Abstract
Ethylene glycol (EG) is a toxic alcohol that causes central nervous system depression and multiple metabolic abnormalities including a high anion gap metabolic acidosis (HAGMA), elevated osmolal gap (OG), and acute kidney injury. Few case reports of EG intoxication report brain MRI findings. We describe an elderly female who was brought to the hospital because of altered mentation. We diagnosed her with EG toxicity and treated with fomepizole based on metabolic abnormalities and presence of urine calcium oxalate crystals. Our patient’s brain MRI showed T2-hyperintense signals located in the midbrain, hippocampi, basal nuclei, and thalami consistent with previous MRI reports with confirmed EG toxicity. As in this case, a clear history of poisoning is not always available. However, antidote therapy with alcohol dehydrogenase blockade should be initiated if clinical data suggests EG intoxication. Our patient’s EG concentration returned elevated two days after admission blood draw confirming the initial diagnosis. MRI of the brain may add diagnostic support to laboratory findings while awaiting a definitive EG concentration.
Introduction
Ethylene glycol (EG) poisoning causes several neurologic effects mediated by alcohol inebriation, metabolic abnormalities, or direct injury to several brain structures [Citation1,Citation2]. In prior case reports of patients with EG poisoning, magnetic resonance imaging (MRI) of the brain have demonstrated characteristic brain imaging abnormalities [Citation3,Citation4]. These images are not as well-known as with other intoxications – like methanol and carbon monoxide – for which brain imaging has diagnostic value [Citation5,Citation6]. Therefore, we present, in accordance with the CARE guidelines [Citation7], MRI abnormalities of the brain caused by severe EG poisoning.
Case report
A 71-year-old woman with history of transient ischemic attack presented to the emergency department with altered mental status. Her family described that she had slurred speech, nausea, and vomiting the day prior to presentation. She had no previous abnormal behavior, history of substance use, or ingestion.
On physical examination, the patient was unresponsive, vital signs were normal, and pupils were symmetric and responsive to light. Laboratory tests showed elevated creatinine 1.77 mg/dL and BUN 24 mg/dL, high anion-gap metabolic acidosis (HAGMA) with bicarbonate <5 mmol/L and anion gap >40 mmol/L. Venous blood gas showed pH 6.93, pCO2 < 20 mmHg, and lactic acid >13 mmol/L. Lactic acid in peripheral blood was 5.96 mmol/L. Ethanol, salicylates, acetaminophen, and ketones were undetectable. Further tests revealed increased serum osmolality 353 mOsm/kg and osmolal gap (OG) 44 mOsm/kg. Urinalysis revealed only microscopic hematuria. Computed tomography of the head initially was unrevealing on admission.
Due to her HAGMA with hyperosmolality suggesting a toxic alcohol ingestion, the consulting toxicologists recommended intravenous fluids, sodium bicarbonate, and an empiric loading dose of fomepizole 15 mg/kg. The patient had progressive renal function deterioration and worsening acid-base disequilibrium that did not improve with these medical interventions, and subsequently she developed refractory tonic-clonic seizures.
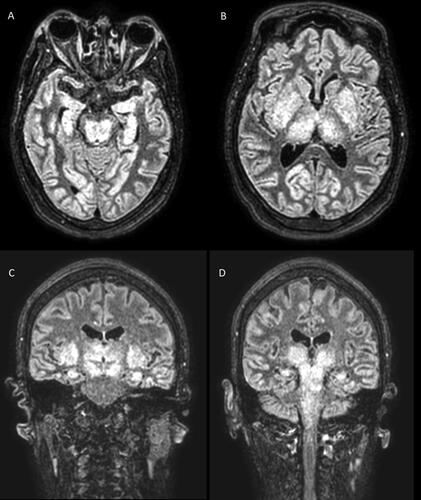
Twenty-four hours after, MRI of the brain without contrast revealed diffuse cortical T2-weighted hyperintensities, bilateral and symmetric enlargement of the basal ganglia and thalami with T2-weighted hyperintensities, as well as T2-weighted hyperintensities involving the midbrain, dorsal pons and medulla ().
Figure 1. Brain MRI axial imaging slides showing diffuse cortical T2-weighted hyperintensities, symmetrical T2-weighted hyperintensities in the midbrain and hippocampi (panel A), basal nuclei, and thalami (panel B). MRI coronal imaging slides show basal ganglia, thalami, and hippocampi involvement (panel C) with extension into midbrain, pons, and medulla (panel D).
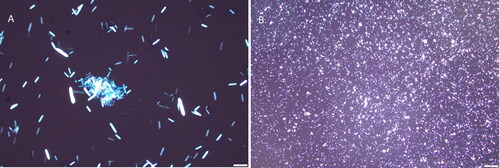
A repeated microscopic analysis of the urine sediment revealed needle-shape calcium oxalate monohydrate (COM) crystals (). The patient was not dialyzed as she had advance directive orders to do not resuscitate, do not intubate, and do not dialyze that were shared by her family. Therefore, she was transitioned to comfort care and passed away about 40 h after admission. Her ethylene glycol concentration from blood drawn on admission resulted at 107 mg/dL two days after admission and confirmed EG poisoning.
Discussion
Ethylene glycol poisoning is a challenging clinical diagnosis because timely EG measurements are usually not available. As a result, clinicians must rely upon clues such as HAGMA, elevated OG, very high lactate measurement, lactate gap, and calcium oxalate crystals in the urine [Citation1,Citation8]. The apparent lactate elevation may also reflect glycolate, as the point-of-care lactate analyzers use lactate oxidase, which cannot distinguish between lactate and glycolate (differing only by one methyl group) [Citation9].
Patients with acute EG poisoning commonly develop neurologic effects, including lethargy, slurred speech, ataxia, seizures, myoclonic jerks, and coma [Citation10]. These symptoms may be multifactorial and related to alcohol inebriation, metabolic abnormalities, and direct injury mediated by the deposition of COM crystals in the wall of cerebral blood vessels [Citation2,Citation11].
Prior case reports have identified brain imaging abnormalities in patients with EG poisoning. CT of the head may be normal in the acute setting, as at the present case, or may show cerebral edema, hypodensities, and hemorrhages. These are mainly located in the basal ganglia, thalami, brain stem, and temporal lobe [Citation3,Citation12,Citation13]. In the recent years, MRI of the brain has demonstrated higher sensitivity to detect brain abnormalities in patients with acute EG poisoning[Citation4]. The most common MRI of the brain findings reported are T2-weighted hyperintense lesions, with a bilateral and symmetrical pattern of distribution, located in deep gray matter structures, such as basal ganglia, thalami, and brain stem, as similarly observed in this case () [Citation3,Citation4]. Less common findings reported include basal ganglia hemorrhage, and cranial nerve enhancement [Citation4,Citation14].
These radiologic abnormalities affecting deep gray matter structures are not specific of EG poisoning. The differential diagnosis includes other acute presentations such as hypoxic ischemic encephalopathy, carbon monoxide and cyanide poisoning, liver disease and hyperammonemia, hyper- and hypoglycemia, osmotic demyelination, thiamine deficiency, and deep cerebral thrombosis [Citation15]. A prior study showed that the majority of brain imaging abnormalities were reversible upon appropriate EG poisoning treatment, and most of these patients had complete neurologic recovery [Citation13] Hence, brain imaging studies do not have prognostication value in EG poisoning, and clinicians most be cautious when interpreting such findings.
In a patient with clinical features suggestive of EG poisoning, MRI of the brain showed T2-weighted hyperintensity signals in the deep gray matter structures. MRI may add diagnostic support in similar cases while awaiting a definitive EG concentration.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The author(s) reported there is no funding associated with the work featured in this article.
References
- Kraut JA, Mullins ME. Toxic alcohols. N Engl J Med. 2018;378(3):270–280.
- Froberg K, Dorion RP, McMartin KE. The role of calcium oxalate crystal deposition in cerebral vessels during ethylene glycol poisoning. Clin Toxicol (Phila). 2006;44(3):315–318.
- Moore MM, Kanekar SG, Dhamija R. Ethylene glycol toxicity: chemistry, pathogenesis, and imaging. Radiol Case Rep. 2008;3(1):122.
- Malhotra A, Mongelluzzo G, Wu X, et al . Ethylene glycol toxicity: MRI brain findings. Clin Neuroradiol. 2017;27(1):109–113.
- Elkhamary SM, Fahmy DM, Galvez-Ruiz A, et al. Spectrum of MRI findings in 58 patients with methanol intoxication: long-term visual and neurological correlation. Egypt J Radiol Nucl Med. 2016;47(3):1049–1055.
- Beppu T. The role of MR imaging in assessment of brain damage from carbon monoxide poisoning: a review of the literature. AJNR Am J Neuroradiol. 2014;35(4):625–631.
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol. 2017;89:218–235.
- Krasowski MD, Wilcoxon RM, Miron J. A retrospective analysis of glycol and toxic alcohol ingestion: utility of anion and osmolal gaps. BMC Clin Pathol. 2012;12:1.
- Verelst S, Vermeersch P, Desmet K. Ethylene glycol poisoning presenting with a falsely elevated lactate level. Clin Toxicol (Phila). 2009;47(3):236–238.
- McQuade DJ, Dargan PI, Wood DM. Challenges in the diagnosis of ethylene glycol poisoning. Ann Clin Biochem. 2014;51(Pt 2):167–178.
- Zeiss J, Velasco ME, McCann KM, et al. Cerebral CT of lethal ethylene glycol intoxication with pathologic correlation. AJNR Am J Neuroradiol. 1989;10(2):440–442.
- Morgan BW, Ford MD, Follmer R. Ethylene glycol ingestion resulting in brainstem and midbrain dysfunction. J Toxicol Clin Toxicol. 2000;38(4):445–451.
- Owen EB, Calhoun AW, McDonald MJ. Reversibility of severe cerebral magnetic resonance imaging changes associated with ethylene glycol toxicity. J Pediatr Intensive Care. 2017;6(3):214–220.
- Lewis LD, Smith BW, Mamourian AC. Delayed sequelae after acute overdoses or poisonings: cranial neuropathy related to ethylene glycol ingestion. Clin Pharmacol Ther. 1997;61(6):692–699.
- Hegde AN, Mohan S, Lath N, et al. Differential diagnosis for bilateral abnormalities of the basal ganglia and thalamus. Radiographics. 2011;31(1):5–30.