Abstract
This is a case of a 23-year-old female who presented to the emergency department (ED) comatose after ingesting 7.8 g of propafenone and 11.7 g of valsartan. Upon arrival to the ED, the patient had seizures and required intubation. Several boluses of sodium bicarbonate were given as well as a continuous infusion to normalize QRS intervals. Norepinephrine and intravenous lipid emulsion (ILE) therapy were initiated. In the intensive care unit (ICU), the patient experienced persistent seizures and a 5-minute period of pulseless ventricular tachycardia. Subsequently, hyperinsulinemic-euglycemic therapy (HIET) was initiated. The patient received a 1 unit/kg intravenous bolus of regular insulin followed by a continuous infusion starting at 1 unit/kg/hr. Norepinephrine infusion was weaned off after 27 h of HIET. Within 48 h of presentation, the patient was transferred out of the ICU. This case exhibits the successful management of a mixed overdose with propafenone and valsartan utilizing a multimodal approach. In addition to supportive care, the patient received sodium bicarbonate, HIET, ILE and vasopressors to manage the cardiovascular collapse associated with these toxicities.
Introduction
In 2019, cardiovascular medications were responsible for 4.79% of all toxic drug exposures reported to the National Poison Data System [Citation1]. Over 1000 cases reported were due to antiarrhythmic agents, while angiotensin receptor blockers (ARBs) were documented in almost 11,000 cases [Citation1].
Propafenone is a potent sodium channel blocker that can cause dose related widening of the QRS complex and slow atrioventricular conduction [Citation2]. It blocks various potassium currents, particularly the inward rectifier, delayed rectifier and transient outward potassium currents [Citation2]. Calcium channel blocking activity is minimal at therapeutic concentrations, but in vitro data suggests higher concentrations of propafenone can inhibit inward calcium currents [Citation2]. Though only 1/40 as potent at beta blockade as propranolol on a per mg basis, beta blockade effects may be more important in overdose. Toxicity can manifest as negative inotropy, sinus bradycardia, QT interval prolongation, seizures, ventricular arrhythmias, and hypoinsulinemia [Citation3, Citation4]. Propafenone’s prime metabolite, 5-hydroxy-propafenone, has similar sodium and calcium channel blocking activities, but causes less beta blockade [Citation2].
Toxicity associated with ARBs is rare but can cause a synergistic effect when combined with other cardiovascular agents in overdose. In accordance with the CARE guidelines (https://www.care-statement.org/case-reports), we present a case of successful management of a combined overdose of propafenone and valsartan.
Case report
A 23-year-old female presented to the emergency department (ED) unresponsive after an apparent intentional overdose. Based on prescription bottles found, the ingestion was believed to be 7.8 g of propafenone and 11.7 g of valsartan. As the timing of ingestion was unknown, gastrointestinal decontamination was not administered to the patient.
In the ED, an initial 20 s seizure resolved with 2 mg of intravenous (IV) lorazepam, and the patient was intubated for airway protection (). Initial vital signs were heart rate 74 bpm and blood pressure of 79/48 mmHg. The initial electrocardiogram (EKG) was abnormal with a first-degree AV block, a ventricular rate of 69 bpm, QRS interval of 172 ms and a QT/QTcB of 568/608 ms. Acetaminophen, ethanol, and salicylate were undetectable. Her urine drug screen (immunoassay) was positive only for cocaine metabolite. No additional drug screens for xenobiotics were obtained. Baseline chemistries were normal, except for a serum creatinine of 1.28 mg/dL and lactate of 3.6 mmol/L.
Figure 1. Case timeline.
BP, blood pressure; EKG, electrocardiogram; HIET, hyperinsulinemic euglycemic therapy; ILE, intravenous lipid emulsion; mEq, milliequivalents; ms, milliseconds; NaHCO3, sodium bicarbonate; NS, normal saline; ROSC, return of spontaneous circulation; VT, ventricular tachycardia.
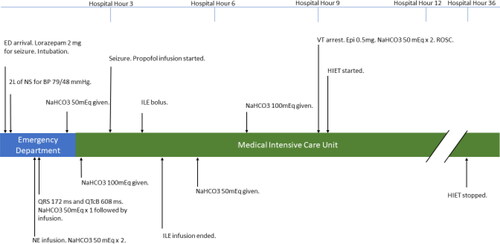
After a two-liter bolus of 0.9% sodium chloride, a norepinephrine infusion was required to maintain a mean arterial pressure of 65 mmHg. The patient received a 50 mEq bolus of sodium bicarbonate, followed by an infusion (150 mEq in 1000 mL of dextrose 5% in water) at 150 mL/hr. The patient was then admitted to the critical care unit. A repeat EKG showed a persistent first-degree AV block, a ventricular rate of 70 bpm, QRS of 186 ms and QT/QTcB (corrected Bazett formula) of 560/604 ms (). Due to continued seizure activity, a propofol infusion and continuous electroencephalography were initiated. Four hours post arrival, the patient received intravenous lipid emulsion (ILE) 20% as a bolus dose of 1.5 mL/kg (87.2 mL) followed by an infusion at 0.25 mL/kg/min for 37 min. After finishing 537.4 mL of ILE infusion, norepinephrine requirements dropped from 60 mcg/min down to 30 mcg/min, and the QTcB decreased from 604 ms to 568 ms. However, three hours after ILE, the QTc increased back to 606 ms, likely due to the short duration (0.5-1 hr) of action of ILE [Citation5].
Figure 2. Electrocardiogram measurements with administration of key therapies.
EKG, electrocardiogram; HIET, hyperinsulinemic-euglycemic therapy.
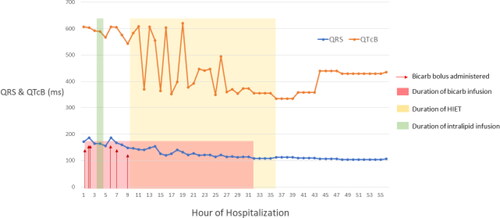
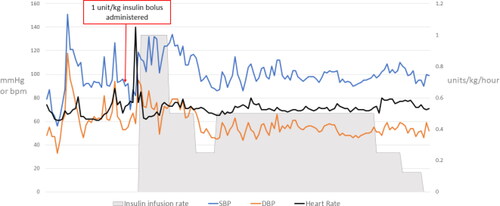
Nine hours after arrival, the patient experienced pulseless ventricular tachycardia, with return of spontaneous circulation (ROSC) achieved after one round of compressions, epinephrine 0.5 mg, and sodium bicarbonate 100 mEq. Of note, the patient’s serum potassium was 3.6 mEq/L and magnesium was 3.5 mg/dL pre-arrest and both the potassium and the magnesium decreased post-arrest to 2.9 mg/dL and 1.9 mg/dL, respectively. The propofol infusion was transitioned to a midazolam infusion, and HIET was initiated with a 1 unit/kg insulin bolus followed by an infusion at 1 unit/kg/hr (). A 10% dextrose infusion at 100 mL/hr was concurrently initiated and titrated to maintain a blood glucose between 100 and 180 mg/dL, with dextrose 50% boluses available as needed. Due to escalating rates of dextrose 10%, the infusion was changed to dextrose 20%. The patient was successfully weaned off of vasopressors after 27 h of HIET.
Figure 3. Hemodynamic parameters during HIET.
**Heart rate and blood pressure from ED arrival to ∼36 h after admission.
By hour 32 of hospitalization, electrolytes and EKG values returned to normal ranges, and the sodium bicarbonate infusion was discontinued. A transthoracic echocardiogram was completed at this time which reported an ejection fraction of 55-60% with no abnormalities except for trace tricuspid regurgitation. At hour 36 the insulin infusion was discontinued, and the patient was extubated. The dextrose infusion was continued for four hours after insulin was discontinued to prevent hypoglycemia. Within 48 h of hospitalization, the patient transferred out of the ICU with no apparent neurological deficits.
Hyperinsulinemic-euglycemic therapy
HIET produces a positive inotropic effect by promoting myocardial cell uptake of glucose as an energy source [Citation6, Citation7]. Additionally, HIET can cause a reduction in systemic vascular resistance, leading to decreased strain on the heart. Propafenone exhibits weak beta-adrenergic receptor and calcium channel receptor blockade causing bradycardia and hypotension [Citation8]. Given this property, it is reasonable to consider HIET as a therapeutic option. Case reports of ARB overdoses are often observed in combination with other cardiovascular agents, therefore, the benefit of HIET specifically in ARB toxicity is unclear.
Our patient was administered HIET after ROSC was obtained to treat cardiogenic shock. HIET may have played a role in successfully preventing cardiovascular collapse as evident by the reduction in need for vasopressors and narrowing of the QRS during the infusion.
Intravenous lipid emulsion
The American College of Medical Toxicology states ILE may be considered for toxicities in drugs with high lipid solubility and recommends initiation when standard measures have failed or in the setting of significant hemodynamic instability [Citation9]. Propafenone is lipophilic with a log P of 2.498 which suggests that ILE would be a reasonable treatment [Citation10]. There is a lack of consensus on when to initiate therapy, given the risk of organ hypoperfusion with a delay in treatment. Case reports on propafenone toxicity have utilized ILE as salvage therapy [Citation11]. Our case differs in that ILE was utilized earlier during treatment and given sequentially with HIET. The optimal dosing regimen is also unclear, but Weinberg recommends a 1.5 mL/kg bolus followed by a 0.25 mL/kg/minute infusion (max of 10 mL/kg) for 30 and 60 min [Citation12]. Ongoing cardiovascular instability may warrant a repeat ILE bolus. As our patient went into cardiac arrest upon arrival to the ICU, we speculate that a repeat administration of the bolus might have prevented ventricular tachycardia and arrest.
Conclusion
We present the successful management of a mixed overdose with propafenone and valsartan through a multimodal approach including ILE, HIET, vasopressors, and sodium bicarbonate.
Acknowledgments
Henry A. Spiller
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Gummin DD, Mowry JB, Beuhler MC, et al. 2019 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 37th annual report. Clin Toxicol (Phila). 2020;58(12):1360–1541.
- Rythmol SR [package insert]. Research Triangle Park (NC): GlaxoSmithKline; 2018.
- Ovaska H, Ludman A, Spencer EP, et al. Propafenone poisoning – a case report with plasma propafenone concentrations. J Med Toxicol. 2010;6(1):37–40.
- Faber TS, Camm AJ. The differentiation of propafenone from other class IC agents, focusing on the effect on ventricular response rate attributable to its beta-blocking action. Eur J Clin Pharmacol. 1996;51(3-4):199–208.
- Lexicomp Online, Lexi-Drugs Online. Waltham (MA): UpToDate, Inc.; February 9, 2022 [accessed 2022 Feb 15]. Available from: https://online.lexi.com
- Hoffman RS, Howland M, Lewin NA, editors, et al. Goldfrank’s toxicologic emergencies, 10th ed. McGraw Hill; 2015 [accessed 2021 Aug 4]. Available from: https://accesspharmacy.mhmedical.com/content.aspx?bookid=1163§ionid=64552562
- Krenz JR, Kaakeh Y. An overview of hyperinsulinemic-euglycemic therapy in calcium channel blocker and β-blocker Overdose. Pharmacotherapy. 2018;38(11):1130–1142.
- Funck-Brentano C, Kroemer HK, Lee JT, et al. Propafenone. N Engl J Med. 1990;322(8):518–525.
- American College of Medical Toxicology. ACMT position statement: guidance for the use of intravenous lipid emulsion. J Med Toxicol. 2017;13:124–125.
- Royal Society of Chemistry. “Propafenone (CSID:4763).” 2022. [accessed 2022 Apr 14]. Available from: http://www.chemspider.com/Chemical-Structure.4763.html
- Bayram B, Kose I, Avci S, et al. Successful treatment of propafenone intoxication with intravenous lipid emulsion. Pharmacotherapy. 2015;35(10):e149–e152.
- Weinberg GL. Lipid emulsion infusion: resuscitation for local anesthetic and other drug overdose. Anesthesiology. 2012;117(1):180–187.
