Abstract
Prallethrin ingestion and/or aspiration can manifest in a wide range of systemic effects. Evidence-based management of chemical pneumonitis following prallethrin aspiration does not exist yet. This case presents an unusual course of illness following prallethrin ingestion/aspiration that responded to antibiotics and steroid use. This is a previously well 22-month-old boy presented to the Emergency Department (ED) with a history of estimated 20 mL ingestion/aspiration of insecticide containing prallethrin 1.6% one hour earlier. The patient had bluish discoloration of his face, with eye tearing, drooling, and frothy secretions from his mouth. The patient was admitted for supportive care and observation. During his four days of admission, he progressively improved and returned to his previously healthy baseline. However, four hours after his discharge, he relapsed and was readmitted with an impression of superimposed pneumonia. During his second course of illness, the patient had worsening of his respiratory status requiring escalation of oxygen therapy and antibiotics. The patient received 21 days of intravenous (IV) antibiotics, and steroids, and was discharged on inhaled salbutamol and fluticasone. In conclusion, pyrethroid aspiration can lead to severe pneumonitis and secondary superimposed bacterial infection.
Background
Prallethrin is a pyrethroid insecticide and repellent for the control of many insects [Citation1]. Generally, prallethrin is considered of low toxicity in humans [Citation1]. This is due to low absorption through skin, resulting in a bioavailability of 1% [Citation2]. In contrast, it has 36% bioavailability following gastric absorption [Citation3]. Both ingestion and/or aspiration of large amounts can induce toxicity and produce various systemic effects [Citation3]. In cases of prallethrin intoxication, gastrointestinal symptoms are the most common (73%). The involvement of the nervous and respiratory systems and lungs are less common, (33% and 29%, respectively) [Citation4, Citation5]. Pyrethroids are usually mixed with nonactive material referred to as inert ingredients. Although these ingredients could be the cause of toxicity, they do not have to be directly identified on a pesticide label since they are nonactive. Hydrocarbon is one of those ingredients that is more likely to cause chemical pneumonitis than prallethrin itself [Citation1]. Overall, systemic manifestations of pyrethroid poisoning occur in 4–48 h [Citation6]. Other routes of intoxication such as dermal or inhalation are less common but reported in the literature [Citation7, Citation8]. There are few reports of prallethrin intoxication in adults, but cases in paediatrics are rare [Citation9, Citation10]. We report, in accordance with the CARE Guidelines (https://www.care-statement.org), a paediatric case of ingestion/aspiration of prallethrin resulting in chemical pneumonitis complicated by superimposed bacterial infection.
Case report
A previously well 22-month-old boy presented to the ED of King Abdulaziz Medical City (KAMC) in Jeddah, Saudi Arabia with a history of an unwitnessed ingestion/aspiration of an estimated 20 mL of an insecticide containing prallethrin (1.6%) about one hour prior to ED arrival. The mother found him with the container in his hands, with blue lips, tearing eyes, drooling, and frothy secretions from his mouth associated with noisy breathing. This event lasted for around 10 min until their arrival at the ED. The mother denied any abnormal movement, abdominal pain, vomiting, or change in bowel habits.
On presentation, the patient appeared drowsy with an oxygen saturation of 80% on room air. We applied 5 L/min oxygen via face mask and his oxygen saturation normalized. On examination, he became alert after oxygenation but was still tachypneic. He had no more drooling, tearing eyes, or cyanosis. His vital signs after oxygenation included respiratory rate (68 breaths/min), heart rate (110 beats/min) with normal rhythm on a cardiac monitor, and blood pressure 104/72 mmHg.
On inspection of the oral cavity, he had no apparent injury to the oral mucosa. Upon chest examination, the child was in respiratory distress with audible stridor, wheeze, tachypnea, and suprasternal retraction. His breath sounds were equal bilaterally with expiratory wheeze. The remainder of the examinations were normal.
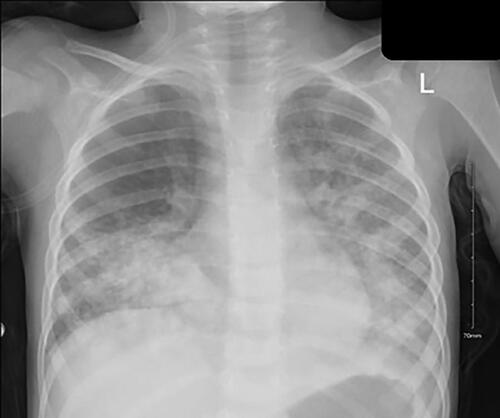
A chest radiograph in the ED revealed opacities in both lower lobes, suggestive of chemical pneumonitis. Laboratory testing revealed hemoglobin of 11.3 g/dL, a leucocyte count of 11.4 × 10^9/L, neutrophil 2.50 × 10^9/L, platelet count of 355 × 10^9/L. Venous blood gas analysis on 5 L/min of oxygen via face mask were pH 7.28, pCO2 45.1 mmHg, and pO2 56.7 mmHg.
After the patient initially was treated supportively in the ED with oxygenation, nebulized epinephrine, salbutamol, IV steroids, and ceftriaxone, he was admitted to the Pediatric Intensive Care Unit (PICU) for respiratory observation of chemical pneumonitis. He was kept nil per os (NPO) with continued intravenous fluids, ceftriaxone, nebulized racemic epinephrine, and IV dexamethasone (0.5 mg/Kg/dose) every 6 h. The patient improved during a one-day stay in the PICU with improved breathing on 2 L/min of oxygen by nasal cannula without need for non-invasive positive pressure ventilation (NIPPV). He was transferred to the paediatrics ward for continuity of care where he received the same management for three days and improved gradually. He had no fever during the admission and once he no longer required additional oxygen, he was discharged home to complete the antibiotic course cefixime.
Within four hours of returning home, the patient recrudesced, developing tachypnea, lethargy, and refusing to eat, prompting parents to return to the hospital. Upon his second presentation, the patient appeared ill, drowsy, and in respiratory distress with an audible expiratory stridor. He also had a fever (38.4 °C) and was tachypneic (70 breaths/min) requiring 5 L/min of oxygen administered via face mask. Chest auscultation revealed decreased air entry in the right lung base with expiratory crepitation bilaterally more in the right lung and bilateral wheeze.
Laboratory testing on the first day after readmission revealed hemoglobin of 10.8 g/dL, leucocyte count of 17.1 × 10^9/L, mainly neutrophil 9.52 × 10^9/L, platelet count of 467 × 10^9/L, high inflammatory markers with C-reactive protein 23.3 mg/L and procalcitonin 2.93 ug/L. Venous blood gas analysis on 5 L/min oxygen via face mask were pH 7.28, pCO2 45.1 mmHg, and pO2 56.7 mmHg. Immediately the patient was started on NIPPV and given acetaminophen, racemic epinephrine, salbutamol nebulization, IV ceftriaxone, and IV dexamethasone and improved clinically over 2 h. He was readmitted to the PICU as a high-risk patient with superimposed pneumonia complicating his initial chemical pneumonitis. The second chest radiograph showed worsening of the previous opacities in bilateral lower lobes ().
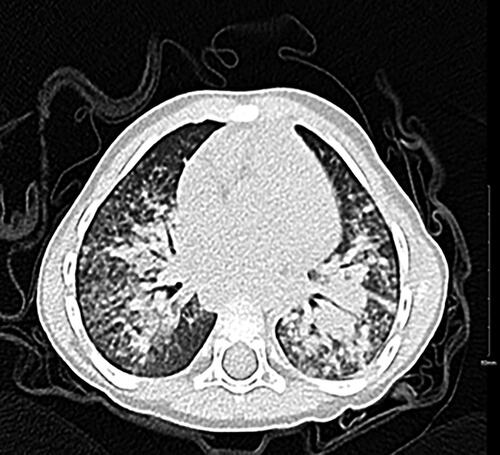
The patient was admitted to the PICU for one day, where his oxygen requirements were weaned from NIPPV to 1 L/min of oxygen on a nasal cannula. He was started on cefepime and transferred to the paediatrics ward. On day two of his second hospital admission, the patient spiked a fever (38 °C) and had worsening of his respiratory status in terms of tachypnea (50s breaths/min) and suprasternal retraction requiring more oxygen reaching 3 L/min on nasal cannula. Clindamycin was added to cefipime at this point. He underwent chest computed tomography (CT), which showed extensive peribronchial consolidation involving the left upper, right middle and bilateral lower lobes with near complete opacification of the left lower lobe and diffuse ground glass opacities (). The infectious diseases consultant recommended changing antibiotics from cefepime and clindamycin to meropenem and vancomycin. They were concerned the patient had developed a superimposed bacterial infection. The patient clinically improved after 21 days on IV antibiotics.
The last chest radiograph done two days before the second discharge revealed mild worsening of the bilateral lung opacities with no evidence of pneumothorax. The patient was then discharged home after clinical improvement without residual respiratory distress for more than 48 h and afebrile for more than 7 days. He was discharged home on inhaled salbutamol and fluticasone with outpatient follow-up with pulmonology and general paediatrics.
Discussion
Although routine antibiotics and steroid use in the treatment of chemical pneumonitis are not recommended, the evidence provided for this included only those with gastric content aspiration [Citation11, Citation12]. Another recommendation by Blame et al. suggests against the routine use of antibiotics in managing patients with chemical pneumonitis due to paraffin aspiration [Citation13]. The decision to give prophylactic antibiotics depends on the likelihood that the patient will develop a secondary infection or not. This likelihood is probably related to the agent causing chemical pneumonitis, its solvents, and their chemical properties [Citation14]. This is why it is still a difficult decision for clinicians to give prophylactic antibiotics or not, and it is still a common practice when managing patients with chemical pneumonitis caused by hydrocarbon-containing compounds [Citation13]. There is a lack of evidence supporting antibiotics and steroid use in prallethrin aspiration, but this case demonstrates that these therapies may be beneficial or even indicated when patients are more severely affected.
Upon first assessment and management, our patient appeared to have chemical pneumonitis with early improvement. However, the first relapse upon first discharge, and the sudden deterioration during his second course of illness, could demonstrate a further impact of prallethrin aspiration that potentially does not appear to be similar to respiratory manifestations in previous reports. Primary respiratory symptoms are likely result from direct irritation to the airways and the development of chemical pneumonitis. A superimposed bacterial infection could be the result of early antibiotic initiation, or alternatively, it could be a direct effect of the prallethrin or its solvents altering lung antimicrobial function [Citation15]. Similar paediatric and adult cases illustrate central nervous system and respiratory system involvement with altered mental status and decreased oxygen saturation [Citation3, Citation4, Citation10]. Other reported cases describe hepatic, cardiac, and dermal effects [Citation4, Citation16–18].
An in-silico study might explain the bacterial superimposed infection by impairment of surfactant proteins (SP) by prallethrin or its solvent carrier. Prallethrin and SP appear to interact through hydrogen bonds, alkyl bonds, Pi–Pi interaction, and Van der Waals interaction. Such interactions could interfere with the antimicrobial function of those SPs and might increase the susceptibility of the lungs to respiratory infections [Citation19].
Our patient initially responded well to supportive management. However, the relapse soon after the first discharge and the sudden deterioration during the second course of illness were unexpected. Although the child ultimately recovered and returned to his previous healthy baseline, there is a lack of evidence supporting our treatment approach ().
Picture 1. Prallethrin chemical structure [Citation20].
![Picture 1. Prallethrin chemical structure [Citation20].](/cms/asset/8bbedef5-157e-4f27-85d2-a9d439781ebb/ttxc_a_2283310_f0003_c.jpg)
Conclusion
Pyrethroid aspiration can lead to chemical pneumonitis and secondary bacterial infection.
Consent to publish
I confirm that any participants (or their guardians if unable to give informed consent, or next of kin, if deceased) who may be identifiable through the manuscript (such as a case report), have been given an opportunity to review the final manuscript and have provided written consent to publish.
Picture 1.tiff
Download TIFF Image (246 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Additional information
Funding
References
- Agency for Toxic Substances and Disease Registry (ATSDR). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. 2014. Toxicological profile for pyrethrins and pyrethroids. https://wwwn.cdc.gov/TSP/PHS/PHS.aspx?phsid=785&toxid=153 [accessed 21 Jun 2023].
- Woollen BH, Marsh JR, Laird WJ, et al. The metabolism of cypermethrin in man: differences in urinary metabolite profiles following oral and dermal administration. Xenobiotica. 1992;22(8):1–5. doi:10.3109/00498259209049904.
- Ray DE, Forshaw PJ. Pyrethroid insecticides: poisoning syndromes, synergies, and therapy. J Toxicol Clin Toxicol. 2000;38(2):95–101. doi:10.1081/clt-100100922.
- Yang PY, Lin JL, Hall AH, et al. Acute ingestion poisoning with insecticide formulations containing the pyrethroid permethrin, xylene, and surfactant: a review of 48 cases. J Toxicol Clin Toxicol. 2002;40(2):107–113. doi:10.1081/clt-120004397.
- Suting E, Bhaskar V, Batra P. Changing epidemiology of poisoning in children: a retrospective study from a tertiary care center in New Delhi, India. Indian J Public Health. 2021;65(4):400–402. doi:10.4103/ijph.IJPH_234_21.
- Bradberry SM, Cage SA, Proudfoot AT, et al. Poisoning due to pyrethroids. Toxicol Rev. 2005;24(2):93–106. doi:10.2165/00139709-200524020-00003.
- Na HG, Kim YD, Choi YS, et al. Allethrin and prallethrin stimulates MUC5AC expression through oxidative stress in human airway epithelial cells. Biochem Biophys Res Commun. 2018;503(1):316–322. doi:10.1016/j.bbrc.2018.06.022.
- Botnariu G, Birsan C, Podoleanu C, et al. Skin necrosis caused by prallethrin – a worldwide used insecticide. Environ Toxicol Pharmacol. 2016;43:103–104. doi:10.1016/j.etap.2016.03.002.
- Bhaskar EM, Moorthy S, Ganeshwala G, et al. Cardiac conduction disturbance due to prallethrin (pyrethroid) poisoning. J Med Toxicol. 2010;6(1):27–30. doi:10.1007/s13181-010-0032-7.
- George J, Malik R, Gogna A. Hypersensitivity reaction and acute respiratory distress syndrome in pyrethroid poisoning and role of steroid therapy. Asia Pac J Med Toxicol. 2015;4(2):91–93. doi:10.22038/apjmt.2015.3732.
- Dragan V, Wei Y, Elligsen M, et al. Prophylactic antimicrobial therapy for acute aspiration pneumonitis. Clin Infect Dis. 2018;67(4):513–518. doi:10.1093/cid/ciy120.
- Mandell LA, Niederman MS. Aspiration pneumonia. N Engl J Med. 2019;380(7):651–663. doi:10.1056/NEJMra1714562.
- Balme K, Stephen C. Paraffin ingestion in children: rationalising antibiotic treatment. S Afr Med J. 2017;107(8):646. doi:10.7196/SAMJ.2017.v107i8.12598.
- Curtis J, Metheny E, Sergent SR. Hydrocarbon toxicity. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023.Available from: https://www.ncbi.nlm.nih.gov/books/NBK499883/
- Joseph B, Barbara B, Adnan SD. Experimental kerosene pneumonia: evaluation of some therapeutic regimens. J. Pediatr. 1974;84(3):396–401. doi:10.1016/S0022-3476(74)80723-7.
- Walters JK, Boswell LE, Green MK, et al. Pyrethrin and pyrethroid illnesses in the pacific northwest: a five-year review. Public Health Rep. 2009;124(1):149–159. doi:10.1177/003335490912400118.
- Anjana KS, Raghunath CN, Arvind C. Acute kidney injury as a rare complication of prallethrin poisoning (“All-Out”) in a child. TOUNJ. 2019;12(1):53–55. doi:10.2174/1874303X01912010053.
- Atashi HA, Zaferani Arani H, Agatha F, et al. Cardiac and respiratory arrest in a 12-year-old girl with acute permethrin oral toxicity: a case report. Clin Case Rep. 2022;10(1):e05245. doi:10.1002/ccr3.5245.
- Ghanty S, Mandi M, Ganguly A, et al. Lung surfactant proteins as potential targets of prallethrin: an in silico approach. Toxicol. Environ. Health Sci. 2022;14(1):89–100. doi:10.1007/s13530-021-00119-0.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 9839306, Prallethrin. https://pubchem.ncbi.nlm.nih.gov/compound/Prallethrin [accessed 17 Sept 2023].