Abstract
Treatment of antimuscarinic toxicity has become more difficult in the United States due to an ongoing national shortage of physostigmine. Rivastigmine has emerged as an alternative, off-label therapy due to its similar mechanism of action as a reversible, centrally-acting acetylcholinesterase inhibitor. We present a case of a diphenhydramine overdose where the patient exhibited classic central antimuscarinic delirium characterized by inattention, floccillation, and deficits in spatial planning manifested by the inability to properly draw a clock. These symptoms rapidly resolved after treatment with oral rivastigmine.
Introduction
Overdose of first-generation antihistamine medications frequently precipitates antimuscarinic toxicity [Citation1,Citation2]. Central nervous system effects are epitomized by floccillation (carphologia), delirium, and agitation [Citation3]. Symptoms of confusion may be subtle, encompassing impairment of executive function, inattention, and diminished visuospatial processing [Citation4]. This may ultimately be characterized by evaluating the patient’s ability to draw a clock.
Physostigmine, a reversible centrally-acting acetylcholinesterase inhibitor, is the antidote of choice to diagnose and treat central antimuscarinic toxicity due to its rapid onset of action. It has been previously demonstrated to reverse visuospatial impairment from antimuscarinic delirium [Citation4]. Unfortunately, there was a national shortage of physostigmine in the years leading up to the abrupt closure of Akorn Pharmaceuticals in the US in 2023. This prompted clinicians to seek alternative treatments for antimuscarinic toxicity [Citation5]. We present a case of antimuscarinic delirium treated with rivastigmine with clinical improvement demonstrated by improved clock drawing.
Case
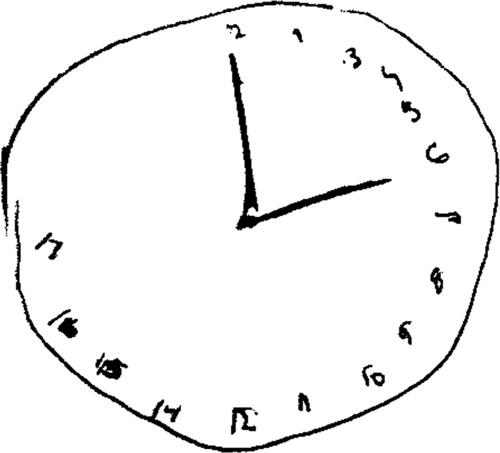
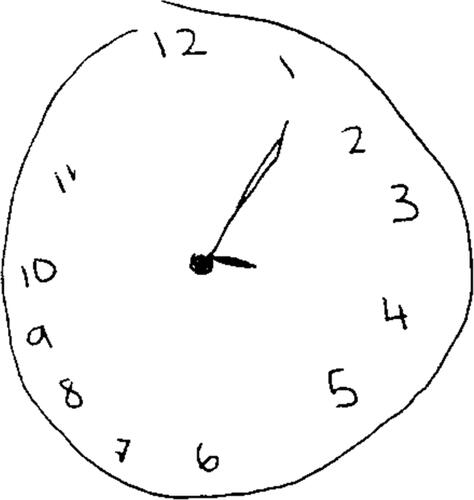
A 20-year-old female presented to the emergency department after an intentional ingestion of an unknown amount of diphenhydramine and ethanol. On arrival, she demonstrated mydriasis, agitation, and confusion. Vital signs were: heart rate, 117 beats per minute; respiratory rate, 20 breaths per minute; blood pressure, 136/92 mmHg, oxygen saturation 100% on ambient air; and an oral temperature of 36.7 °C. Initial serum ethanol, drawn about 90 min post-ingestion, was 201 mg/dL. Despite receiving four milligrams of intravenous lorazepam over the ensuing six hours, she continued to exhibit central and peripheral antimuscarinic symptoms. Upon bedside toxicology evaluation later that morning (10.5 h after ingestion), we observed mydriasis, floccillation (carphologia), garbled speech, and inattention. Our team prompted her to draw a clock () to further characterize her cognitive function. We noted that she exhibited significant deficits with spatial planning and perseveration as demonstrated by the clustering of hours 1–12 in the right half of the clock and numerical extension to ‘hour 17′ on the left, respectively [Citation6]. Due to a hospital shortage of physostigmine, we recommended 6 mg of oral rivastigmine. Upon re-evaluation, 1.5 h after medication administration (3.5 h after the initial toxicology encounter and 14 h after reported ingestion), her peripheral symptoms and sensorium were improved. She was no longer agitated, her speech was clearer, and she demonstrated near normalization in her visuospatial processing when asked to draw another clock (). No further medication was required and she was subsequently discharged home that afternoon.
Discussion
Despite a persistent national shortage of physostigmine within the US, off-label application of rivastigmine for antimuscarinic toxicity has gained traction [Citation5,Citation7,Citation8]. It is a newer cholinesterase inhibitor that was initially approved for dementia in 1997. Like physostigmine, it is able to cross the blood-brain barrier because of its uncharged tertiary amine moiety. Other acetylcholinesterase inhibitors, such as pyridostigmine or neostigmine, exhibit solely peripheral effects because the positively charged quaternary ammonium atom these compounds contain limits their ability to freely cross into the cerebral spinal fluid.
In the setting of antimuscarinic toxicity, rivastigmine may competitively overcome peripheral and central xenobiotic-induced muscarinic-receptor antagonism due to an increase in unmetabolized acetylcholine. Unlikely physostigmine, which is administered parenterally, rivastigmine is only available in oral and transdermal formulations. The onset of action of the oral medication takes about one hour [Citation8]. At two hours, peak cerebral spinal fluid concentrations are achieved [Citation9]. This is much slower than intravenous physostigmine where maximum clinical reversal of antimuscarinic effects can be expected within fifteen minutes [Citation10]. Nevertheless, the duration of acetylcholinesterase inhibition (and by extension, the expected clinical effects) is considerably longer, lasting up to 8–10 h [Citation9].
These differential pharmacokinetics are advantageous in the treatment of antimuscarinic toxicity. The duration of action of oral rivastigmine more closely mirrors the usual time course of antimuscarinic symptoms and may avoid the need for frequent redosing, which is often required with physostigmine [Citation10]. Seizures and bradycardia, previously considered perennial risks with physostigmine administration, appear to be uncommon in contemporary literature [Citation1,Citation2]. Further, the much slower onset of oral rivastigmine theoretically reduces the risk of acutely precipitating peripheral cholinergic symptoms which may occur with overzealous physostigmine administration. Although adverse-effect data on rivastigmine in this scenario is limited, they, too, are likely rare considering the suggested dosing is within the FDA-recommended guidelines for dementia (a physiologic state where the cholinergic tone can be presumed to be higher than in an individual exhibiting antimuscarinic symptoms) [Citation8]. Whether there is any role for transdermal rivastigmine in the treatment of antimuscarinic delirium has yet to be fully elucidated and requires further study.
In the case described herein, the patient we treated with rivastigmine demonstrated clear and rapid improvement in her central antimuscarinic symptoms demonstrated by her ability to correctly draw a clock. This test was initially designed to assess for constructional apraxia, a neurologic disorder related to the inability to draw objects [Citation11]. It is generally caused by structural disorders of the parietal lobe, such as Alzheimer’s Disease or stroke, but functional impairment has been noted previously in diphenhydramine-induced antimuscarinic delirium [Citation4]. The dose of 6 mg was chosen based on the previous experience of clinicians within our consulting service; these recommendations were subsequently published by Hughes and colleagues [Citation8].
Limitations
The main limitation of this report pertains to the timing at which rivastigmine was administered. Although it is possible that some of the improvement in her mental status was spontaneous and related to endogenous metabolization of the diphenhydramine, we feel that it does not fully explain the dramatic extent to which she improved after the administration of rivastigmine. In a comparative study of physostigmine and benzodiazepines published by Burns and colleagues, the time to recovery (resolution of delirium and agitation) for the benzodiazepine subgroups were about 31 h, whether or not physostigmine was administered. This is much longer than the time to clinical recovery for our patient which was about 14 h after presentation and comparable to the time of recovery for patients only given physostigmine or physostigmine as the initial treatment [Citation2].
The effects of alcohol may be considered a contributing factor to her mentation and agitation on arrival to the hospital, but at the time of toxicology evaluation, her cognitive state was much more consistent with antimuscarinic poisoning. It can also be expected that she would have metabolized the majority of the alcohol in the nine hours from her initial blood draw until our team’s encounter.
In summary, clinicians may consider prescribing rivastigmine as an off-label therapy to patients with suspected antimuscarinic delirium if physostigmine is unavailable. Further study on the overall effectiveness of rivastigmine is needed, but a growing bank of literature is supportive of our observations.
Additional information
Funding
References
- Wang GS, Baker K, Ng P, et al. A randomized trial comparing physostigmine vs lorazepam for treatment of antimuscarinic (anticholinergic) toxidrome. Clin Toxicol (Phila). 2021;59(8):1–3. doi:10.1080/15563650.2020.1854281.
- Burns MJ, Linden CH, Graudins A, et al. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374–381. doi:10.1016/S0196-0644(00)70057-6.
- Boley SP, Olives TD, Bangh SA, et al. Physostigmine is superior to non-antidote therapy in the management of antimuscarinic delirium: a prospective study from a regional poison center. Clin Toxicol (Phila). 2019;57(1):50–55. doi:10.1080/15563650.2018.1485154.
- Richardson WH, 3rd, Williams SR, Carstairs SD. A picturesque reversal of antimuscarinic delirium. J Emerg Med. 2004;26(4):463. doi:10.1016/j.jemermed.2004.01.005.
- Greene SC. Rivastigmine use in the treatment of antimuscarinic delirium. J Med Toxicol. 2023;19(3):284–287. doi:10.1007/s13181-023-00947-1.
- Rouleau I, Salmon DP, Butters N, et al. Quantitative and qualitative analyses of clock drawings in alzheimer’s and huntington’s disease. Brain Cogn. 1992;18(1):70–87. doi:10.1016/0278-2626(92)90112-y.
- Van Kernebeek MW, Ghesquiere M, Vanderbruggen N, et al. Rivastigmine for the treatment of anticholinergic delirium following severe procyclidine intoxication. Clin Toxicol (Phila). 2021;59(5):447–448. Maydoi:10.1080/15563650.2020.1818768.
- Hughes AR, Moore KK, Mah ND, et al. Letter in response to rivastigmine for the treatment of anticholinergic delirium following severe procyclidine intoxication. Clin Toxicol (Phila). 2021;59(9):855–856. doi:10.1080/15563650.2020.1869757.
- Excelon® (Rivastigmine Tartrate) Capsules. Accessed on July 27, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2000/20823lbl.pdf.
- Rosenbaum C, Bird SB. Timing and frequency of physostigmine redosing for antimuscarinic toxicity. J Med Toxicol. 2010;6(4):386–392. doi:10.1007/s13181-010-0077-7.
- Aprahamian I, Martinelli JE, Neri AL, et al. The clock drawing test: a review of its accuracy in screening for dementia. Dement Neuropsychol. 2009;3(2):74–81. doi:10.1590/S1980-57642009DN30200002.