Abstract
Calcium channel blockers (CCB) overdose can result in cardiogenic shock and that high dose insulin euglycaemic therapy (HIET) is increasingly used to treat CCB poisoning. This case illustrates the risk of severe hypokalaemia resulting from HIET for calcium channel blocker poisoning.
Introduction
Calcium channel blockers (CCB) overdose can result in cardiogenic shock. High dose insulin euglycemic therapy (HIET) is often used to treat CCB poisoning. This case presented in accord with the CARE guidelines (www.care-statement.org), illustrates the risk of severe hypokalaemia resulting from HIET for calcium channel blocker poisoning.
Case report
A 51-year-old gentleman weighing 100 kg with a past medical history of hypertension and gastro oesophageal reflux disease arrived t to the emergency department. Two hours prior his ingestion of his own medications that included a total of 3.6 gm of extended release diltiazem, 3 gm irbesartan and 250 mg hydrochlorothiazide (HCTZ).
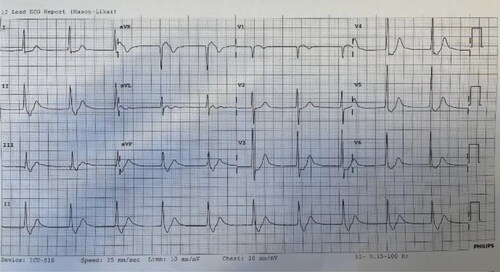
On arrival to the ED, he was oriented with a blood pressure of 125/85 mmHg, a heart rate of 75 bpm, a respiratory rate of 18 and oxygen saturations of 98% on room air. His initial ECG showed sinus rhythm with normal cardiac intervals.
His initial venous blood gas () noted a potassium 3.1 mmol/L (55 mg/dL) and normal blood sugar concentration 5.8 mmol (104 mg/dL). The patient vomited immediately after receiving 50 g of activated charcoal shortly after arrival. His repeat vitals were blood pressure of 80/45 mmHg, and bradycardia at 45 bpm. His ECG showed a prolonged PR interval of 220 msec with normal QRS width. He responded to 2 boluses of atropine 600 mcg, temporarily improving heart rate to 60 bpm and blood pressure to 92/51. Over the course of the following 2 h in a sequential manner, he received 60 mL calcium gluconate 10%, a total of 60 mcg adrenaline boluses which improved his heart rate and blood pressure. An adrenaline infusion (0.1 mcg/kg/min) followed. He was subsequently received High-dose Insulin Euglycemic Therapy (HIET) (bolus 1 unit/kg then 0.5 unit/kg/h). He was intubated and re-administered 50 g activated charcoal via nasogastric tube.
Table 1. Serial Blood Biochemistry.
Six hours after ingestion, blood pressure was 85/35 mmHg with a junctional rhythm on ECG. His cardiac intervals included QRS interval of 110 msec and no visible P waves (). Repeat arterial blood gas showed metabolic acidosis with profound hypokalaemia 1.3 mmol/L. Bedside echo demonstrated features of left ventricular outflow tract obstruction (LVOTO) restricting stroke volume and subsequent tissue perfusion manifesting as persistent hypotension. HIET was ceased due the profound hypokalaemia and adrenaline ceased due to concerns about LVOTO. He received potassium replacement at 40 mmol/h, dobutamine (2.5 mcg/kg/h), noradrenaline (0.5-1 mcg/kg/min) and argipressin (0.03 mcg/kg/min) targeting heart rate greater than 50 bpm and mean arterial pressure of 60–70 mmHg. At 14 h, he deteriorated further to a junctional rhythm of 30 bpm and blood pressure of 65/30 mmHg. Dobutamine was increased (5 mcg/kg/min) and a transvenous pacing wire was inserted for emergent temporary pacing.
Over the following 24 h, his laboratory values improved. His potassium had normalised as well as a slight improvement in his pH. His lactate had worsened (increasing 7.3 mmol/L and 7 mmol/L) before improving several hours later. His creatinine worsened from 98 mmol/L to 194 mmol/L meeting criteria for acute kidney injury. An ECG revealed a heart rate of 79 bpm, normalisation of PR interval to 110 msec, QRS width of 110 msec and an absolute QT of 410 msec. He continued on dextrose 25% (25 g/100 mL) at 50–100 mL/h for persisting hypoglycaemia maintaining blood sugar between 5 and 10 mmol/L (90–180 mg). Over 24 h, he had received a total 150 mmol potassium. He was discharged from ICU to a psychiatric ward on day 5.
Discussion
This case of massive diltiazem ingestion with evidence of cardioplegic and vasoplegic shock demonstrates life threatening hypokalaemia as complications of management. This case illustrates an unusually profound drop in potassium.
While there have been no clinical trials comparing the use of HIET to other treatments in humans, many case reports report the beneficial effects of HIET in CCB poisoning with cardiogenic shock [Citation1]. Recognized side effects of HIET include hypokalemia and hypoglycemia [Citation2,Citation3]. However, in most of these cases, this has been mild. Page et al. in a retrospective analysis of 22 patients utilising HIET reported 2 cases of severe hypokalaemia of 1.6 mmol/L and 2.1 mmol/L respectively [Citation4]. The former patient died attributed to the underlying poisoning as opposed to the profound hypokalaemia. Furthermore, the analysis suggests that although HIET can disrupt potassium homeostasis, there was no complications associated with its use. HIET doses up to 10 U/kg/h was deemed as safe as low dose therapy (1–2 U/kg/h). HIE therapy reflects a redistribution of potassium intracellularly as opposed to total body depletion. Arrythmias including atrioventricular blocks, atrial fibrillation, and ventricular tachycardias have been reported with total body potassium depletion [Citation4].
The unexpected and profound drop in potassium likely aggravated the electrophysiologic effects of diltiazem poisoning and likely further impaired myocardial contractility. The ingestion of diuretics must also be considered as contributing to the profound hypokalemia. There is evidence suggesting that acute diuretic ingestion causes significant hypokalaemia [Citation5]. The case demonstrates that careful attention to potassium replacement is vital in administering HIET to prevent severe hypokalaemia particularly in patients who have ingested diuretics. Cessation of HIET is associated with worsening cardioplegic shock which can be difficult to manage with traditional inotropes and vasopressors. After potassium repletion, restarting HIET at a lower infusion rate may be safe.
Author contributions
All authors contributed to the diagnosis and management of this patient.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Levine MD, Boyer E. Hyperinsulinemia-euglycemia therapy: a useful tool in treating calcium channel blocker poisoning. Crit Care. 2006;10(4):149.
- Megarbane B, Karyo S, Baud FJ. The role of insulin and glucose (hyperinsulinaemia/euglycaemia) therapy in acute calcium channel antagonist and beta‐blocker poisoning. Toxicol Rev. 2004;23:215–222.
- Lheureux PE, Zahir S, Gris M, et al. Bench-to-bedside review: hyperinsulinaemia/euglycaemia therapy in the management of overdose of calcium-channel blockers. Crit Care. 2006;10(3):212.
- Page C, Ryan N, Isbister G. The safety of high dose insulin euglycaemic therapy in toxin induced cardiac toxicity. Clin Toxicol. 2018;56(6):389–396.
- Siegel D, Hulley SB, Black DM, et al. Diuretics, serum and intracellular electrolyte levels, and ventricular arrhythmias in hypertensive men. JAMA. 1992;267(8):1083–1089.