Abstract
With rising therapeutic use worldwide, prescription stimulants are increasingly implicated in poisonings. Most of the limited research on stimulant poisoning focuses on illicit amphetamines. We aimed to investigate the clinical effects of intentional prescription stimulant poisoning. This is a retrospective review of hospitalised patients referred to a Poisons Information Centre and a toxicology unit following intentional exposures to prescription stimulants (dexamfetamine, lisdexamfetamine and methylphenidate) from 1 January 2018 through 31 December 2021. Patients were identified from both units’ databases and data extracted from medical records. To facilitate comparison a dexamfetamine-equivalent dose was calculated. There were 186 intentional exposures in 178 patients (median age 17 years, males 54%) over the study period, consisting of 44 (24%) dexamfetamine, 50 (27%) lisdexamfetamine and 92 (49%) methylphenidate exposures. Stimulant exposures rose annually from 34 presentations in 2018 to 61 in 2021. Deliberate self-poisoning accounted for 139 (75%) presentations and the remaining 47 (25%) were recreational. Co-ingestions were taken in 101 (54%) presentations, leaving 85 (46%) single-agent exposures with a median dexamfetamine-equivalent dose ingested of 135 mg (range 15-4500 mg). Of the single-agent exposures tachycardia (69%), hypertension (47%) and agitation (58%) were the commonest clinical features. Most patients (58%) had mild-moderate symptoms, managed solely with supportive cares or oral sedation. Twenty-three (27%) patients had severe agitation receiving parenteral sedation. Rhabdomyolysis occurred in 3 single-agent exposures (4%) and 8 (9%) had an acute kidney injury. Severe cardiovascular toxicity was rare, with one patient developing hypertensive crisis and another having a supraventricular tachycardia. The median length of stay for single-agent exposures was 11 h (IQR 5-19 h). Prescription stimulant poisoning appears to be rising and commonly results in tachycardia, hypertension, and agitation. Severe cardiovascular toxicity was rare.
Introduction
Dexamfetamine, lisdexamfetamine, and methylphenidate are stimulants used for the treatment of attention deficit hyperactivity disorder (ADHD). They act by inhibiting the reuptake of dopamine and noradrenaline and facilitating their release, resulting in increased synaptic concentrations of both neurotransmitters [Citation1]. In Australia, dexamfetamine was first subsidised for the treatment of ADHD in 1973. Subsequently, immediate-release methylphenidate was subsidised in 2005, and modified-release variations in 2007 [Citation2].
Lisdexamfetamine is the most recent addition to the class of prescription stimulants. It was first registered by the Australian Therapeutic Goods Administration in 2013 for ADHD in children and adolescents. In 2021, its listing was broadened on the Pharmaceutical Benefits Scheme (PBS) to include adult diagnoses of ADHD [Citation2]. Lisdexamfetamine requires enzymatic hydrolysis of the lysine from dexamfetamine before exhibiting pharmacologic effects. The peak plasma concentration of lisdexamfetamine-derived dexamfetamine after a therapeutic dose has been observed to be lower and delayed 3–6 h post ingestion, with clinical effects lasting up to 13–14 h [Citation3]. According to the World Health Organisation (WHO), the ‘defined daily dose’ (DDD) is the assumed average maintenance dose per day for a drug used for its main indication in adults [Citation4]. The DDD may provide a better reflection of average maintenance dosing than the prescribed daily dose. This is because the latter is affected by variations outside maintenance dosing, such as lower doses during initial dose titration [Citation4]. The DDD for lisdexametamine is 30 mg [Citation5]. Its pro-drug design has been postulated to reduce the potential for abuse [Citation6]. In comparison, dexamfetamine reaches peak plasma concentration at 2.6 h [Citation7] and has a DDD of 15 mg [Citation5]. Immediate-release methylphenidate reaches peak plasma concentration at 2 h [Citation8] and has a DDD of 30 mg (for both immediate and controlled-release forms) [Citation5]. In Australia, sustained release methylphenidate is available in the formulations Concerta® and Ritalin LA®. Both formulations exhibit a biphasic peak following oral dosing. An initial peak occurs at approximately 2 h with a second peak at approximately 6 h [Citation9]. In overdose, prescription stimulants are expected to cause symptoms consistent with sympathomimetic toxidrome, such as tachycardia, hypertension, hyperthermia, diaphoresis, and agitation [Citation10].
The rates of prescribing of stimulant medications continue to grow annually worldwide [Citation11]. This may be attributable to rising awareness of ADHD [Citation12]. On the other hand, there are growing concerns regarding the misuse of prescription stimulants [Citation13]. Stimulant misuse may occur recreationally to induce euphoria or improve cognitive performance [Citation3]. Recent evidence suggests that the COVID-19 pandemic resulted in a decrease in illicit methamphetamine-related hospital presentations [Citation14], presumably due to increased border security and travel restrictions [Citation15]. Whether reduced access to methamphetamine has been associated with a reciprocal increase in the misuse of prescription stimulants has not yet been investigated.
There is limited literature describing the specific clinical effects of intentional stimulant poisoning, despite their increasing availability and misuse [Citation10]. In this study, we investigate intentional exposures to prescription stimulants (overdoses and recreational use) reported to a state Poisons Information Centre (PIC) and a local clinical toxicology unit over a 4-year period.
Methods
Study design and settings
This is a retrospective review of patients with prescription stimulant poisoning (dexamfetamine, lisdexamfetamine, or methylphenidate) reported to a state PIC and a local clinical toxicology unit from 1 January 2018 to 31 December 2021. The PIC receives about 37,000 calls per year from the public and health care professionals.
The local clinical toxicology unit is located within a tertiary hospital with an emergency department that has approximately 64,000 presentations annually. The unit admits approximately 2000 patients each year. Data on each patient presentation are prospectively recorded in both units’ database that undergoes regular audit. The use of both databases and patient medical records for research has been granted by the respective local area human research ethics committee (HREC/14/QPAH/308 and LNR/19/QCHQ/53256).
Patient selection
We identified patients with intentional exposures to dexamfetamine, lisdexamfetamine, and methylphenidate through both databases. We identified intentional exposures using database documentation and confirmed intent using ingestion details in patients’ electronic medical records. Doses greater than two times the ‘defined daily dose’ (DDD) as defined by the World Health Organization (WHO) were included in the study. For children and adolescents, doses greater than two times the child’s usual dose were included. Patients with medical records that were inaccessible were excluded from the study.
Data collection
We reviewed patients’ electronic health records to retrieve additional information from their hospital admission. Data extracted for all patients included baseline characteristics (age, sex), ingestion details (time, dose, intent, co-ingestions), complications (rhabdomyolysis, acute kidney injury), treatment (chemical sedation, intubation, vasodilators), length of stay (LOS), and Intensive Care Unit (ICU) admission.
In patients with single-agent stimulant exposure, we extracted further data from the medical record. These included: baseline characteristics (prescribed medications), observations (peak heart rate [HR], systolic blood pressure [SBP], respiratory rate [RR], and temperature) clinical effects consistent with sympathomimetic toxidrome (including agitation, mydriasis, pressured speech, abnormal movements, hallucinations, and diaphoresis), and complications (rhabdomyolysis, acute kidney injury, intracranial haemorrhage, myocardial infarction [Citation16], and seizures).
We used the Kidney Disease Improving Global Outcomes (KDIGO) guidelines to define acute kidney injury (AKI) [Citation17]. If the baseline creatinine was not available for adult patients, it was estimated using the Modification of Diet in Renal Disease (MDRD) Study equation, assuming that eGFR is 75 mL/min per 1.73 m2, as suggested in the KDIGO guidelines. Baseline creatinine for children and adolescents was estimated using the height-based Full Age Spectrum (FAS) equation with an assumed eGFR of 125 mL/min per 1.73 m2 [Citation18,Citation19].
We defined rhabdomyolysis as a creatine kinase activity >1000 IU/L. We recorded outcomes by the Poisoning Severity Score (PSS) [Citation20].
To investigate the relationship between dose and clinical effects, doses reported of lisdexamfetamine and methylphenidate were converted to a dexamfetamine-equivalent dose. For lisdexamfetamine, the dose was multiplied by 0.4, and for methylphenidate, the dose was multiplied by 0.5. Based on mole weight, 40 mg of dexamphetamine is approximately equivalent to 100 mg of lisdexamphetamine and 80 mg of methylphenidate respectively [Citation21–23].
Analysis
Continuous variables are reported as medians, interquartile ranges (IQR), and ranges. All analysis was performed in GraphPad Prism 9 for Mac OS (GraphPad Software, San Diego, CA, USA; www.graphpad.com).
Results
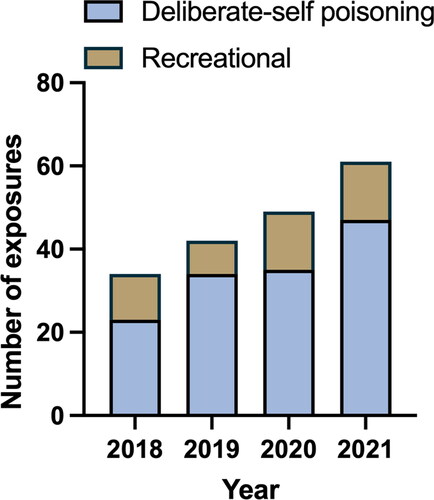
We extracted 236 records of exposures from the databases over the 4-year period. After removing records with an inaccessible medical record (26), duplicate calls (15), and exposures where the dose recorded was less than double the DDD (9), there were a total of 186 exposures in 178 patients. The number of exposures increased consistently each year, from 34 presentations in 2017 to 61 in 2021 (). The baseline characteristics of presentations are presented in .
Figure 1. Total number of stimulant presentations over the four-year period (2018–2021) comparing dexamfetamine, lisdexamfetamine and methylphenidate.
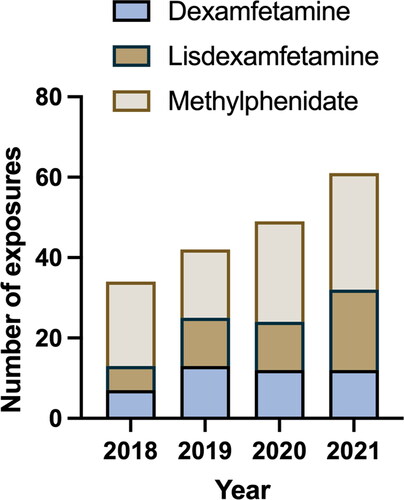
Table 1. Baseline characteristics of 186 presentations with stimulant poisonings over four-year period 2018–2021.
Isolated prescription stimulant exposure
There were a total of 85 presentations in which a prescription stimulant was taken alone. The main route of exposure was oral ingestion with a median dexamfetamine-equivalent dose of 135 mg (IQR 60–300 mg) (). Tachycardia, agitation, and hypertension occurred commonly in 59 (69%), 49 (58%), and 40 (47%) presentations respectively. There were no instances of myocardial infarction, intracranial haemorrhage, or seizures. The most common specific treatment was sedation, which was given in 51 (60%) presentations, with agitation being severe enough to necessitate parenteral sedation in 23 (27%) patients (). Oral sedation was predominantly with diazepam (90%) and parenteral sedation with droperidol (68%). Compared to other stimulants, dexamfetamine exposures appeared to have more agitation (91%), with more patients receiving parenteral sedation (45%). Dexamfetamine had the longest median length of stay (17 h).
Table 2. Clinical features of 85 presentations with isolated stimulant exposures.
There were 2 exposures with severe (Poisoning Severity Scale of 3) cardiovascular toxicity. One 51-year-old male patient developed a hypertensive crisis following a deliberate overdose of 4500 mg of dexamfetamine. The patient had a background of hypertension, usually on antihypertensives, with a baseline systolic blood pressure of 150 mmHg and no other cardiovascular disease. He developed a peak systolic blood pressure of 200 mmHg, was agitated and persistently tachycardic to 120 beats per minute. He was managed with a glyceryl trinitrate infusion and oral diazepam in the clinical toxicology unit and discharged at 36 h. This was the highest dose ingested in the series. The patient described having filled a bag with nine bottles containing 100 tablets that he had stockpiled over two years, consuming them over an hour. This dose was a significant outlier – the next highest dexamfetamine-equivalent dose ingested was 1700 mg (involving a 12-year-old female who ingested 3400 mg of modified-release methylphenidate). She had a peak systolic blood pressure of 140 mmHg, peak heart rate of 135 beats per minute, and was hyperthermic to 38.1 °C. She developed mild agitation managed with oral diazepam and had a length of stay of 19 h.
The other exposure with severe toxicity was a 17-year-old male with no significant medical history, who developed supraventricular tachycardia (peak heart rate 180 beats per minute) and agitation 8 h after a deliberate overdose of 180 mg of lisdexamfetamine. He was managed in a high-dependency unit with a single 6 mg dose of intravenous adenosine that successfully reverted the patient to sinus rhythm. He was managed with both intravenous and oral diazepam, and was discharged home at 63 h.
Discussion
Prescription stimulant poisoning increased over the four years in our series, most following deliberate self-poisonings. Tachycardia, hypertension, and agitation were common, while severe cardiovascular toxicity was rare. Patients taking dexamfetamine appeared to have more agitation compared to other agents.
The rising number of stimulant poisonings correlates with the rising number of patients treated for ADHD in Australia, as well as the rising prescription rate (). Between 2013 and 2017, the number of new patients treated for ADHD increased annually by 10.5% [Citation11]. The number of ADHD prescriptions subsidised by the PBS is rising rapidly. In 2020, over 1.9 million ADHD prescriptions were subsidised, compared to 806,000 in 2013. Between 2013 and 2020, the average annual growth rate of prescribing was 14%. Growth from 2018–2019 and 2019–2020 rose even more substantially at 20% each year [Citation2].
Figure 2. Number of stimulant presentations over four-year period comparing deliberate-self poisonings and recreational exposures.
Almost all the patients in this series were symptomatic, with 35% experiencing moderate toxicity, usually severe agitation. This differs from a U.S. Poisons Centre series of 23,553 exposures that found that stimulant exposures typically had no effects or minor toxicity [Citation10]. This was likely due to the inclusion of unintentional paediatric exposures and therapeutic errors, which were the two most common reasons for exposure, respectively.
Our findings were more consistent with those found by Danish and New South Wales Poison Information Centres’ series. Notably, both studies included co-ingestants and did not report on the effects of isolated stimulant exposures, which may have clouded the picture [Citation24,Citation25]. This is the first case series on prescription stimulants to demonstrate and compare the clinical effects of single-agent exposures to dexamfetamine, lisdexamfetamine, and methylphenidate.
Most patients had relatively short-lived toxicity, with a median length of stay of 11 h. Interestingly, although some stimulants are available in longer acting preparations, like methylphenidate or lisdexamfetamine, their median lengths of stay were less than or similar to dexamfetamine, an immediate-release formulation. It is possible this reflects dexamfetamine having relatively more severe effects in overdose compared to methylphenidate and lisdexamfetamine. Immediate release methylphenidate appeared to have a relatively shorter duration of toxicity.
Dexamfetamine appeared to be associated with more severe toxicity, predominantly severe agitation, compared to lisdexamfetamine and methylphenidate. Furthermore, recreational exposures were more common. One explanation is that recreational users are less likely to present to hospital than those that have taken deliberate self-poisonings, selecting for more severe exposures. Another possible explanation is that individuals with recreational exposures may under-report their ingested dose. Alternatively, dexamfetamine may cause more severe toxicity in overdose compared to other prescription stimulants. In our series, the median equivalent dose for dexamfetamine exposures of 60 mg was lower than the grouped median of 135 mg. Despite this, patients taking dexamfetamine received more parenteral sedation compared to other prescription stimulants. Increased severity did not appear to increase the likelihood of complications, which remained uncommon across all stimulants.
This study is limited by its retrospective design and potential inaccuracies in the documented risk assessment. It is possible clinical features and complications were under-reported. Similarly, our definition of severe agitation assumed clinicians always gave parenteral sedation to patients with severe agitation, which may have underestimated the true rate of severe agitation.
Both the Poisons Information Centre and the clinical toxicology unit prospectively enter all exposures into their respective clinical databases, which include detailed information on the most important clinical effects, complications and management, minimising some of the limitations of the retrospective design. Accuracy was also strengthened by access to patients’ electronic medical records, which included detailed progress notes, pathology, and imaging results. A further limitation of our study was that stimulants were not confirmed by analytical testing, the agent and dose ingested relies on the patient’s report. Previous literature has supported the reliability of patient history when reporting deliberate self-poisoning exposure to a single drug [Citation26].
Conclusion
Prescription stimulant poisoning is increasing. For single-agent exposures, most patients were symptomatic with tachycardia, agitation, and hypertension. Severe cardiovascular toxicity was rare. Most toxicity in isolated stimulant exposures resolved within 24 h, even when longer acting preparations were taken.
Contributors
C.M, C.W, and K.Z.I conceived the study. C.M and K.Z.I completed data collection, analysed the data and performed statistical analysis. C.M drafted the manuscript, and all authors contributed substantially to its revision. C.M takes responsibility for the paper as a whole.
Data availability
Data available on request from the authors.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Solanto MV. Neuropsychopharmacological mechanisms of stimulant drug action in attention-deficit hyperactivity disorder: a review and integration. Behav Brain Res. 1998;94(1):1–7.
- Pharmaceutical Benefits Scheme. Attention deficit hyperactivity disorder: utilisation analysis. Canberra: Commonwealth of Australia; 2021 [cited 16 Nov 2022]. Available from: https://www.pbs.gov.au/industry/listing/participants/public-release-docs/2018-05/adhd-dusc-prd-2018-05-final.pdf
- Steer C, Froelich J, Soutullo CA, et al. Lisdexamfetamine dimesylate: a new therapeutic option for attention-deficit hyperactivity disorder. CNS Drugs. 2012;26(8):691–705.
- WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC classification and DDD assignment 2023. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2022.
- WHO Collaborating Centre for Drug Statistics Methodology. Centrally acting sympathomimetics. [Internet]. Oslo: WHO Collaborating Centre for Drug Statistics Methodology; 2022. [cited 2022 Dec 26]. Available from: https://www.whocc.no/atc_ddd_index/?code=N06BA&showdescription=no
- Heal DJ, Smith SL, Gosden J, et al. Amphetamine, past and present–a pharmacological and clinical perspective. J Psychopharmacol. 2013;27(6):479–496.
- Wong YN, Wang L, Hartman L, et al. Comparison of the single-dose pharmacokinetics and tolerability of modafinil and dextroamphetamine administered alone or in combination in healthy male volunteers. J Clin Pharmacol. 1998;38(10):971–978.
- Kimko HC, Cross JT, Abernethy DR. Pharmacokinetics and clinical effectiveness of methylphenidate. Clin Pharmacokinet. 1999;37(6):457–470.
- Markowitz JS, Straughn AB, Patrick KS, et al. Pharmacokinetics of methylphenidate after oral administration of two modified-release formulations in healthy adults. Clin Pharmacokinet. 2003;42(4):393–401.
- Kaland ME, Klein-Schwartz W. Comparison of lisdexamfetamine and dextroamphetamine exposures reported to U.S. poison centres. Clin Toxicol (Phila). 2015;53(5):477–485.
- Trends in attention-deficit hyperactivity disorder medication use: a retrospective observational study using population-based databases. Lancet Psychiatry. 2018;5(10):824–835.
- Kooij SJ, Bejerot S, Blackwell A, et al. European consensus statement on diagnosis and treatment of adult ADHD: the European Network Adult ADHD. BMC Psychiatry. 2010;10:Article 67.
- Sembower MA, Ertischek MD, Buchholtz C, et al. Surveillance of diversion and nonmedical use of extended-release prescription amphetamine and oral methylphenidate in the United States. J Addict Dis. 2013;32(1):26–38.
- Fry M, Harris K, Isoardi KZ. Falling methamphetamine-related presentations to a clinical toxicology unit during the COVID-19 pandemic. Emerg Med Australas. 2021;33(1):179–180.
- Mitchell P. Australia’s illegal drug supply down, prices up as pandemic hits cartels. 2020 [Cited 19 Aug 2020]. Available from URL: https://indaily.com.au/news/2020/06/09/australias-illegal-drug-supply-down-prices-up-as-pandemic-hits-cartels/.
- Thygesen K, Alpert JS, Jaffe AS, Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction, et al. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231–2264. Epub 2018 Aug 25. PMID: 30153967.
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84.
- Pottel H, Hoste L, Dubourg L, et al. An estimated glomerular filtration rate equation for the full age spectrum. Nephrol Dial Transplant. 2016;31(5):798–806.
- Braun C, Fazlur Rahman AKM, Macomb E, et al. Derivation and evaluation of baseline creatinine equations for hospitalized children and adolescents: the AKI baseline creatinine equation. Pediatr Nephrol. 2022;37(12):3223–3233.
- Persson HE, Sjöberg GK, Haines JA, et al. Poisoning severity score: grading of acute poisoning. J Toxicol Clin Toxicol. 1998;36(3):205–213.
- Jasinski DR, Krishnan S. Abuse liability and safety of oral lisdexamfetamine dimesylate in individuals with a history of stimulant abuse. J Psychopharmacol. 2009;23(4):419–427.
- Dolder PC, Strajhar P, Vizeli P, et al. Pharmacokinetics and pharmacodynamics of lisdexamfetamine compared with D-amphetamine in healthy subjects. Front Pharmacol. 2017;8:Article 617.
- Janowsky DS, Davis JM. Methylphenidate, dextroamphetamine, and levamfetamine: effects on schizophrenic symptoms. Arch Gen Psychiatry. 1976;33(3):304–308.
- Jensen LS, Pagsberg AK, Dalhoff K. Methylphenidate misuse in adult patients and the impact of therapeutic use. Hum Exp Toxciol. 2015;34:460–467.
- Cairns R, Daniels B, Wood DA, et al. ADHD medication overdose and misuse: the NSW Poisons Information Centre experience, 2004-2014. Med J Aust. 2016;204(4):154. Article 154.
- Bentur Y, Lurie Y, Tamir A, et al. Reliability of history of acetaminophen ingestion in intentional drug overdose patients. Hum Exp Toxicol. 2011;30(1):44–50. Epub 2010 Mar 30. PMID: 20354060.
