Abstract
Introduction
Our study investigated whether lipid emulsion therapy could act as an antidote for intravenous amiodarone toxicity in a rat model.
Methods
20 rats were randomised to receive lipid emulsion (ILE) or saline. Rats were infused with amiodarone at 1 mg/kg/min for 20 min. ILE rats then received 6 mL/kg of IV 20% ILE, with controls receiving saline. Amiodarone infusion then recommenced at 0.25 mg/kg/minute for 15 min. Heart rate and mean arterial pressure (MAP) were recorded at the commencement and end of the first amiodarone infusion, and every 5 min during the second amiodarone infusion until experiment termination. Blood was sampled for amiodarone concentration at the conclusion of each infusion.
Results
At experiment termination MAP was greater for the ILE group (85 vs 60 mmHg, p = 0.01), with no difference in heart rate between groups (224 vs 232 bpm, p = 0.19). Amiodarone concentration decreased after saline treatment and was stable after ILE (change −6.4 micromol/L saline, 0.11 micromol/L lipid p < 0.001)
Conclusions
ILE therapy mitigated intravenous amiodarone-induced hypotension in this rat model. While amiodarone was retained in lipid treated blood the quantum of this increase did not support the “lipid sink” hypothesis. Further research is required to evaluate the clinical relevance of these findings.
Keywords:
Introduction
Amiodarone is one of the most frequently used anti-arrhythmic drugs, with over 3 million prescriptions in the USA in 2018 [Citation1]. It remains in both the Adult and Paediatric Advanced Life support algorithms and is frequently used to treat non-arrest related ventricular and supra ventricular tachyarrhythmias [Citation2,Citation3]. Side effects of intravenous amiodarone include hypotension, symptomatic bradycardia and heart block in patients with sinus/atrioventricular node disease [Citation4]
Intravenous lipid emulsion or ILE (Intralipid ®, Fresnius Kabi, Uppsala, Sweden) is, an oil-in-water emulation of soya oil stabilised in egg lethicin which was initially approved in 1962 for use in parenteral nutrition. Its role as a potential antidote first emerged after an observation that it increased the dose of bupivacaine required to produce asystole in rats [Citation5]. Use in drug overdose/toxicity has expanded to treating local anaesthetic systemic toxicity and refractory tricyclic antidepressant toxicity [Citation6]. Use of ILE has also been associated with success in case reports of reducing toxicity from lipophilic drugs including calcium channel blockers, beta-blockers, and flecainide [Citation7–9].
Amiodarone is highly lipophilic with a log P of 7.635 [Citation10] a property shared by local anaesthetic agents. A proposed mechanism of action for ILE is the “lipid sink” into which lipophilic intoxicant preferentially distributes, holding it away from the site of toxic action in the heart or brain [Citation11–13]. Three previous animal studies have investigated the effect of ILE in models of amiodarone toxicity. All used ILE prior to amiodarone with two reporting blood amiodarone concentrations. Pantazopoulos et al demonstrated ILE prior to amiodarone infusion increased the amiodarone concentration in plasma and eliminated amiodarone’s hypotensive effects when compared to normal saline [Citation14]. Xanthos et al investigated the effects of an intravenous bolus of lipid emulsion followed by an infusion on four-hour survival in pigs who were subjected to intravenous amiodarone toxicity [Citation15]. All animals in both groups survived. A third study performed by Niiya et al investigated the effect of ILE on amiodarone induced hypotension [Citation16]. ILE pretreatment completely prevented the decrease in arterial blood pressure caused by amiodarone, with a steeper rise in blood amiodarone concentration in the lipid group. All three previous animal studies used porcine models prone to a pseudo-allergenic reaction to ILE [Citation17] which has not been reported in humans. ILE has previously been demonstrated as successful in eliminating the hypotensive effects in verapamil toxicity in a rat model [Citation18]. There are two case reports in which ILE has been used with mixed overdoses involving amiodarone. The first of which was a mixed overdose of amiodarone, diltiazem and metoprolol (doses unknown) in a patient with known hypertrophic cardiomyopathy. The administration of a bolus of ILE was associated with improvement in mean arterial pressure (MAP) [Citation19]. The second report used intravenous lipid emulsion in mixed oral flecainide and amiodarone toxicity and was associated with a positive outcome in terms of haemodynamics and survival [Citation14].
In this study we aimed to demonstrate the effects of ILE in a rat model of intravenous amiodarone toxicity with concomitant blood amiodarone concentration measurement. The objective was to look at ILE as a rescue treatment after onset of amiodarone toxicity in a manner simulating clinical poisoning.
Materials and methods
Animals
The animal study was conducted at the Ruakura Animal Research facility, Hamilton, New Zealand. All study protocols were reviewed and approved by the Ruakura Animal Ethics committee.
20 Female Sprague-Dawley rats were studied, all born from April 2020 with an age range of 135-141 days and weight range 310-345 grams. Animals were kept in single gender enclosures with no chance of pregnancy. Twelve-hour light-dark cycles (lights on/off at 07:00/19:00 h) and climate control were maintained. Access to feed and water was allowed ad libitum until the day of animal utilization.
Animal manipulations
On the day of study animals were sedated with ketamine at 50 mg/Kg (Mayne Pharma Ltd, Auckland, New Zealand), and xylazine at 4 mg/Kg (Bayer HealthCare, Leverkusen, Germany) via intraperitoneal injection. Animals were then placed on a warming board at 38.5 °C. An intravenous (IV) cannula was placed in the tail vein using a needle over catheter technique (24 G x 0.75in Insyte, BD, Switzerland). Following dissection of the anterior neck, a tracheostomy tube (14 G x 1.77in Insyte, BD, Switzerland) was placed under direct vision. Mechanical ventilation using a small animal ventilator (Inspira ASV, Harvard Apparatus, US) was then undertaken with oxygen as the inspired gas admixed with 2% isoflurane (Merial, Auckland, New Zealand) via a vaporizer (Somnosuite Small Animal Anesthesia System, Kent Scientific Corporation, Torrington, US) to maintain anesthesia.
A carotid arterial line was placed under direct vision using a catheter over needle technique and tied off proximally and distally (24 G x 0.75in Insyte, BD, Switzerland) allowing for arterial blood sampling and monitoring of arterial pressure (BP Amp, AD Instruments, Bella Vista, Australia). Anesthesia, mechanical ventilation, and blood pressure and ECG monitoring were continued for the duration of the experiment. Hemodynamic metrics were recorded on a standardized template at predetermined time points in the experiment.
Experimental protocol
Following completion of animal manipulations and collection of baseline metrics, Amiodarone injectable solution (Hamelin Pharmaceuticals, Hamelin, Germany) diluted in 5% glucose at 1 mg/mL was infused into the tail vein at 1 mg/kg/min for 20 min. We used this dose/rate of amiodarone based on previous work [Citation20] and dose ranging experiments using 3 subjects with a goal of survival while reducing MAP by at least 40%. The end of the 20-minute infusion was nominated time 0 (T0). The infusion was discontinued, and rats were randomly assigned to receive injection of either 6 mL/kg 20% ILE or an equal volume of 0.9% NaCl over 1 min. Infusion commenced immediately after arterial blood sampling, which typically took 20 s. After completion of study drug injection amiodarone infusion was recommenced at 0.25 mg/kg/minute for 15 min . We chose to continue the infusion at this rate, found to stably maintain amiodarone induced hypotension in dose ranging experiments, to maximise any potential treatment separation between experimental groups. Heart rate and blood pressure were recorded at the commencement of first amiodarone infusion, T 0 and 5, 10 and 15 min (T5, T10 and T15) after the amiodarone infusion recommenced at the lower rate. At T 0 and T15, 1 mL of blood was taken concurrently from the arterial line for amiodarone concentration measurement.
At the end of the experimental period mechanical ventilation was ceased and a terminal bleed was taken from the carotid arterial line. Death was confirmed by absent respiratory efforts and examination of the ECG trace.
Amiodarone assay
Amiodarone concentrations in plasma were determined using a HPLC method. Internal standard was mixed with standard, quality control or sample and sodium acetate (pH 5.4), and the drugs were then extracted by vortexing blood samples with the solvents, a mixture of methyl tert-butyl ether and isopropyl alcohol. The solvent layer was dried down and reconstituted with mobile phase. An aliquot was injected into a reverse phase liquid chromatography column. The drugs are detected at 254 nm and quantitated using peak area ratios.
The method used a one point calibration linear through zero with two levels of quality control samples run with each batch of samples.
Statistical analysis
We calculated that 10 animals in each group would provide an 80% power to detect a difference of 15 mmHg in mean blood pressure with alpha of 0.05. We chose a large effect size at 1.3 standard deviations [Citation21]. We used GraphPad Prism GraphPad Prism (version 9.0, GraphPad Software Inc, La Jolla, USA) for analysis. We compared continuous metrics using Students t test and calculated means with 95% confidence intervals.
Results
Baseline characteristics
Baseline characteristics are outlined in . There were no statistically significant differences for any baseline variable between groups.
Table 1. Baseline characteristics.
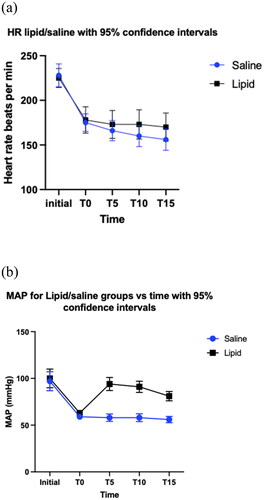
Effects of saline and ILE on heart rate and MAP
The heart rate was not significantly different at T 15 between groups (p = 0.19). Mean heart rate over time is shown in . At T15 MAP was greater for the lipid emulsion treated rats than the saline or group (p = 0.001). MAP over time is shown in .
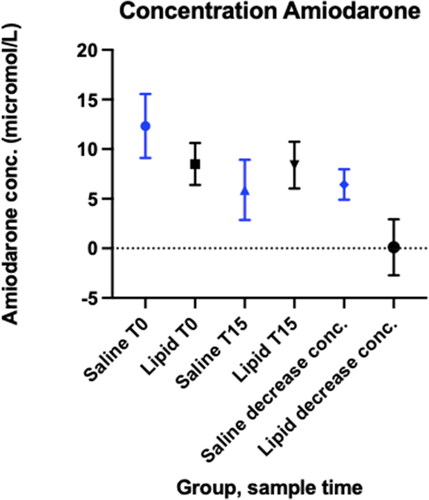
Amiodarone concentrations
Amiodarone concentrations at T 0 prior to any treatments was higher for saline treated rats; mean (95% CI) (micromole/L) Saline 12.3 (9.5-15.1); Lipid 8.5 (6.7-10.3) (p 0.04). At T15 lipid treated rats had a higher amiodarone concentration, though this difference did not reach statistical significance (Saline 5.9 (2.9-8.9); Lipid 8.3 (6.0- 10.7), p = 0.16). The change in amiodarone concentration from T0 to T15 was greater for saline treated rats than lipid treated rats (Saline − 6.4 (- 4.9 to − 8.0); Lipid 0.11 (-1.71 to 1.93), p < 0.001), .
Discussion
This experiment demonstrated that ILE increased mean arterial pressure (MAP) from intravenous amiodarone induced hypotension when compared with saline in whole rats. We demonstrated an improvement in MAP both directly after the ILE bolus, but also with ongoing amiodarone infusion. There are several mechanisms which may explain why there was both an immediate and sustained improvement in MAP.
The “lipid sink” is a theory whereby preferential partitioning of lipophilic molecules into introduced lipid microdroplets traps the pharmacologically active agent within the circulation, with entrapment of drug away from the tissues in which they are causing toxicity. Based on estimates of blood volume and measured concentrations, only 1% of infused amiodarone was in the “sink”, which we would regard as insufficient to mediate the effects seen in our model. A more modern theory of the pharmacokinetic mechanism for ILE is the “lipid shuttle” whereby drug within the lipid micro droplets remains pharmacokinetically active, augmenting redistribution to tissues with high affinity for lipid soluble drugs. Evidence for such effects has been documented for the highly lipophilic agents bupivacaine [Citation22]. A “lipid shuttle” effect may explain the improvement in MAP seen in our model.
Pharmacodynamic effects for ILE may also have mediated the increased MAP seen in our model. Petersen et al observed increases in heart rate and blood pressure in 10 human volunteers given ILE post-treatment with 60 mg of IV metoprolol (moderately lipophilic beta-blocker) [Citation23]. Fettiplace et al found positive inotropic effects of ILE alone in in vivo and ex vivo in rat models [Citation24, Citation25]. In this model ILE directly improved left ventricular contractility. Potential mechanisms for the pharmacodynamic effect for lipid emulsion include effects on myocyte calcium channels, attenuation of mitochondrial dysfunction, effects on sodium and calcium channels and decreased nitric oxide release. While our methodology does not permit comment on whether any of these effects were at play in our model, it is plausible that pharmacodynamic effects may have complemented the lipid shuttle in causing the increased blood pressure seen with ILE.
Our experiment has limitations. The timeframe may not have been sufficient for pharmacokinetic equilibrium between cardiac myocytes and intravascular lipid, and in the absence of lipid infusion blood lipid concentrations will have decreased over the observation period. The effect of anaesthetic agents, though not systematically different between groups, would most frequently be absent in the clinical situation. Our study was not blinded to individuals delivering ILE due to difference in colour of ILE and 0.9% NaCl. Endpoints were however objective and measured at specific timepoints, mitigating any effects from lack of blinding. The baseline difference in amiodarone concentrations at the point of toxicity, while not due to any experimental factors, may have affected the time course of recovery from amiodarone toxicity between groups. Saline treated rats were heavier than amiodarone treated rats (median 423 vs 350 g), though this difference did not reach statistical significance (p = 0.06). The difference in amiodarone concentration at T0 may have been due to random variation or a pharmacokinetic difference due to difference in weights. A further pertinent limitation of this study is that an animal model cannot truly represent actual clinical outcomes in humans.
Conclusion
Intravenous lipid emulsion increased MAP in rats poisoned with amiodarone, while saline had no effect on MAP. There was no difference in heart rate between ILE and saline groups. While amiodarone was retained in lipid treated blood the quantum of this increase did not support the “lipid sink” hypothesis. Further research is required to evaluate the clinical relevance of these findings.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Amiodarone Hydrochloride. Drug Usage Statistics, ClinCalc DrugStats Database. Available from: https://clincalc.com/DrugStats/Drugs/AmiodaroneHydrochloride.
- Advanced Life Support for Adults. Available from: https://www.nzrc.org.nz/assets/Uploads/Advanced-Life-Support-for-Adults-Jan-2016.pdf (2016).
- Advanced Life Support for Infants and Children. Available from: https://www.nzrc.org.nz/assets/Guidelines/Algorithms/Advanced-Life-Support-for-Infants-and-Children-Jan-2016.pdf (2016).
- Medsafe. New Zealand Data Sheet - Cordarone X. 1–28. 2019.
- Weinberg GL, VadeBoncouer T, Ramaraju GA, et al. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. Anestheiology. 1998;88(4):1071–1075.
- Angel-Isaza AM, Bustamante-Cristancho LA, Uribe-B FL. Successful outcome following intravenous lipid emulsion rescue therapy in a patient with cardiac arrest due to amitriptyline overdose. Am J Case Rep. 2020;21:1–5.
- Mullins ME, Miller SN, Nall CE, et al. Intravenous lipid emulsion therapy for flecainide toxicity. Toxicol Commun. 2017;1(1):34–36.
- Wilson BJ, Cruikshank JS, Wiebe KL, et al. Intravenous lipid emulsion therapy for sustained release diltiazem poisoning: a case report. J Popul Ther Clin Pharmacol. 2012;19:218–222.
- Le Fevre P, Gosling M, Acharya K, et al. Dramatic resuscitation with intralipid in an epinephrine unresponsive cardiac arrest following overdose of amitriptyline and propranolol. BMJ Case Rep. 2017;2017:1–5.
- chemicalize.com. Available from: https://chemicalize.com/app/calculation/amiodarone.
- Weinberg G, Ripper R, Murphy P, et al. Lipid infusion accelerates removal of bupivacaine and recovery from bupivacaine toxicity in the isolated rat heart. Reg Anesthesia Pain Med. 2006;31(4):296–303.
- Buysa M, Scheepersa PA, Levina AI. Lipid emulsion therapy: Non-nutritive uses of lipid emulsions in anaesthesia and intensive care. South Afr J Anaesthesia Analg. 2015;21:5–11.
- Weinberg G, Lin B, Zheng S, et al. Partitioning effect in lipid resuscitation: further evidence for the lipid sink. Crit Care Med. 2010;38(11):2268–2269.
- Pantazopoulos C, Pantazopoulos I, Stratigopoulou P, et al. Amiodarone overdose in swine. Is lipid emulsion effective in preventing amiodarone-related hypotension? Resuscitation. 2012;83:e76.
- Xanthos T, Psichalakis N, Russell D, et al. IntralipidTM administration attenuates the hypotensive effects of acute intravenous amiodarone overdose in a swine model. Am J Emergency Med. 2016;34(8):1389–1393.
- Niiya T, Litonius E, Petäjä L, et al. Intravenous lipid emulsion sequesters amiodarone in plasma and eliminates its hypotensive action in pigs. Ann Emerg Med. 2010;56(4):402–408.
- Bedocs P, Capacchione J, Potts L, et al. Hypersensitivity reactions to intravenous lipid emulsion in swine: relevance for lipid resuscitation studies. Anesth Analg. 2014;119(5):1094–1101.
- Ok S-H, Shin I-W, Lee SH, et al. Lipid emulsion alleviates the vasodilation and mean blood pressure decrease induced by a toxic dose of verapamil in isolated rat aortae and an in vivo rat model. Hum Exp Toxicol. 2018;37(6):636–646.
- Bologa C, Lionte C, Popescu A, et al. First case of acute poisoning with amiodarone and flecainide in attempted suicide successfully managed with lipid emulsion therapy in the emergency department: case report and literature review. Healthcare (Switzerland). 2021;671:1–18.
- Somberg JC, Cao W, Cvetanovic I, et al. Pharmacology and toxicology of a new aqueous formulation of intravenous amiodarone (Amio-Aqueous) in comparison to cordarone Iv. J Investig Med. 2005;53(2):S368.1–S368.
- Wang Y, et al. Comparison of invasive blood pressure measurements from the caudal ventral artery and the femoral artery in male adult SD and wistar rats. PLoS One. 2013;8(4):1–7.
- Shi K, Xia Y, Wang Q, et al. The effect of lipid emulsion on pharmacokinetics and tissue distribution of bupivacaine in rats. Anesth Analg. 2013;116(4):804–809.
- Petersen KM, Bøgevig S, Petersen TS, et al. Hemodynamic effects of intravenous, high dose intravenous lipid emulsion with and without metoprolol infusion in healthy volunteers; a randomised clinical trial. Clin Pharmacol Ther. 2019;105(4):1009–1017.
- Fettiplace MR, et al. Rapid cardiotonic effects of lipid emulsion infusion. Crit Care Med. 2013;41:156–162.
- Fettiplace MR, Weinberg G. The mechanisms underlying lipid resuscitation therapy. Reg Anesth Pain Med. 2018;43(2):138–149.