Abstract
Lithium poisoning remains common. Symptomatic lithium poisonings often require hemodialysis, especially when the patient has impaired renal function. An effective and non-invasive treatment to remove excess lithium would be desirable. We tested two recently approved, orally administered potassium binders in an in-vitro model. We used lithium-heparin tubes as both the source of lithium based prior studies showing the different volumes of serum in lithium-heparin tubes will produce apparent lithium concentrations in the range of toxicity (2.5 mmol/L and 5 mmol/L). We added three different volumes (0.5 mL, 0.75 mL, or 1.0 mL) of normal saline (NS) to the tubes. We calculated concentrations of sodium zirconium cyclosilicate (SZC, Lokelma®) and patiromer (Veltassa®) to simulate the ratio of drug to total body water for a 70 kg human. We prepared stock suspensions with different concentrations above and below the estimated ratios. We added varying concentrations of SZC or patiromer to tubes containing of NS. We measured sodium and lithium concentrations in duplicate for each concentration. Neither SZC nor patiromer reduced the lithium concentration across the range of concentrations in this in-vitro study.
Introduction
Lithium (Li+) poisoning remains a frequent problem and often requires consultation with toxicologists and nephrologists. The American Association for Poison Control Centers reported 7,085 lithium carbonate exposures and four deaths in 2019 [Citation1]. In severe cases, lithium toxicity may warrant hemodialysis, which may not be immediately available in all settings [Citation2].
Previous studies suggested the enteral use of sodium polystyrene sulfonate (SPS, Kayexalate®, Sanofi-Aventis, Bridgewater NJ) to bind Li+ [Citation3–6], although this is controversial due to adverse gastrointestinal effects [Citation7–10].
Sodium zirconium cyclosilicate (SZC, ZS-9, Lokema®, AstraZeneca, Wilmington DE) and patiromer (Veltassa®, Vifor Pharma, Redwood City CA) are both insoluble cation exchangers recently approved for the treatment of hyperkalemia in adults [Citation11–13]. Like SPS, they bind potassium ions in the gastrointestinal lumen. The FDA New Drug Application to for SZC suggested a drug-drug interaction by a reduction in the lithium concentration during an in-vitro experimentation [Citation14].
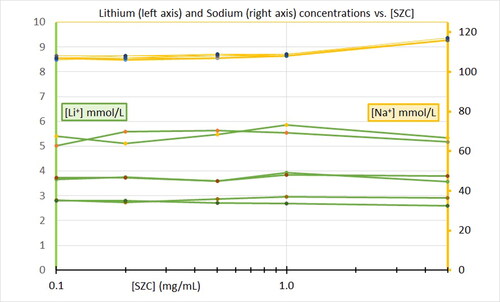
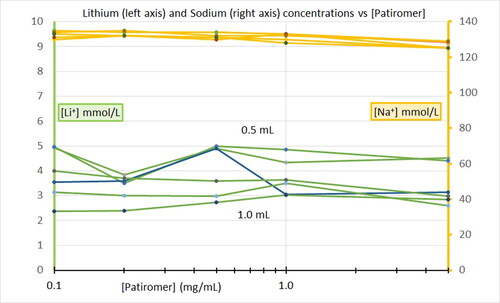
It is unknown whether SZC () or patiromer () will bind lithium in the gastrointestinal tract in the setting of lithium poisoning or overdose. The objective of this pilot experiment was to determine whether either SZC or patiromer would reduce Li+ concentration in an in-vitro model.
Methods
We used 6 mL lithium-heparin blood collection tubes (BD Vacutainer® Reference 367886, Franklin Lakes, NJ) as our source of lithium. Each 6 mL tube contains 95 units USP of lithium heparin. Previous case reports and experiments have shown that these tubes yield apparent Li+ concentrations from 1.5 mmol/L to over 5 mmol/L [Citation15–18]. The apparent [Li+] is inversely proportional to the blood volume in the tubes. These concentrations match in the range of clinical concern for lithium toxicity prompting decisions about hemodialysis [Citation2]. We obtained medical grade SZC and patiromer from our hospital pharmacy. We performed experiments with SZC and patiromer on separate study days.
We added normal saline (NS, 0.9% sodium chloride in deionized water) to 6 mL green-top lithium-heparin tubes to simulate serum. We added varying volumes of NS to create target [Li+] >/= 2.5 mmol/L. We identified 0.5, 0.75, and 1.0 mL as our target volumes of NS for the rest of the study.
We selected our target SZC and patiromer concentrations by estimating the ratio of prescription doses to total body water for a 70 kg human. The recommended doses of SZC are 5 to 10 g. For a 70 kg patient, a 10 g dose of SZC is roughly in equilibrium with about 50 L of total body water. This approximate ratio of 10 g/50 L equals 1 mg/5 mL or 0.2 mg/mL. The recommended doses of patiromer are 8.4 g to 25.2 g. For a 70 kg patient, the high dose of patiromer gives an approximate ratio of 25.2 g/50 L equals 2.5 mg/5 mL or 0.5 mg/mL.
We created suspensions of SZC by adding 5 mg, 10 mg, 25 mg, 50 mg and 250 mg of the compound to 50 mL of NS in conical tubes. The resultant stock solutions of suspended SZC had concentrations of 0.1 mg/mL, 0.2 mg/mL, 0.5 mg/mL, 1 mg/mL, and 5 mg/mL.
Likewise, we added 250 mg of patiromer to 50 mL of NS in a conical vial and suspended by vortex mixing. We added aliquots of 0.4, 0.8, 2.0, 4.0, and 20 mL of this stock suspension to conical vials and volume balanced with NS to total of 20 mL, achieving concentrations of 0.1 mg/mL, 0.2 mg/mL, 0.5 mg/mL, 1 mg/mL, and 5 mg/mL of patiromer in NS.
We vortex mixed each suspension and pipetted aliquots of 0.5 mL, 0.75 mL, and 1 mL of either SZC or patiromer suspension into the lithium-heparin Vacutainer® tubes (two tubes for each concentration of each drug). We placed the tubes on a rocker panel for 10 min, and centrifuged for 5 min at 4400 rpm.
We pipetted 350 µL of the supernatant from sample into 1.5 mL microcentrifuge tubes and measured [Li+] and [Na+] using a Roche® 9180 Electrolyte Analyzer (Indianapolis, IN) with an ion-selective electrode. All measurements occurred within four hours of preparation.
Addition of 0.5 mL, 1 mL, 2 mL, 3 mL, 4 mL, and 5 mL of NS (without SZC or patiromer) to 6 mL lithium-heparin blood collection tubes produced apparent [Li+] of 5.78 mmol/L, 2.84 mmol/L, 1.34 mmol/L, 0.76 mmol/L, 0.61 mmol/L, and 0.51 mmol/L, respectively. For the remainder of the experiment we used volumes of 0.5 mL, 0.75 mL, and 1.0 mL.
Results
Increasing concentrations of sodium zirconium cyclosilicate had no effect on lithium concentrations at any of the volumes tested (). Likewise, increasing concentrations from 0.1 to 5.0 mg/mL of patiromer had no effect on Li+ concentrations ().
Discussion
This novel model of lithium poisoning takes advantage of a well-known laboratory error in which lithium-heparin coated tubes are inappropriately used for blood collection resulting in [Li+] ranging from 1.05 to 4.04 mmol/L, even in the absence of any lithium in the patient’s blood [Citation15–18]. These apparent concentrations are in the range of clinical concern for lithium poisoning.
The FDA New Drug Application (NDA) for sodium zirconium cyclosilicate reported that SZC reduced [Li+] by 39% in an in-vitro model [Citation14]. The NDA attributed this to the pH of the base solution. The regulatory process did not require human volunteer trials for this interaction. Unpublished preclinical data indicated that lithium carbonate reduced potassium binding by 12% but did not report the opposite interaction [Citation19].
We found no association between either [SZC] or [patiromer] and [Li+] within the range that we tested. Our study concentrations included [SZC] and [patiromer] above and below the ratios estimated to represent therapeutic use. These ranges might be too low. Future experiments with higher concentrations of sodium zirconium cyclosilicate or patiromer in physiologic gastric, buffered, and intestinal fluid models are necessary.
Another limitation is that the duration of interaction between SZC or patiromer with the lithium-heparin tubes may have been too short. The therapeutic effects in lowering potassium concentrations occur over hours to days with successive use. It is possible that a longer exposure time might have had different results.
Another explanation for lack of effect of SZC on [Li+] relates to the ionic sizes. The crystalline structure of SZC has a mean pore diameter of 3.0 Å [Citation19]. The unhydrated ionic diameters for sodium (Na+) and potassium (K+) are 2.34 Å and 2.98 Å, respectively [Citation20, Citation21]. These favor the release of Na+ in exchange for K+, which becomes trapped inside the pores. Li+ has an even smaller ionic diameter of 1.88 Å [Citation21], and may bind even more poorly than Na+ does.
This pilot study shows the feasibility of using a lithium blood collection tube filled with normal saline as a model for drug-drug interaction between lithium and a potassium binding therapeutic. It is possible that longer interaction times between the potassium binders and the lithium-heparin tubes might have produced greater effect.
In this novel lithium heparin blood tube model for drug-drug interactions with lithium, no reduction in lithium concentrations was observed over a range of sodium zirconium cyclosilicate concentrations from 0.1 mg/mL to 5.0 mg/mL or patiromer concentrations from 0 mg/ml to 5.0 mg/mL.
Conclusion
Neither sodium zirconium cyclosilicate nor patiromer had any effect in reducing lithium concentrations in this in-vitro model. Future study in animals, human volunteers, or lithium poisoned patients may better determine whether either potassium binder would have clinical benefit in lithium poisoning.
Disclosure statement
No potential competing interest was reported by the authors.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Gummin DD, Mowry JB, Beuhler MC, et al. 2019 Annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 37th Annual Report. Clin Toxicol (Phila)). 2020;58(12):1360–1541.
- Decker BS, Goldfarb DS, Dargan PI, et al. Extracorporeal treatment for lithium poisoning: Systematic review and recommendations from the EXTRIP workgroup. Clin J Am Soc Nephrol. 2015;10(5):875–887.
- Linakis JG, Lacouture PG, Eisenberg MS, et al. Administration of activated charcoal or sodium polystyrene sulfonate (kayexalate) as gastric decontamination for lithium intoxication: an animal model. Pharmacol Toxicol. 1989;65(5):387–389.
- Gehrke JC, Watling SM, Gehrke CW, Zumwalt R. In-vivo binding of lithium using the cation exchange resin sodium polystyrene sulfonate. Am J Emerg Med. 1996;14(1):37–38.
- Linakis JG, Savitt DL, Wu TY, et al. Use of sodium polystyrene sulfonate for reduction of plasma lithium concentrations after chronic lithium dosing in mice. J Toxicol Clin Toxicol. 1998;36(4):309–313.
- Ghannoum M, Lavergne V, Yue CS, et al. Successful treatment of lithium toxicity with sodium polystyrene sulfonate: a retrospective cohort study. Clin Toxicol (Phila)). 2010;48(1):34–41.
- Harel Z, Harel S, Shah PS, et al. Gastrointestinal adverse events with sodium polystyrene sulfonate (kayexalate) use: a systematic review. Am J Med. 2013;126(3):264.e9–e24.
- Noel JA, Bota SE, Petrcich W, et al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med. 2019;179(8):1025–1033.
- Laureati P, Xu Y, Trevisan M, et al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant. 2020;35(9):1518–1526.
- Wu YH, Chou JW, Lai HC, et al. Adverse gastrointestinal effects with kayexalate or kalimate: a comprehensive review. Clin Exp Gastroenterol. 2021;14:1–18.
- Georgianos PI, Agarwal R. Revisiting RAAS blockade in CKD with newer potassium-binding drugs. Kidney Int. 2018;93(2):325–334.
- Rosano GMC, Spoletini I, Agewall S. Pharmacology of new treatments for hyperkalaemia: patiromer and sodium zirconium cyclosilicate. Eur Heart J Suppl. 2019;21(Suppl A):A28–A33.
- Li L, Harrison SD, Cope MJ, et al. Mechanism of action and pharmacology of patiromer, a nonabsorbed cross-linked polymer that lowers serum potassium concentration in patients with hyperkalemia. J Cardiovasc Pharmacol Ther. 2016;21(5):456–465.
- FDA Center for Drug Evaluation and Research. Clinical Pharmacology Biopharmaceutics Review, Application Number: 207078Orig1s000. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/207078Orig1ClinPharmR.pdf. Accessed September 24, 2021.
- Lee DC, Klachko MN. Falsely elevated lithium levels in plasma samples obtained in lithium containing tubes. J Toxicol Clin Toxicol. 1996;34(4):467–469.
- Nordt SP, Cantrell FL. Elevated lithium level: a case and brief overview of lithium poisoning. Psychosom Med. 1999;61(4):564–565.
- Wills BK, Mycyk MB, Mazor S, et al. Factitious lithium toxicity secondary to lithium heparin-containing blood tubes. J Med Toxicol. 2006; 2(2):61–63.
- Brizzee L, Stone A, Palmer MC. False lithium toxicity secondary to lithium heparin test tube: a case report and review. Ment Health Clin. 2020; 10(3):90–94.
- Rafique Z, Peacock WF, LoVecchio F, et al. Sodium zirconium cyclosilicate (ZS-9) for the treatment of hyperkalemia. Expert Opin Pharmacother. 2015;16(11):1727–1734.
- Stavros F, Yang A, Leon A, et al. Characterization of structure and function of ZS-9, a K+ selective ion trap. PLoS ONE. 2014; 9(12):e114686.
- Volkov AG, Paula S, Deamer DW. Two mechanisms of permeation of small neutral molecules and hydrated ions across phospholipid bilayers. Bioelectrochem Bioenerg. 1997;42(2):153–160..