Abstract
Introduction
Bupropion overdose can produce seizures, arrhythmias, and shock. The toxicokinetics of massive bupropion ingestions are not well characterized.
Case report
A 22-year-old female ingested an estimated 40.5 g (644 mg/kg) of extended release bupropion. Subsequently she experienced seizures, required intubation, developed torsades des pointes that progressed to cardiac arrest, and required cannulation with venous-arterial extracorporeal membrane oxygenation (VA-ECMO). Intravenous lipid emulsion was administered without adversely affecting the ECMO circuit. The patient was successfully decannulated after 84 h of ECMO support and discharged neurologically intact. Serial bupropion and hydroxybupropion serum concentrations were drawn every 6-12 h starting on hospital day one and continuing for seven days, for a total of 22 serum concentrations each.
Discussion
The patient’s first bupropion and hydroxybupropion serum concentrations were 4000 ng/mL and 5300 ng/mL, respectively. Clearance of bupropion followed first order kinetics (t ½ = 20.6 h) while hydroxybupropion had zero order kinetics (t ½ = 118.5 h).
Conclusion
This bupropion overdose was treated with VA-ECMO with 20% lipid emulsion therapy, without complications. In this patient, the toxicokinetics of bupropion were first-order.
Introduction
Bupropion is a cathinone derivative approved by the FDA for the treatment of depressive disorders and smoking cessation. In 2018, there were 6,903 single substance exposures and 14,824 total bupropion exposures reported to US poison centers [Citation1]. Ingestions greater than 600 mg may cause generalized tonic-clonic seizures [Citation2], and larger doses can cause cardiovascular collapse [Citation2, Citation3]. No specific antidotes exist. Case reports describe successful treatment with either ECMO or lipid emulsion [Citation3–5]. Potential concerns about using both together include lipid emulsion disrupting the ECMO circuit with fat agglutination, clogging, increased blood clot formation, and cracking of parts of the circuit [Citation6]. This case, presented in accord with the CARE guidelines (https://www.care-statement.org/), illustrates clinical features and pharmacokinetics of severe bupropion toxicity with successful outcome with combined ECMO and lipid emulsion in addition to supportive care.
Case report
A 22-year-old female presented to a community ED hemodynamically stable and neurologically intact 1 h after reportedly ingesting a 90-day supply of 450 mg bupropion extended release tablets (estimated maximum total bupropion dose 40.5 g; 644 mg/kg). Over the next 9 h, she received a total of 12 mg IV lorazepam (2 mg twice and 4 mg twice) after experiencing 4 generalized tonic-clonic seizures. She subsequently required intubation for airway protection and sedation with a propofol infusion. Ultimately, the patient became hypotensive necessitating a norepinephrine infusion and was transferred to our facility. Prior to being transferred, she received a total of 450 mEq (7.2 mEq/kg) of sodium bicarbonate IV push. Upon arrival at our hospital, the patient’s initial EKG demonstrated sinus rhythm (rate 70 bpm, QTc 597 msec, QRS 162 msec) with frequent premature ventricular contractions (PVCs) and right bundle branch block.The patient’s serum and urine toxicologic screens were negative for acetaminophen, amphetamine, baclofen, barbiturate, benzodiazepine, buprenorphine, cannabinoids, cocaine, ethanol, gabapentin, methadone, opiates, oxycodone, and salicylates.
Approximately 3 h after arriving at our facility, the patient’s cardiac rhythm devolved into torsades des pointes and then pulseless electrical activity. Return of spontaneous circulation followed 11 min of chest compressions, 4 mg of epinephrine, 8 g of magnesium, and 50 mEq of sodium bicarbonate. She continued to be hypotensive despite continuous infusions of norepinephrine, dopamine, phenylephrine, isoproterenol, vasopressin, and methylene blue; thus, veno-arterial (VA) ECMO was initiated emergently via femoral cannulation.
We obtained bupropion and hydroxybupropion serum concentrations every 6-12 h starting 20 h after ingestion and for a total of 6 days (). Determination of both bupropion and hydroxybupropion levels was via high-performance liquid chromatography with ultraviolet detection. The first bupropion serum concentration was 4000 ng/mL and the hydroxybupropion serum concentration was 5300 ng/mL. The patient’s serum bupropion concentrations followed an exponential curve with rate constant 0.0337 (ng/mL/hr), corresponding to a half-life of 20.6 h (). The reported half-life of bupropion is 21 (±9) hours (coefficient of determination 0.97). [Citation2] The active metabolite, hydroxybupropion followed zero-order kinetics with a half-life of 118.5 h (coefficient of determination 0.709). The reported half-life of hydroxybupropion is 24 (±5) hours [Citation2].
Figure 1. Bupropion and hydroxybupropion serum concentrations over time with significant clinical events.
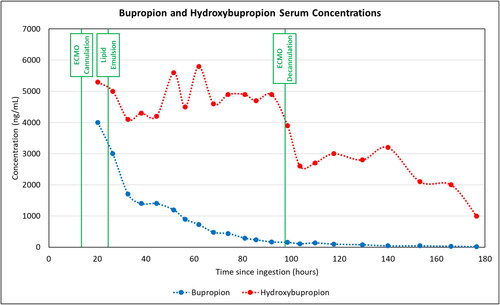
During the 10 h following ECMO cannulation, the patient experienced recurrent ventricular tachycardia requiring 13 defibrillations, electrolyte repletion, amiodarone, and lidocaine. She received a bolus of 94 mL (approximately 1.5 mL/kg) of 20% lipid emulsion. She required only one further defibrillation 1 h after the lipid emulsion bolus. At this time, the patient exhibited no evidence of brainstem reflexes or motor responses. Her neurologic status improved over the following week. She was extubated on hospital day 8. She was verbally interactive and fully oriented at that time.
This patient remained on VA-ECMO (Cardiohelp System) for 84 h without circuit or oxygenator (Quadrox-Id Bioline) complications due to the lipid emulsion. The patient was medically cleared and discharged to the inpatient psychiatry unit on hospital day 14.
Discussion
Toxicities from bupropion and hydroxybupropion include seizures, cardiac dysrhythmias (tachycardia, QT prolongation, torsades des pointes), and, ultimately, cardiogenic shock. The seizures may be convulsive or nonconvulsive, and in some cases bupropion toxicity has mimicked brain death [Citation7]. Hydroxybupropion is the major, active metabolite of bupropion with approximately one-half the potency of bupropion and an unclear role in development of toxicity [Citation2, Citation8]. Bupropion-related seizures should be treated with GABAergic agents, beginning with benzodiazepines, and escalating to propofol or barbiturates as needed. Lipid emulsion therapy and/or ECMO have been used in symptomatic massive overdoses, although not usually in concert [Citation3–5].
Limitations of this case report include potentially incomplete documentation of interventions, procedures, and vital signs. We are not able to establish a cause-and-effect relationship between the use of lipid emulsion and any patient centered outcomes. There are potential confounding effects of both ECMO and lipid emulsion on bupropion and hydroxybupropion serum concentrations and the calculation of their respective half-lives. Additionally, the administration of lipid emulsion occurred while bupropion was rapidly accumulating in the serum. The fall in serum concentration could reflect lipid emulsion reducing the effective serum concentration of bupropion by redistributing some bupropion to the organic (lipid) phase, that is to say lipid emulsion could have rescued first-order kinetics with no change to the metabolism or excretion of bupropion.
This case includes a serum bupropion serum concentration of 4000 ng/mL and hydroxybupropion level of 5300 ng/mL, as well as ant extensively documented course of drug clearance, with 22 bupropion and hydroxybupropion concentrations. We found that bupropion followed first-order kinetics (half-life 20.6 h) while its main active metabolite, hydroxybupropion, followed zero-order kinetics (). Her decreased need for subsequent defibrillations following lipid emulsion suggests beneficial effect. There were no adverse effects related to the ECMO circuit following a single bolus of lipid emulsion.
Conclusion
This bupropion overdose resulted in cardiogenic shock and refractory arrhythmias treated with VA-ECMO with 20% lipid emulsion therapy, without complications to the patient or ECMO circuit. In this patient, the kinetics of bupropion were first-order.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gummin DD, Mowry JB, Spyker DA, et al. 2018 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS): 36th annual report. Clin Toxicol (Phila)). 2019;57(12):1220–1413.
- Wellbutrin [package insert]. Bridgewater, NJ: Bausch Health US, 2019.
- Heise CW, Skolnik AB, Raschke RA, et al. Two cases of refractory cardiogenic shock secondary to bupropion successfully treated with veno-arterial extracorporeal membrane oxygenation. J Med Toxicol. 2016;12(3):301–304.
- Chhabra N, Deslauriers C, Wahl M, et al. Management of severe bupropion poisoning with intravenous lipid emulsion. Clin Toxicol (Phila)). 2018;56(1):51–54.
- Sirianni AJ, Osterhoudt KC, Calello DP, et al. Use of lipid emulsion in the resuscitation of a patient with prolonged cardiovascular collapse after overdose of bupropion and lamotrigine. Ann Emerg Med. 2008;51(4):412–415.e1.
- Lee HM, Archer JR, Dargan PI, et al. What are the adverse effects associated with the combined use of intravenous lipid emulsion and extracorporeal membrane oxygenation in the poisoned patient? Clin Toxicol (Phila)). 2015;53(3):145–150.
- Wu P, Juurlink D. 2016. Bupropion. In: Brent J, Burkhart K, Dargan P, Hatten B, Megarbane B, Palmer R., editors. Critical care toxicology. Cham (Switzerland): Springer.
- Al-Abri SA, Orengo JP, Hayashi S, et al. Delayed bupropion cardiotoxicity associated with elevated serum concentrations of bupropion but not hydroxybupropion. Clin Toxicol (Phila)). 2013;51(10):1230–1234.
