Abstract
Paracetamol-induced nephrotoxicity in the absence of hepatotoxicity is only described in a few studies. The aim of this retrospective cohort study with data from the Capital Region of Denmark 2010-2017, was to examine the incidence of possible paracetamol-induced nephrotoxicity in absence of hepatotoxicity. Only one out of 5,827 admissions (0.02%) included in the study developed clinically relevant acute kidney injury (AKI) that could be attributed to paracetamol in absence of acute liver injury. This study demonstrates that clinically relevant AKI due to paracetamol overdose rarely occurs without concomitant hepatic injury when excluding other prerenal, renal, and postrenal causes of renal dysfunction, NAC interference and chronic kidney injury.
Introduction
Paracetamol has a well-known safety profile when used in recommended doses. However, in overdose it may cause liver injury. N-acetylcysteine (NAC) is an effective antidote to prevent the occurrence of liver injury. Paracetamol poisoning may also cause acute kidney injury (AKI) as a secondary effect of liver damage (hepato-renal syndrome). However, only a few studies have described a direct nephrotoxic effect of paracetamol in the absence of hepatotoxicity [Citation1–4]. The aim of this study was to examine the incidence of possible paracetamol-induced AKI in absence of acute liver injury (ALI).
Materials and methods
Study design and setting
In this retrospective cohort study, we included patients admitted to hospitals in the Capital Region of Denmark from 1 Jan 2010 through 31 Dec 2017 with either a peak plasma paracetamol concentration over 0.167 mmol/L (25.24 µg/mL) or a paracetamol poisoning diagnosis. All patients with a suspected intake of more than 6 gram paracetamol are coded with a paracetamol poisoning diagnosis, and according to the Danish guidelines they are all treated with NAC without the use of the risk stratification nomogram [Citation5]. The lower limit of detection (LLOD) of plasma paracetamol is defined as ≤130 µmol/L (19.65 µg/mL). The inclusion of all patients with a paracetamol poisoning diagnosis allowed us to determine whether AKI was related to paracetamol as both patients with a plasma paracetamol concentration below and above the LLOD at admission were included. Furthermore, follow-up of kidney function after discharge was assessed when possible. The study is reported according to the STROBE statement [Citation6].
Study population
The population of the Capital Region of Denmark is approximately 1.7 million [Citation7]. In Denmark all residents with paracetamol poisoning are admitted to hospitals in their local region. Patients with severe liver failure are transferred to the national center for liver transplantation located in the Capital Region.
Data collection and outcome
We obtained age, gender, procedures and prior diagnostic codes from a regional version of The Danish National Patient Register via the Danish unique personal identification number given to all Danish residents at birth or upon immigration [Citation8]. We collected measurements of plasma paracetamol and other laboratory markers from the Clinical Laboratory Information System using the personalized identification number [Citation9]. The widely accepted thresholds for classifying paracetamol poisoning are ALT ≥ 100 U/L for moderate hepatotoxicity and ALT ≥ 1000 U/L for severe hepatotoxicity. Hence, the primary outcome was the incidence of AKI caused by paracetamol in the absence of ALI defined as a peak alanine aminotransferase (ALT) < 100 U/L. The medical charts of the patients with AKI without ALI were reviewed critically by the author A. Daoud, who was trained specifically on this chart abstraction before the study started. A. Daoud reviewed each chart and was in frequent contact with T. Petersen to resolve disputes. Most of the records were also reviewed by T. Petersen to ensure agreement. We reviewed the medical charts to determine the causes of AKI using standardized abstraction forms to maintain consistency in chart review. Causes of AKI were classified as either prerenal, renal (intrinsic), postrenal, chronic kidney disease or NAC interference. NAC interference (the effect of NAC on plasma creatinine) was assessed after NAC administration by observing a decrease in plasma creatinine below the reference range with a subsequent increase after cessation of NAC while observing no effect on BUN. If the cause of AKI was unknown and not affected by NAC interference it was classified as unexplained.
AKI was defined according to the KDIGO (Kidney Disease: Improving Global Outcomes) AKI guidelines [Citation10] by the International Society of Nephrology as an increase in the plasma creatinine to 1.5-1.9 times the baseline concentration (stage 1), 2.0-2.9 times baseline concentration (stage 2) and ≥3.0 times baseline concentration (stage 3) or as urine volume <0.5 mL/kg/h for 6-12 h (stage 1), <0.5 mL/kg/h for ≥12 h (stage 2) and <0.5 mL/kg/h for ≥24 h or anuria for ≥12 h (stage 3). The baseline concentration of plasma creatinine was defined as the first plasma creatinine measurement during index hospitalization or as the median plasma creatinine from a year up to hospitalization, whichever was lower. Initiation of renal replacement therapy, a plasma creatinine ≥353.6 µmol/L or a decrease in eGFR to <35 mL/min per 1.37 m2 in children (<18 years) were also defined as AKI stage 3. Furthermore, we described AKI as clinically relevant if AKI had a prognostic or therapeutic consequence for the patient.
Ethics
The study was approved by the Danish Patient Safety Authority (No. 3-3013-1884/1/) and the Danish Data Protection Agency (No. BFH-2016-058). According to Danish law, a registry-based study does not require approval from an ethics review board.
Statistical analysis
Data are reported as median and interquartile range [IQR] or absolute numbers and percentages out of the total number of patients, as applicable, unless otherwise stated. However, no cell counts below three could be reported due to data privacy regulations. Fisher’s exact test was used to compare proportions of renal dysfunction between groups. Missing data were omitted and not replaced by imputation, unless otherwise stated. All available data were used for analyses performed with R version 4.0.2 [Citation11].
Results
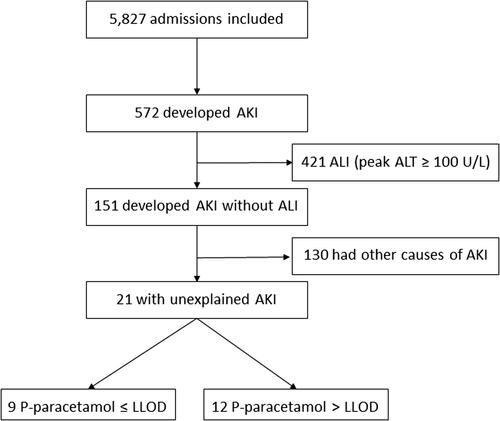
In total, 572 out of 5,827 admissions included in the study developed AKI. Dialyses were performed in 88, and 67 ended with a fatal outcome. The KDIGO AKI stage was 2-3 for 165 with ALI (153 with severe hepatotoxicity). Of the 5,827 admissions, 860 had ALI of which 626 developed severe hepatotoxicity. Among patients without any signs of ALI, 151 developed AKI, and of these 44 had plasma paracetamol concentrations below the LLOD at admission. Of the 151 admissions, the cause of AKI remained unexplained in 21 admissions after chart review (, ). Of these, 14 were females. The median age was 21 (IQR: 17-55) years. The proportion of patients with an unexplained AKI did not differ between the patients with plasma paracetamol concentration below and above LLOD at admission (). In 18 cases the KDIGO AKI stage was 1 and 2-3 in the remaining 3 cases. In accordance with the KDIGO AKI guidelines we staged the 18 cases with 1 due to increases in the plasma creatinine to 1.5-1.9 times the baseline concentration, e.g. we staged a case with 1 due to the increase in plasma creatinine from 43 to 68 µmol/L (0.49 to 0.77 mg/dL). Despite fulfilling the KDIGO AKI stage 1 criteria none of them developed clinically relevant acute kidney injuries. In the two cases with stage 2, no change in the baseline concentration of plasma urea was observed. In the case staged 3, the measurements of plasma paracetamol concentrations were not available since the patient was transferred from a hospital outside the Capital Region. However, the patient was included and classified as having a plasma paracetamol concentration above LLOD as it was mentioned in the medical chart that the paracetamol concentration was elevated. The estimated ingested dose of paracetamol was 17,500 (IQR: 11,750-25,000) mg for the 12 of 21 with a paracetamol concentration above LLOD and 8,125 (IQR: 5,000-10,500) mg for the remaining 9 with a paracetamol concentration below LLOD. Information about the ingested dose of paracetamol was available for 19 of 21 patients. The median time from the suspected ingestion to the first blood sample was 9 (IQR: 3-12) hours and 8.5 (IQR: 4-13) hours for those with a paracetamol concentration above and below LLOD, respectively. This information was available for 18 of 21 patients. However, all patients were treated with NAC in accordance with the Danish recommendations. All 21 patients fully recovered without dialysis, and no deaths during hospitalization occurred. Follow-up of kidney function after discharge was feasible for 15 of 21 patients re-admitted to hospital until this day and disclosed completely normal kidney function.
Table 1. Causes of renal dysfunction.
Discussion
The incidence of unexplained AKI in the absence of ALI was low in our study (21/5,827; 0.36%) and largely of minor severity except for one case in stage 3. We found no correlation between a detectable plasma paracetamol concentration and the development of unexplained AKI. This suggests that the mild AKI was not caused by paracetamol. Only one case revealed clinically relevant unexplained AKI that could be attributed to paracetamol in the absence of ALI.
The use of the KDIGO AKI guidelines led to the detection of clinically irrelevant acute kidney injuries as even negligible stochastic fluctuations in plasma creatinine values fulfilled the definition of minor acute kidney injuries.
Previous studies [Citation1–4] have not used the KDIGO AKI criteria, instead they have relied on different definitions, e.g. a plasma creatinine concentration over a certain value. In addition, they have given limited details on kidney function before admission, post-hospitalization outcomes and they have not considered the effect of NAC on creatinine concentrations. NAC interferes with the analytical method used for creatinine measurement [Citation12] and the paracetamol metabolite NAPQI may as well interfere with the method used [Citation13]. The renal function may then be underestimated in a patient treated with NAC if only creatinine is used as a marker of renal function. It is therefore difficult to compare prior findings with the current study. In addition, in our study the NAC interference was more pronounced in patients with measurable paracetamol. The interference is significant () and may be due to the paracetamol metabolite NAPQI interference [Citation13].
The retrospective design of the study may lead to underestimation of the incidence as AKI may develop several days after admission at a time when most patients normally would have been discharged. Occurrence of clinically relevant post-hospitalization AKI would likely have required readmission of these patients to hospital, but no subsequent readmissions necessitating treatment occurred. Similarly, our method to group causes of renal dysfunction () does not allow us to estimate the contribution of paracetamol to the development of AKI, since paracetamol may be a potential nephrotoxic contributor in patients overdosing NSAIDs or presenting with other causes.
Conclusion
Clinically relevant acute kidney injury due to paracetamol overdose alone is extremely rare in the absence of concomitant liver injury when excluding other prerenal, renal, and postrenal causes of renal dysfunction, NAC interference and chronic kidney injury.
Data availability statement
The data were derived from a regional version of The Danish National Patient Register via the Danish unique personal identification number given to all Danish residents at birth or upon immigration. Owing to data privacy regulations, patient data on the individual level are only accessible to authorized researchers after application to the Danish Health Data Authority.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This study is noncommercial and did not receive any financial support.
References
- Cobden I, Record CO, Ward MK, et al. Paracetamol-induced acute renal failure in the absence of fulminant liver damage. Br Med J (Clin Res Ed). 1982;284(6308):21–22.
- Campbell NR, Baylis B. Renal impairment associated with an acute paracetamol overdose in the absence of hepatotoxicity. Postgrad Med J. 1992;68(796):116–118.
- Prescott LF, Proudfoot AT, Cregeen RJ. Paracetamol-induced acute renal failure in the absence of fulminant liver damage. Br Med J (Clin Res Ed). 1982;284(6313):421–422.
- Mach M-Av, Hermanns-Clausen M, Koch I, et al. Experiences of a poison center network with renal insufficiency in acetaminophen overdose: an analysis of 17 cases. Clin Toxicol (Phila). 2005;43(1):31–37.
- Daoud A, Dalhoff KP, Petersen TS. Comment on “The changing face of paracetamol toxicity and new regimens for an old antidote acetylcysteine”. Br J Clin Pharmacol. 2021;87(4):2160–2161.
- Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–349.
- Daoud A, Dalhoff KP, Christensen MB, et al. Two-bag intravenous N-acetylcysteine, antihistamine pretreatment and high plasma paracetamol levels are associated with a lower incidence of anaphylactoid reactions to N-acetylcysteine. Clin Toxicol. 2020;58(7):698–704.
- Lynge E, Sandegaard JL, Rebolj M. The danish national patient register. Scand J Public Health. 2011;39(7 Suppl):30–33.
- Grann AF, Erichsen R, Nielsen AG, et al. Existing data sources for clinical epidemiology: the clinical laboratory information system (LABKA) research database at Aarhus University, Denmark. Clin Epidemiol. 2011;3:133–138.
- International Society of Nephrology. Summary of recommendation statements. Kidney Int Suppl. 2012;2:8–12.
- R Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2020. https://www.R-project.org/
- Huang JW, Lahey B, Clarkin OJ, et al. A systematic review of the effect of N-Acetylcysteine on serum creatinine and cystatin C measurements. Kidney Int Rep. 2021;6(2):396–403.
- ROCHE – eLabDoc. CREP2 method sheet. Available from: https://pim-eservices.roche.com/eLD/web/pi/en/home [cited 2022 Jul 8].