Abstract
Acetaminophen overdose is a leading cause of liver failure in the United States. Macrophage migration inhibitory factor (MIF) is a cytokine that is released early and promotes acetaminophen toxicity in preclinical models. This cytokine could prove a useful biomarker in emergency department (ED) patients immediately following an acute acetaminophen overdose. We selected a convenience sample of thirteen patients from a prospective consecutive cohort of ED patients with suspected acute overdose. Research associates collected waste specimens for MIF analysis that remained after use for clinical care. Our team compared patients with confirmed acetaminophen overdose (n = 9) to patients without acetaminophen exposure or liver injury (n = 3) and a patient with liver injury in the absence of detectable acetaminophen (n = 1). In our acetaminophen group, all nine patients had measurable acetaminophen concentrations. Median MIF serum concentrations were 16.08 ng/mL (In this pilot study, MIF was feasible to measure in specimens from an ED drug overdose cohort, and was significantly elevated in the acetaminophen group compared to non-acetaminophen controls without liver injury.
Introduction
Acetaminophen (APAP) overdose is a prominent cause of acute liver failure in the United States [Citation1]. Early prognostication is essential in this disease process since timely administration of the antidote acetylcysteine can prevent clinical decompensation and subsequent liver failure.
Macrophage migration inhibitory factor (MIF) is a proinflammatory cytokine and enzyme that has been studied as a biomarker in a wide range of disease processes, including sepsis, multiple sclerosis, and various cancers [Citation2,Citation3]. MIF has been the subject of preclinical acetaminophen overdose studies that suggest it promotes toxicity, possibly by proinflammatory mechanisms. Attenuation of MIF activity by genetic deletion and small molecule inhibitors improved survival in a murine acetaminophen model [Citation4–6]. A 2002 study indicated that mice given an overdose of acetaminophen rapidly released hepatocyte MIF into serum prior to release of transaminases [Citation4]. In this pilot study we attempted to determine if elevated concentrations of MIF could be similarly detected in the sera of human patients with acetaminophen overdose, which could improve clinical management of acetaminophen-induced liver injury.
Methods
Study sample
We selected a convenience sample from a prospective cohort of ED patients who presented with acute drug overdose at two urban teaching hospitals from 2016 to 2017, as previously described [Citation7,Citation8]. Acetaminophen overdose was defined as patients in the cohort (i.e. patient report of overdose) with positive acetaminophen concentrations. Non-acetaminophen controls were randomly selected from cohort subjects with available waste specimens. We selected patients without acetaminophen overdose or liver injury (negative controls, Patients 1–3, n = 3), patients with acetaminophen overdose (Patients 4–12, n = 9), and one patient with acute liver injury with a negative acetaminophen concentration (positive control, Patient 13, n = 1). The Institutional Review Board at The Mount Sinai Hospital approved of this study.
Sample collection and storage
Research coordinators collected waste specimens from initial blood collections in the emergency department after their use for clinical care. We included patients with non-acetaminophen exposures in this study. Samples were stored at −80 °C in a secure research facility until used for research studies, and freeze-thaw cycles were minimized and held consistent among samples. Clinical parameters of overdose such as acetaminophen concentrations and transaminase measurements were available to the investigators by chart review.
Transaminase measurements
Transaminase measurements were gleaned from chart review, and the researchers did not repeat these measurements during this study. Transaminase normal limits were 1–45 U/L for aspartate transaminase and 1–35 U/L for alanine transaminase. Clinical laboratories obtained results using an Abbott Architect C16000 chemistry analyzer (Abbott Park, IL).
Sample analysis
To analyze MIF concentrations, we used an enzyme-linked immunosorbent assay (Duoset Kit, R&D Systems, Minneapolis, MN), which has a linear detection range of 31.2–2000 pg/mL; samples were run in serial dilutions to ensure detection within this range. Samples with frank hemolysis by visual inspection were documented qualitatively by the investigators during the study before being subjected to MIF analysis.
Statistics
Given the pilot nature of the study, no formal a priori sample size calculation was performed. We obtained demographic and overdose data along with clinical values, when available, by chart review. Our group undertook post-hoc comparison of non-APAP patients without liver injury to APAP patients. We compared median and interquartile range (IQR) of MIF serum concentrations using a Mann Whitney U test, and statistical calculations were done using Graphpad Prism 9 (San Diego, CA). Patients with only a single transaminase measurement were treated as having identical peak and initial transaminases.
Results
Demographics and descriptive statistics
Of the three negative controls included in our study, the median age was 23 years, and all were male. Our acetaminophen cohort consisted of older individuals, with a median age of 59 years. The median peak transaminases for our study were near normal limits ().
Table 1. Demographics and descriptive statistics.
Overdose and serum parameters
Only three patients had acetaminophen as their sole overdose agent. When patients had co-ingestions, none of the co-ingested medications were significant hepatotoxins. All patients in our acetaminophen overdose cohort had a measurable serum acetaminophen. Only three had a listed time of overdose relative to ED presentation. All except one (patient 5) received acetylcysteine. Patient 10 did not have liver function testing results available to our research team ().
Table 2. Overdose and serum parameters by patient.
Measurement of MIF
MIF serum concentrations were measurable for all patients included in the study. Multiple study participants had lower values than previously published studies in normal human subjects [Citation9]. As MIF is present in red blood cells, we investigated whether hemolysis could impact our MIF measurements [Citation10,Citation11]. In our study, samples from Patients 1, 6, 11, and 12 had visible frank hemolysis noted during sample analysis. Patient 1 had MIF concentrations lower than the normal range expected for healthy controls ().
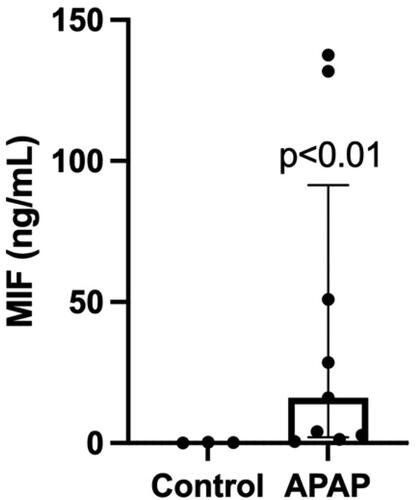
Our non-acetaminophen overdose controls without liver failure had a median serum MIF of 0.19 ng/mL with an IQR of 0.27 ng/mL (0.05–0.32 ng/mL). Our overdose cohort had a median serum MIF of 16.08 ng/mL with an IQR of 89.34 ng/mL (2.06–91.40 ng/mL), an elevation that was statistically significant compared to negative controls (p = 0.0091) (, ). Increased serum MIF was also noted in our acetaminophen-negative overdose patient with acute liver injury (MIF 65.16 ng/mL).
Figure 1. MIF is elevated in serum from an overdose population. MIF serum concentrations were compared between an overdose cohort (acetaminophen overdose) and negative controls (no acetaminophen overdose or liver injury). Results are displayed as box plots representing median/IQR, with individual patient values represented for each group; p = 0.0091 using a Mann-Whitney U test.
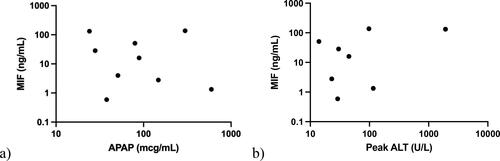
Among our acetaminophen overdose cohort, patient 8 was noted with the lowest acetaminophen concentration, but had the highest peak transaminases and the second highest MIF concentration. Their timing of ED arrival after ingestion of acetaminophen is unknown. Among patients with unknown time to ED arrival, patient 6 had the highest acetaminophen concentration, highest MIF concentration, and had only mild elevations in peak transaminases not exceeding 100 U/L. Patient 4 had the highest measured acetaminophen concentration and arrived to the ED two hours post-ingestion; they did have elevations in their transaminases during their hospital stay (not exceeding 200 U/L) but also had the second lowest MIF concentration in the cohort. When MIF serum concentrations in patients with acetaminophen overdose were graphed against peak ALT (where available) and initial serum acetaminophen, there was no significant correlation ().
Discussion
Our pilot study indicates that MIF is measurable in patients presenting to the ED with acute overdose, and that MIF serum concentrations are significantly elevated in acetaminophen overdose compared to patients without acetaminophen overdose or acute liver injury. In a patient with acute liver injury in the absence of detectable acetaminophen, MIF serum concentrations were elevated compared to normal ranges suggested by prior studies [Citation9], which may suggest that elevated serum MIF is not specific to acetaminophen overdose. The effects of hemolysis on the assay are unclear, given the low number of samples with hemolysis in our study; however, given the high concentrations of MIF in human red blood cells, further studies should continue to consider this potential confound.
Several biomarkers have been pursued in an effort to improve diagnosis and prognostication in acetaminophen overdose, including NAPQI-protein adducts and damage-associated molecular patterns (DAMPs) like high mobility box group 1 (HMGB1) [Citation12,Citation13]. These biomarkers continue to await clinical validation, and may be complicated to measure in an emergency setting; notably, a NAPQI-protein adduct point-of-care assay is currently under investigation [Citation14]. Although it is not yet being used as a biomarker in clinical settings, MIF can be easily measured using an ELISA assay adaptable to high throughput analysis, and may have distinct release and detectability patterns compared to these biomarkers. We hope that our study acts as a proof of concept that opens further, larger studies on the clinical use of this biomarker.
Limitations
This was a pilot study performed without a priori sample size calculation in two urban EDs in the American Northeast, which limits the generalizability of our findings. Demographics were not controlled between non-acetaminophen and acetaminophen overdose patients, and multiple patients took additional xenobiotics with acetaminophen in overdose.
The sample analyzed from a patient with acute liver injury and a negative acetaminophen concentration may represent a non-acetaminophen overdose with acute liver injury, or a late presenting acetaminophen patient who developed subsequent liver injury. Unfortunately, we did not have access to adduct testing or similar methods to assess this, nor did we have access to other types of hepatic injury patients in order to pursue the question of MIF’s specificity further.
Our research group did not have access to clinical data on outcomes for patients aside from peak transaminases. Based on this readout, only four of nine patients experienced elevated transaminases as a result of their overdose, suggesting a low disease burden in our overdose cohort. Only two of these patients had peak transaminases over 100 U/L: patient 4 had the highest measured acetaminophen concentration, but their concentration of MIF was only minimally increased compared to controls; patient 8 had the highest peak ALT, and the second highest MIF concentration. Of note, most patients with acetaminophen overdose received acetylcysteine, despite some (e.g. Patient 11) not meeting criteria as described by the nomogram of Rumack and Matthew [Citation15]. This may have attenuated liver injury from their overdose.
Conclusion
This pilot study highlights the potential of MIF as a clinical biomarker in acetaminophen overdose. Unlike other investigated biomarkers, MIF is easily measured and may be elevated early in the disease process. Future studies of MIF in early risk stratification of acetaminophen overdose are warranted.
Disclosure statement
Joshua Bloom holds a patent for the use of the drug iguratimod as an inhibitor of macrophage migration inhibitory factor. The authors have no other relevant affiliations, financial involvements, or financial interests to disclose.
Additional information
Funding
References
- Yoon E, Babar A, Choudhary M, et al. Acetaminophen-induced hepatotoxicity: a comprehensive update. J Clin Transl Hepatol. 2016;4(2):131–142.
- Bernhagen J, Calandra T, Mitchell RA, et al. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365(6448):756–759.
- Bloom J, Sun S, Al-Abed Y. MIF, a controversial cytokine: a review of structural features, challenges, and opportunities for drug development. Expert Opin Ther Targets. 2016;20(12):1463–1475.
- Bourdi M, Reilly TP, Elkahloun AG, et al. Macrophage migration inhibitory factor in drug-induced liver injury: a role in susceptibility and stress responsiveness. Biochem Biophys Res Commun. 2002;294(2):225–230.
- Ohkawara T, Okubo N, Maehara O, et al. Protective effect of ISO-1 with inhibition of RIPK3 up-regulation and neutrophilic accumulation on acetaminophen-induced liver injury in mice. Toxicol Lett. 2021;339:51–59.
- Bloom J, He M, Al-Abed Y. Abstract 056: macrophage migration inhibitory factor as a therapeutic target in acetaminophen-induced liver injury. J Med Toxicol. 2019;15(2):70.
- Manini AF, Nair AP, Vedanthan R, et al. Validation of the prognostic utility of the electrocardiogram for acute drug overdose. J Am Hear Assoc Cardiovasc Cerebrovasc Dis. 2017;6(2):e004320.
- Shastry S, Ellis J, Loo G, et al. Antidotal sodium bicarbonate therapy: delayed QTc prolongation and cardiovascular events. J Med Toxicol. 2021;17(1):27–36.
- Petrovsky N, Socha L, Silva D, et al. Macrophage migration inhibitory factor exhibits a pronounced circadian rhythm relevant to its role as a glucocorticoid counter-regulator. Immunol Cell Biol. 2003;81(2):137–143.
- Karsten E, Hill CJ, Herbert BR. Red blood cells: the primary reservoir of macrophage migration inhibitory factor in whole blood. Cytokine. 2018;102:34–40.
- Sobierajski J, Hendgen-Cotta UB, Luedike P, et al. Assessment of macrophage migration inhibitory factor in humans: protocol for accurate and reproducible levels. Free Radic Biol Med. 2013;63:236–242.
- James LP, Letzig L, Simpson PM, et al. Pharmacokinetics of acetaminophen-protein adducts in adults with acetaminophen overdose and acute liver failure. Drug Metab Dispos. 2009;37(8):1779–1784.
- Antoine DJ, Williams DP, Kipar A, et al. High-mobility group box-1 protein and keratin-18, circulating serum proteins informative of acetaminophen-induced necrosis and apoptosis in vivo. Toxicol Sci. 2009;112(2):521–531.
- Roberts DW, Lee WM, Hinson JA, et al. An immunoassay to rapidly measure acetaminophen protein adducts accurately identifies patients with acute liver injury or failure. Clin Gastroenterol Hepatol. 2017;15(4):555–562.e3.
- Rumack BH, Matthew H. Acetaminophen poisoning and toxicity. Pediatrics. 1975;55(6):871–876.