mist, the worker reported that his symptoms were remarkably improved. At follow-up with the worker 3 months after his cessation of exposure to ethanol vapor and mist he was still doing well. His symptoms did not return even though he had returned to work full time. This is in contrast to the reemergence of his symptoms when he returned to full time work after undergoing chelation.
For ethical reasons it was not possible obtain any biological markers of exposure in this case as this would have required the worker to resume his former hazardous practice of using ethanol as a cutting fluid. Therefore, the biological markers of effect, exposure modeling, and a time-exposure-symptoms-line (TESL) were used to determine if there was a relationship between the worker’s symptoms and his exposure history. The TESL provides an organizational structure for exposure and clinical data that can be used as an aid in formulating the diagnosis of neurotoxic disease based on the coincidence of the exposure episodes and the appearance of illnesses along the TESL in the absence of any other reasonable explanation to account for the findings and complaints of the patient. The relationship between the exposure and symptoms plotted on the TESL is further supported by a review of the literature for effects of exposure to the same chemical substances [Citation10, Citation11] ().
Figure 2. Time-exposure-symptoms-line. This time line shows the chronology of events in this case report from onset of the worker’s symptoms when he first switched to using ethanol as cutting fluid, through to the time when he finally stopped using it and began to recover from the effects of his chronic exposure.

His non-occupational exposure to chemicals (e.g. pharmaceutical and recreational drugs) that may have caused or exacerbated his symptoms was also reviewed. Interview of the worker and review of his medical records both indicated that he had no history of alcohol abuse or recreational drug use (see ). Because ethanol can increase the neurological side effects of escitalopram which include dizziness, drowsiness, and difficulty concentrating his use of this medication was considered when interpreting the observations reported herein. In addition, because he did not recognize that ethanol was playing a role in the etiology of his symptoms, he did not alter his nightly habit of having a glass of wine with dinner.
Table 1. Occupational exposure and medical histories.
Industrial hygiene evaluation of exposure circumstances
The worker used an average of 1 gallon (range: 1 quart to 2 gallons) of ethanol per week applied during 3.5 h each day. The volume of the machine room where he worked was 240 m3 (4 × 17 x 3.5 m). The volume of the entire basement shop was 2970 m3. The air change rate was 6.73 air changes per hour (ACH). The height of the toolroom lathe at the head stock/chuck was 115 cm. The worker himself is 165 cm tall and stood at the machine while he worked (see Fig1D). He reported that his face was typically 30 to 60 cm from the cutting surface. The ethanol was applied to the tool with a 1-quart squirt bottle intermittently. As previously stated, he could at times feel ethanol droplets hit his face indicating that in addition to being vaporized via heating, the ethanol was also being dispersed into his breathing zone as a mist. The machine was not equipped with a mist collection system. Due to cessation of exposure being used to confirm the etiology of the worker’s symptoms, no measurements were made in his personal breathing zone while he used ethanol as a cutting fluid. However, it was possible to conservatively model his daily exposure to ethanol vapor alone, not including the additional exposure from ethanol droplets thrown into his breathing zone.
Using the above parameters, the molecular weight of ethanol (46.07 g/mol) and its density (0.789 mg/ml) estimates of the worker's exposure were calculated assuming he applied the material at a steady rate of 0.25 liters per hour we estimate that his typical exposure averaged approximately 94 part per million (ppm) of ethanol in the ambient air. At his highest reported use of 2 gal (approximately 7.6 L) per week, during which time the ambient concentration was likely twice as high (188 ppm).” The maximum ethanol concentration he could have experienced was about 1000 parts per million (ppm). The ethanol in his inspired air would be expected to move down the concentration gradient into his blood which could lead to ethanol intoxication.
In the case presented herein, the worker also reported actually feeling droplets of liquid ethanol hitting his face during the machining process demonstrating that he was exposed to mist as well as ethanol vapor. Machine turning operations include the use of lathes, inherently generate mists which, include liquid particles smaller than 20 microns in diameter. When coolant impacts the cutting tool, the tool spins the liquid off at high speeds, creating mechanically formed airborne mist which serves as a source of exposure to toxic chemicals [Citation12].
Mini review
Workers seeking to reduce fumes from smoke which are inherent to the chemical nature of heating cutting oils, will occasionally try substituting other lubricants including alcohols for these cutting oils putting themselves and their coworkers at risk for neurotoxic effects associated with inhalation of ethanol vapor and mist [Citation13–15]. Unfortunately, heating ethanol vaporizes the liquid and, there is a linear relationship between the concentration of ethanol vapor in inhaled air and blood ethanol concentrations [Citation14]. Blue collar workers are also not sedentary and therefore, their exposure circumstances are similar to those of subjects engaged in a moderate level of physical activity (e.g. 12 min per hour of exercise) who show increased blood concentrations of ethanol as compared with sedentary subjects. Dumas-Campagna and colleagues [Citation14] have shown that even at a moderate level, physical activity causes a significant increase (two to three times) of blood ethanol concentrations. This occurs because blood flow to muscle tissue is increased while blood flow to the liver is decreased leading to reduced metabolism of ethanol [Citation16]. Inhalation of ethanol vapor is associated with elevated urine ethyl glucuronide concentrations [Citation15].
Dumas-Campagna and colleagues [Citation14] have also demonstrated that blood ethanol concentrations rise rapidly during acute exposure and that a steady state concentration is reach within 1 h when human subjects are exposed to ethanol vapor concentrations ranging from 125 to 1,000 ppm. Peak blood alcohol concentrations of 0.03 mg/dL were measured in male subjects after 4 h of exposure to 1000 ppm. One hour after acute exposure ended blood ethanol concentrations decline toward baseline.
Generation of ethanol vapors and aerosols can be achieved by with heat and/or physical agitation [Citation17]. Inhaled ethanol vapors move down the concentration until the concentrations in the inhaled air and blood reach equilibrium. Therefore, a subject inhaling air with an ethanol concentration greater than the concentration in his blood will absorb ethanol. Ethanol also readily crosses the blood-brain-barrier. Within the central nervous system, ethanol disrupts neuronal function producing dose dependent clinical manifestations including ataxia, attention, delayed reaction times and memory dysfunction. The effects of unintentional or experimenter-delivered ethanol vapor on the brain have been investigated in animal models from an addiction perspective [Citation18, 19] but little research has been done from an industrial hygiene perspective.
Conclusions
This report highlights the potential risks associated with substituting alcohols such as ethanol for cutting fluids and, calls attention to the need for additional studies to further elucidate the neurobehavioral effects associated with chronic exposures to ethanol vapor and mist.
Consent
The worker discussed in this report has provide his consent.
Disclosure of interest statement
The authors report no conflict of interest.
References
- Amoore JE, Hautala E. Odor as an aid to chemical safety: odor thresholds compared with threshold limit values and volatilities for 214 industrial chemicals in air and water dilution. J Appl Toxicol. 1983;3(6):272–290.
- Lester D, Greenberg LA. The inhalation of ethyl alcohol by man. I. Industrial hygiene and medicolegal aspects. II. Individuals treated with tetraethylthiuram disulfide. Q J Stud Alcohol. 1951;12(2):168–178.
- Reiss B, Simpson CD, Baker MG, et al. Hair manganese as an exposure biomarker among welders. Ann Occup Hyg. 2016;60(2):139–149.
- Josephs KA, Ahlskog JE, Klos KJ, et al. Neurologic manifestations in welders with pallidal MRI T1 hyperintensity. Neurology. 2005;64(12):2033–2039.
- Criswell SR, Perlmutter JS, Huang JL, et al. Basal ganglia intensity indices and diffusion weighted imaging in manganese-exposed welders. Occup Environ Med. 2012;69(6):437–443.
- Emara AM, el-Ghawabi SH, Madkour OI, et al. Chronic manganese poisoning in the dry battery industry. Br J Ind Med. 1971;28(1):78–82.
- Rutchik J, Ratner MH. Should age at onset of Parkinsonism be the end point of interest in investigations of the link between exosomal α-Synuclein and manganese exposure in Welders? J Occup Environ Med. 2019;61(12):e530–e531.
- Day AM, Celio MA, Lisman SA, et al. Acute and chronic effects of alcohol on trail making test performance among underage drinkers in a field setting. J Stud Alcohol Drugs. 2013;74(4):635–641.
- American College of Medical Toxicology. ACMT recommends against use of post-chelator challenge urinary metal testing. J Med Toxicol. 2017;13(4):352–354.
- Feldman RG. Occupational and environmental neurotoxicology. Philadelphia (PA): Lippincott-Raven; 1999.
- Feldman RG, Ratner MH, Ptak T. Chronic toxic encephalopathy in a painter exposed to mixed solvents. Environ Health Perspect. 1999;107(5):417–422.
- Donaldson Torit. Coolant & oil mist technical reference guide. F118000 ENG (06/18) Mist Collection of Metalworking Fluids ©2011-2018 Donaldson Company, Inc. Minneapolis, MN, 2018.
- McManus TN, Haddad AN. Use of methanol as a coolant during machining of aluminum in a shipbuilding environment: a failure to assess and manage risk. AMR. 2014;955-959:1061–1064.
- Dumas-Campagna J, Tardif R, Charest-Tardif G, et al. Ethanol toxicokinetics resulting from inhalation exposure in human volunteers and toxicokinetic modeling. Inhal Toxicol. 2014;26(2):59–69.
- Arndt T, Schrofel S, Gussregen B, et al. Inhalation but not transdermal resorption of hand sanitizer ethanol causes positive ethyl glucuronide findings in urine. Forensic Sci Int. 2014;237:126–130.
- Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev. 2015;95(2):549–601.
- MacLean RR, Valentine GW, Jatlow PI, et al. Inhalation of alcohol vapor: measurement and implications. Alcohol Clin Exp Res. 2017;41(2):238–250.
- Sabino V, Cottone P, Koob GF, et al. Dissociation between opioid and CRF1 antagonist sensitive drinking in Sardinian alcohol-preferring rats. Psychopharmacology. 2006;189(2):175–186.
- Richardson HN, Chan SH, Crawford EF, et al. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol Dis. 2009;36(1):1–10.
