Abstract
Pesticide self-poisoning is one of the three most important global means of suicide, killing an estimated 110–168,000 people each year, mostly in poor rural Asian communities. Organophosphorus (OP) and carbamate anticholinesterase insecticides are responsible for about two-thirds of these deaths. Calcium channel blocking medicines (CCB) may reduce the effect of pesticides and prevent deaths. Two preclinical rodents’ studies and eight clinical studies utilising nimodipine and magnesium sulphate (MgSO4), respectively, showed mixed results. We have established a multi-centre randomised controlled trial (RCT) of patients with OP or carbamate self-poisoning admitted to at least six major hospitals in Bangladesh. The study aims to recruit maximum 3,243 patients over four years. One-third of the patients selected at random will receive standard treatment, while one-third will be treated with additional nimodipine and one-third with additional MgSO4. The additional treatments will be given for 48 h. We will check mortality (currently an estimated 11% die with standard treatment) and need for intensive care for mechanical ventilation across the three groups. This could lead to development of the first novel treatment for anticholinesterase poisoning in 50 years and its introduction into routine hospital practice worldwide.
Background
Pesticide self-poisoning is one of the three most important global means of suicide [Citation1], killing an estimated 110–168,000 people annually [Citation2]. The pesticide used is often available in home and grabbed at a moment of crisis or anger [Citation3,Citation4]. The outcome is predominantly determined by the toxicity of the pesticide ingested [Citation5]. Bangladesh has banned several acutely toxic highly hazardous OP insecticides (all WHO hazard class I compounds [Citation6] removed from agricultural use in 2000), thereby reducing the number of deaths [Citation7]. However, pesticide self-poisoning remains a serious problem across the country [Citation8].
The anticholinesterase (OP) and carbamate insecticides are responsible for about two-thirds of poisoning related deaths globally. They both inhibit the enzyme acetylcholinesterase (AChE, EC 3.1.1.7), resulting in an excess of acetylcholine (ACh) and an acute cholinergic crisis due to overstimulation of cholinergic receptors throughout the body [Citation9]. This results in reduced consciousness, pulmonary oedema, and acute respiratory failure [Citation10]. Patients require resuscitation with oxygen, fluids, the muscarinic antagonist atropine, and AChE-reactivating oxime drugs [Citation9].
Escalating, titrated dose regimens of atropine are now the cornerstone of therapy for OP and carbamate poisoning [Citation11] after a RCT that was performed in Chattogram, Bangladesh, by the authors [Citation12]. This therapy is life-saving; however, the effectiveness of oxime drugs in management remains unclear [Citation13,Citation14]. Unfortunately, treatment overall is often ineffective and many patients die during initial course of treatment [Citation15]. Others die later, due to sustained dysfunction of the neuromuscular junction (NMJ) that causes respiratory failure that may require mechanical ventilation for many days. Facilities for invasive ventilation are scarce in the Bangladeshi hospitals where most patients present and often die in general ward [Citation16]. No new therapy has been introduced into clinical practice for 50 years, despite hundreds of animal studies and many millions of deaths [Citation17,Citation18]. New adjuvant treatments are urgently required.
Pre-synaptic ACh release is controlled by the flow of calcium into the nerve terminal. We predict that reduction of the pre-synaptic calcium load, using MgSO4 or nimodipine, after anticholinesterase poisoning will reduce ACh release and the overstimulation that causes both acute (cholinergic crisis) and delayed (respiratory, intermediate syndrome) toxicity. Two preclinical rodent studies have shown that dihydropyridine CCBs, including nimodipine, offer benefit [Citation19,Citation20]. Both drugs can rapidly reverse the effect of the OP pesticide omethoate at the murine NMJ (MgSO4 [Citation21] Nimodipine, Richard Ribchester–unpublished data, personal communication).
We performed a systematic review for clinical studies of MgSO4 or CCBs in OP or carbamate insecticide poisoning, finding five published and three unpublished studies () [Citation22]. Dihydropyridine medicines were studied in animals, but no human studies have been reported [Citation22]. Only four of eight studies were RCTs; all studies were of MgSO4, of small to modest size, and at risk of bias. They included 441 patients, with 239 patients receiving MgSO4 and 202 control patients. The pooled odds ratios for magnesium sulphate for mortality and need for intubation and ventilation for all eight studies were 0.55 (95% confidence interval 0.32–0.94) and 0.52 (95% CI 0.34–0.79), respectively. However, there was heterogeneity in the results with the highest quality phase III RCTs (Dawson, Vijayakumar) providing more conservative estimates (). Although a small dose-escalation study suggested benefit from higher doses of MgSO4, there was no evidence of a dose effect across the studies. Adverse effects were reported rarely, with only 11.1% of patients in the RCT receiving the highest dose of MgSO4 requiring their infusion to be stopped due to hypotension (Dawson unpublished). This study used doses of MgSO4 (4 g over 30 min, then 1 g/hr for at least 24 hrs) that are used routinely and safely in pregnant women with eclampsia [Citation23].
Figure 1. Systematic review of clinical studies of magnesium sulphate or dihydropyridines in OP or carbamate insecticide poisoning. Reproduced with permission from [Citation22].
![Figure 1. Systematic review of clinical studies of magnesium sulphate or dihydropyridines in OP or carbamate insecticide poisoning. Reproduced with permission from [Citation22].](/cms/asset/f9793969-1feb-497c-83ff-18ae2ef1601e/ttxc_a_2272073_f0001_b.jpg)
Methods
The aim of this study is to determine whether the addition of intravenous MgSO4 or nimodipine to standard therapy (supportive care plus, for all patients, atropine and, for OP insecticide poisoned patients, pralidoxime) benefits patients after self-poisoning with OP or carbamate insecticides. The study will be a large, definitive, multi-centre, open pragmatic, phase III RCT with three parallel groups: standard treatment versus standard treatment plus MgSO4 versus standard treatment plus nimodipine.
Clinical data will be collected via handheld computers using bespoke programming by MAXSOL PRO Pvt Ltd Sri Lanka, with a unified analysis database created across all sites. Study doctors will not be able to predict allocation before randomization. Allocation will not be blinded and will be indicated by instructions on the handheld computer. However, the next treatment allocation cannot be predicted in advance. The randomization list is hidden until the point of randomization. The primary outcome (vital status at discharge) is unambiguous and secondary outcomes are objective. Analysis will be performed on an intention-to-treat basis. All clinical events (until discharge) will be entered at site and laboratory data will be collected and linked with the unified analysis database.
The study will last for 4–5 years enrolling a maximum of 3,243 patients. The independent Data Monitoring Committee (iDMC) will keep this number under review. The investigational medicinal product (IMP) treatment phase will last for 48 h. The primary outcome will be vital status at hospital discharge. There will be no ongoing treatment of participants with study drug after discharge. The trial has been designed and will be reported consistent with the CONSORT Statement [Citation24].
Study hypothesis & principal comparisons
The main hypothesis is that the addition of Intravenous magnesium sulphate or nimodipine to standard therapy will reduce all-cause mortality rate from 11% to 7%. Hence, the two comparisons the study is powered for are (i) IV magnesium sulphate with standard therapy and (ii) IV nimodipine with standard therapy, each compared with (iii) standard therapy alone. A comparison of the two additional therapies (i) vs. (ii) will be exploratory.
Patients
The RCT will be performed in at least six major Bangladeshi hospitals namely, Chittagong Medical College Hospital (CMCH) in Chittagong, Dhaka Medical College Hospital (DMCH) in Dhaka, MAG Osmani Medical College Hospital (SOMCH) in Sylhet, Shaheed Ziaur Rahman Medical College Hospital (SZMCH) in Bogura, Rajshahi Medical College Hospital (RMCH) in Rajshahi and Khulna Medical College Hospital (KMCH) In Khulna.
All patients admitted to the adult medical wards of the six study hospitals with suspected OP or carbamate insecticide poisoning will be seen on presentation by ward doctors. These doctors will perform a routine bedside AChE analysis using a CheckMate (Securetec, Germany) or TestMate chE400 (EQM research Inc, USA) device. If cholinesterase activity is inhibited (<30 IU/L) and the patient/relative has given approval for an approach by the research team, GCP-trained Bangladeshi clinical researchers will inform them about the trial and seek consent for recruitment. Similar to other RCTs of acute poisoning, 30 min will be available for the patient and relatives to consider their decision. This brevity will ensure that the patient can be treated early and able to obtain the full potential benefit of the treatment. Written informed consent will be obtained from the patient or, if the patient lacks capacity, their relatives (first-degree legally acceptable representative like spouse, parent, adult child, brother and sister). A signature or thumbprints will be taken from the participants.
Follow up will be by telephone three months after hospital discharge (or sooner if the patient self-discharges against medical advice) to check the person’s health. Consent will be sought to contact participants in future by telephone or in person to discuss additional separately funded studies of the complications of poisoning and treatment with study drugs. The ward staff following standard treatment guidelines as per the hospital treatment policy will care for ineligible and non-recruited patients.
Inclusion criteria
Patients aged 16 years or older (no upper age limit) with suspected OP (including combined products) or carbamate insecticide self-poisoning admitted to medical wards with the cholinergic toxidrome requiring atropine. And, inhibited blood AChE activity as shown by clinical bedside test (value less than 30 IU/L) [Citation25].
Exclusion criteria
Children aged <16 years and patients who do not require atropine, known occupational and homicidal poisoning, known case of CKD (grade IV and V), hypersensitivity to MgSO4 and nimodipine, patients who have had a myocardial infarction or unstable angina in the last month, patients with traumatic subarachnoid haemorrhage and pregnancy and lack of informed consent (unaccompanied unconscious patients and others)
Patient management
All participants will receive standard treatment for OP or carbamate poisoning, as per published protocols [Citation12,Citation26]. Management will be guided by Standard Operating Procedures (SOPs) for which the clinical research staff will receive training. Each patient will initially receive 1–3 mg of atropine as a fast bolus intravenous (IV) push, aiming for clear lungs, adequate blood pressure (>80 mmHg systolic) and heart rate (>80 bpm), with doses doubled every 5 min until these targets are obtained (= “atropinisation”). At this point, when patients are switched to IV infusions of atropine rather than boluses, will be recorded as “time to atropinisation.”
Patients poisoned with OP insecticides, or an unknown anticholinesterase insecticide will receive pralidoxime (1 g over 30 min q6h for 48 h); those known to be poisoned by a carbamate will not receive pralidoxime. The responsible insecticide will be identified where possible from the history, the bottle brought in by the patient or relative, the patient/relative identifying the pesticide on a chart showing all locally available pesticides, and/or relatives sending a photo of the bottle.
The medical team independently of study doctors will make decisions about intubation transfer of patients to intensive care, weaning of ventilation and extubation. All decisions will be based on the patient’s clinical condition and the available hospital resources, as per usual hospital practice. Most decisions about initiating and weaning of ventilation as well as extubation are expected to take place after 48 h, when most participants are predicted to be off trial medication.
Trial intervention and study procedure
Participants allocated to magnesium will receive a 4 g IV bolus of magnesium sulphate (10% solution in 0.9% NaCl [sourced from clinical stock]) over 60 min, as soon as possible after the first atropine dose, using an infusion pump, followed by an infusion of 1 g/h for max 48 h or until atropine is no longer required.
Participants allocated to nimodipine will receive an initial infusion of nimodipine in 0.9% NaCl [sourced from clinical stock] of 0.5 mg/h for 2 h, as soon as possible after the first dose of atropine, using an infusion pump. In the absence of hypotension unresponsive to atropine, the dose will then be up-titrated carefully to 2 mg/h for a maximum of 48 h or until atropine is no longer required.
Control patients will receive standard therapy with 0.9% sodium chloride solution for infusion for 48 h. Drugs will be dispensed as soon as possible after recruitment and infused through I/V cannula.
Blood and urine samples of all the patients will be collected to measure magnesium and nimodipine concentration (to confirm correct allocation), pesticide concentrations (to identify responsible pesticide), cholinesterase activity (to monitor progression of poisoning), and for collaborative research (serum, plasma, peripheral blood mononuclear cells and others) (). Ten mL of urine will be collected at each time point.
Table 1. Schema of sample collection timing, as appropriate for patient survival or discharge.
Serum magnesium and calcium concentrations will be measured, and values fed back to the ward and research clinicians to ensure the protocol does not induce moderate-severe hypermagnesemia or hypocalcaemia. Other samples will be stored in 2–8 °C fridge and −20 °C or −80 °C freezers as per standard protocols. They will be stored at individual study sites (for fridge and −20 °C freezer) before being transferred to Bangabandhu Sheikh Mujib Medical University (BSMMU), Dhaka, for longer −80 °C storage. Samples will be shipped on dry ice for both local and overseas shipment. Analyses will be performed in study sites, a central laboratory in BSMMU or in collaborators’ laboratories such as those in Edinburgh (M Eddleston, University of Edinburgh). All stored specimens will be kept for up to 10 years after the end of the study and will be available for further analyses by study investigators for example immunological studies, after ethics review. Prior consent will be sought from every participant for storing and utilizing the samples in future. DNA and RNA will not be collected.
Randomisation
Once consent has been obtained, participants will be randomized by a study physician at the bedside using a handheld computer incorporating a bespoke randomization program, as per previous studies [Citation27]. This can be done quickly, with baseline information typically captured in under 5 min. Recruitment & randomization will be performed contemporaneously with assessment/resuscitation of patients by a second person so that medical care is not delayed by recruitment.
Patients will be randomized in a 1:1:1 ratio to standard treatment, standard treatment plus magnesium sulphate, or standard treatment plus nimodipine, stratified by site in randomly permuted block size with allocation concealed. The primary outcome (mortality at discharge) in this unblinded study is objective. Analysis will be performed on an intention-to-treat basis.
Outcome
The primary outcome will be all-cause mortality at hospital discharge. This easily measured outcome is the single most important outcome for patients and health care services after poisoning. Secondary outcomes will include time to atropinisation, proportion of patients requiring intubation, duration of ventilation and intubation, and duration of hospital stay. These outcomes are of key importance to health services due to resource and cost implications.
Sample size
On average, 11% of patients eligible for the study die before discharge under routine care in the six study hospitals. The anticipated treatment effect for magnesium sulphate or nimodipine is an absolute reduction of 4% in all-cause mortality at hospital discharge. This reduction would be sufficient to adopt either treatment as the new standard of care. The study would have 80% power at an overall two-sided 5% level of significance (calculated at 2.69% according to Dunnett’s test [Citation28]) to detect a reduction from 11% to 7% in all-cause mortality with 954 patients per arm (“power.prop.test” function in R version 3.5.1 used) [Citation29]. Under a 1:1:1 randomisation ratio, the study would need to recruit a total of 2,862 patients. The study is not formally powered to compare the two novel interventions of magnesium sulphate and nimodipine.
To allow the study to drop a novel intervention arm for efficacy or futility, we will use a group sequential design (Lan De Mets alpha spending approach with Fleming and O’Brien boundaries) to conduct formal interim analyses at 25%, 50%, 75% and 100% of patients with mature data on the primary outcome (all-cause mortality at hospital discharge). The group sequential design was considered in the “gsDesign” (version 2.0) package in R (version 3.5.1) and increased the required maximum sample size to 3,210 (1,070 per arm). Two-sided non-binding stopping boundaries for futility and efficacy will be considered at each formal interim analysis stage. The non-binding two-sided futility stopping boundaries at the second, third and fourth interim analyses are Z = 0.79, 1.62 and 2.25, and the two-sided efficacy stopping boundaries are Z = 4.81, 3.31, 2.64 and 2.25. To account for unexpected loss to follow-up, the maximum sample size was increased by 1% to 3,243.
Statistical analysis
The analysis will be performed on an intention-to-treat basis, using a fixed effects logistic regression on the primary outcome of all causes in-hospital death, with the two novel interventions fitted as two indicator variables (one for each of the new treatments). The logistic regression model will be adjusted for site, analysis stage, age, sex, and time from ingestion to randomization. The treatment effect estimates will be expressed as odds ratios and confidence intervals calculated at the appropriate level. The Absolute Risk Reduction (ARR) and Number Needed to Treat (NNT) will also be reported.
We will explore the pre-specified subgroup analyses (including sex, age, time from ingestion, pesticide class, GCS on admission) on the primary outcome by including treatment by baseline factor interaction terms. The study is not formally powered for these subgroup analyses, which will be exploratory in nature. The secondary outcome of proportion of patients needing intubation will be analysed using a logistic regression model. The other secondary outcomes will be analysed using linear models.
Independent data monitoring and ethics committee (IDMEC)
An independent DMC (termed a Data Safety Monitoring Board, DSMB) in Bangladesh) will be established to oversee the safety of participants in the trial. In light of interim data, and evidence from other relevant studies, the DMC will inform the Chief Investigator whether to stop parts of the study, based in part on statistical considerations.
The DMC will be asked to closely observe the number of deaths and secondary outcomes in the control arm to provide information on the accuracy of the power calculation. It will also be asked to monitor the incidence of hypotension unresponsive to atropine that results in discontinuation of medicine across the two study arms, as an event of special interest.
A TSC will be established to monitor, review and supervise the trial progress, to consider recommendations from DMC, to report the sponsor (MRC) on the progress of the trial and advice the CI and MRC on the presentations of all aspects of the trial. [Citation30]. The terms of reference are detailed in DMC & TSC Charters [Citation31].
Discussion
This trial will test the ability of two widely available, affordable medicines–MgSO4 and nimodipine–to reduce the severity of OP and carbamate self-poisoning, reducing deaths and duration of mechanical ventilation in intensive care, when added to standard care. Reducing the need for intubation and ventilation, and reducing the duration of ventilation when required, will markedly lower the risk of death since ventilation in resource-poor hospitals is a high-risk activity, with many patients dying while being ventilated. There is now an urgent need to gain this definitive evidence. To generate enough evidence and subsequently incorporate these treatment options into clinical practice, a large multi-centre RCT is required. As per the clinicaltrials.gov registry, no RCT is currently registered or enrolling patients to study the effect of calcium channel blockers to treat OP poisoning. In fact, as per the registry, this is the only RCT of novel treatments for this OP and carbamate poisoning that is actively enrolling cases.
This RCT may lead to the introduction of the first novel treatment for these poisonings in conventional hospital practice around the world in the last 50 years. Better treatment offers the potential to save tens of thousands of lives in some of rural Asia’s most impoverished communities and lessen the tragedy that suicide causes for children, families, and communities.
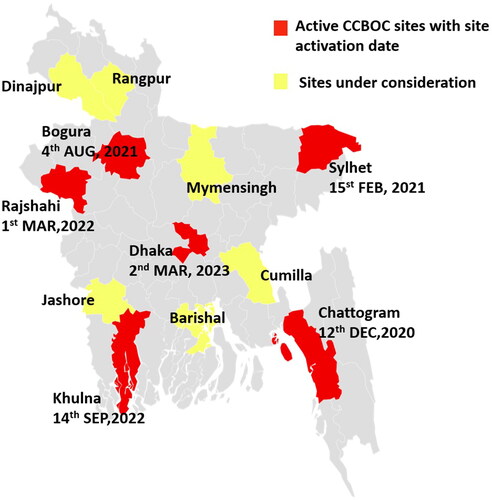
COVID-19 pandemic seriously hampered our study set up, staff recruitment, and procurement of logistics. The first site, CMCH was activated on 12 December 2020 and enrolled the study’s first case the same day. The second site SOMCH was activated on 15 February 2021. We subsequently activated our third site in SZMC (04 August 2021), fourth site in RMCH (01 March 2022), fifth site in KMCH (14 September 2022), sixth site in DMCH (02 March 2023) (). Since we are lagging in our enrolment target, we are looking for up to six more sites to expand the trial. These are Rangpur medical college hospital (RpMCH), Rangpur; Sher-e-Bangla medical college hospital (SBMCH), Barisal; Jashore medical college hospital (JMCH), Jashore; M Abdur Rahim medical college hospital (MARMCH), Dinajpur; Mymensingh medical college hospital (MMCH); and Cumilla medical college hospital (CuMCH) (). We are collecting the data from the sites and the feasibility assessments will be completed soon.
Figure 2. Map of Bangladesh showing the districts of currently (as of March 2023) active (red colour) study hospitals and possible future (yellow colour) study hospitals throughout the country. The active study hospitals cover an estimated 33% of the country’s population.
This RCT will provide an opportunity to establish high-quality infrastructure for studies of other diseases in patients being admitted to these hospitals. We expect that doctors and researchers will be able to benefit from this infrastructure and experience by setting up studies for patients outside clinical toxicology. It will also hopefully encourage junior doctors in an academic career path and encourage a good practice that will carry on to their clinical application.
Ethical approval
This study is ethically approved by the Research Ethics Committee of the Bangladesh medical research council (BMRC), Mohakhali, Dhaka, Bangladesh (BMRC/NREC/2016-2019/891) and was reviewed by the Research Ethics Committee of the Liverpool School of Tropical Medicine, Pembroke Place Liverpool L3 5QA UK (LSTM 18-081). Ethical approval has also been taken from local IRBs where needed.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- World Health Organization. Preventing suicide. WHO Libr Cat Data. 2014; p. 1.
- Mew EJ, Padmanathan P, Konradsen F, et al. The global burden of fatal self-poisoning with pesticides 2006–15: systematic review. J Affect Disord. 2017;219:93–9. doi:10.1016/j.jad.2017.05.002.
- Eddleston M, Karunaratne A, Weerakoon M, et al. Choice of poison for intentional self-poisoning in rural Sri Lanka. Clin Toxicol. 2006;44(3):283–286. doi:10.1080/15563650600584444.
- Eddleston M, Phillips MR. Self poisoning with pesticides. BMJ. 2004;328(7430):42–44. doi:10.1136/bmj.328.7430.42.
- Buckley NA, Fahim M, Raubenheimer J, et al. Case fatality of agricultural pesticides after self-poisoning in Sri Lanka: a prospective cohort study. Lancet Glob Health. 2021;9(6):e854–e862. doi:10.1016/S2214-109X(21)00086-3.
- WHO. The WHO recommended classification of pesticides by hazard and guidelines to classification. 2020.
- Chowdhury FR, Dewan G, Verma VR, et al. Bans of WHO class i pesticides in Bangladesh – suicide prevention without hampering agricultural output. Int J Epidemiol. 2018;47(1):175–184. doi:10.1093/ije/dyx157.
- Dewan G. Analysis of recent situation of pesticide poisoning in Bangladesh: is there a proper estimate? Asia Pacific J Med Toxicol. 2014;3:76–83.
- Eddleston M, Chowdhury FR. Pharmacological treatment of organophosphorus insecticide poisoning: the old and the possible new. Br J Clin Pharmacol. 2016;81(3):462–470. doi:10.1111/bcp.12784.
- Eddleston M, Juszczak E, Buckley NA, et al. Multiple-dose activated charcoal in acute self-poisoning: a randomised controlled trial. Lancet. 2008;371(9612):579–587. doi:10.1016/S0140-6736(08)60270-6.
- Connors NJ, Harnett ZH, Hoffman RS. Comparison of current recommended regimens of atropinization in organophosphate poisoning. J Med Toxicol. 2014;10(2):143–147. doi:10.1007/s13181-013-0324-9.
- Abedin MJ, Sayeed AA, Basher A, et al. Open-label randomized clinical trial of atropine bolus injection versus incremental boluses plus infusion for organophosphate poisoning in Bangladesh. J Med Toxicol. 2012;8(2):108–117. doi:10.1007/s13181-012-0214-6.
- Buckley NA, Eddleston M, Li Y, et al. Oximes for acute organophosphate pesticide poisoning. Cochrane Database Syst Rev. 2011;2011(2):CD005085. doi:10.1002/14651858.CD005085.PUB2/APPENDICES.
- Blumenberg A, Benabbas R, deSouza IS, et al. Utility of 2-pyridine aldoxime methyl chloride (2-pam) for acute organophosphate poisoning: a systematic review and meta-analysis. J Med Toxicol. 2018;14(1):91–98. doi:10.1007/s13181-017-0636-2.
- Eddleston M, Eyer P, Worek F, et al. Differences between organophosphorus insecticides in human self-poisoning: a prospective cohort study. Lancet. 2005;366(9495):1452–1459. doi:10.1016/S0140-6736(05)67598-8.
- Chowdhury FR, Rahman MM, Ullah P, et al. Salbutamol in acute organophosphorus insecticide poisoning – a pilot dose-response phase II study. Clin Toxicol. 2018;56(9):820–827. doi:10.1080/15563650.2018.1440587.
- Buckley NA, Karalliedde L, Dawson A, et al. Where is the evidence for treatments used in pesticide poisoning? Is clinical toxicology fiddling while the developing world burns? J Toxicol Clin Toxicol. 2004;42(1):113–116. doi:10.1081/clt-120028756.
- Karunarathne A, Gunnell D, Konradsen F, et al. How many premature deaths from pesticide suicide have occurred since the agricultural green revolution? Clin Toxicol. 2020;58(4):227–232. doi:10.1080/15563650.2019.1662433.
- Dretchen KL, Bowles AM, Raines A. Protection by phenytoin and calcium channel blocking agents against the toxicity of diisopropylfluorophosphate. Toxicol Appl Pharmacol. 1986;83(3):584–589. doi:10.1016/0041-008x(86)90241-3.
- Rohatgi S, Bhattacharya R, Gupta D. S. Efficacy of calcium channel blocker as an adjunct in therapy of organophosphate poisoning. Indian J Physiol Pharmacol. 1993;37:255–256.
- Dissanayake KN, Chou RCC, Thompson A, et al. Impaired neuromuscular function by conjoint actions of organophosphorus insecticide metabolites omethoate and cyclohexanol with implications for treatment of respiratory failure. Clin Toxicol. 2021;59(12):1239–1258. doi:10.1080/15563650.2021.1916519.
- Brvar M, Chan MY, Dawson AH, et al. Magnesium sulphate and calcium channel blocking drugs as antidotes for acute organophosphorus insecticide poisoning – a systematic review and meta-analysis. Clin Toxicol. 2018;56(8):725–736. doi:10.1080/15563650.2018.1446532.
- British Medical Assosiation and Royal Pharmaceutical Society of Great Britain. BNF 78 (Biritsh National Formularly). London: BMJ Group, pharmaceutical press; 2019. p.1051; Available from: https://bnf.nice.org.uk.
- Moher D, Schulz KF, Altman DG, et al. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357(9263):1191–1194. doi:10.1016/S0140-6736(00)04337-3.
- Worek F, Mast U, Kiderlen D, et al. Improved determination of acetylcholinesterase activity in human whole blood. Clin Chim Acta. 1999;288(1–2):73–90. doi:10.1016/s0009-8981(99)00144-8.
- Eddleston M, Dawson A, Karalliedde L, et al. Early management after self-poisoning with an organophosphorus or carbamate pesticide – a treatment protocol for junior doctors. Crit Care. 2004;8(6):R391–R397. doi:10.1186/cc2953.
- Eddleston M, Juszczak E, Buckley NA, et al. Study protocol: a randomised controlled trial of multiple and single dose activated charcoal for acute self-poisoning. BMC Emerg Med. 2007;7(1):2. doi:10.1186/1471-227X-7-2.
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50(272):1096–1121. doi:10.1080/01621459.1955.10501294.
- R core team. 2021. R: a language and environment for statistical computing. R foundation for statistical computing. Vienna, Austria; [cited 2023 Mar 26]. Available from: https://www.r-project.org/.
- Medical Research Council. MRC guidelines for management of global health trials: involving clinical or public health interventions. Environ Manage. 2017; p. 1–41.
- Charles H. Data monitoring committee & trial steering committee charters. 2019;p. 3–7. Available from: https://www.accord.ed.ac.uk/cr015-data-monitoring-committee-and-trial-steering-committee-charters.
