Abstract
Background
Bongkrekic acid (BA) is a mitochondrial toxin, produced by the bacteria Burkholderia gladioli pv. cocovenenans during fermentation. Outbreaks of BA poisoning have been reported in China, Indonesia, and Mozambique, but have not been previously observed in North America.
Case discussion
A 67-year-old male with a past medical history of hypertension presented with two days of generalized weakness, nausea, and vomiting. He had consumed ogi, a home-fermented cornmeal pudding two days earlier. The patient developed acute liver failure, encephalopathy, acute kidney injury, coagulopathy, methemoglobinemia, and metabolic acidosis. Despite intensive care, he died on hospital day 8. Workup for common causes of acute liver failure was negative. Blood testing was positive for BA, estimated at 1,000 ng/mL. This case illustrates poisoning by BA, a rare and often lethal toxin resulting from improperly fermented food.
Introduction
Globalization of food markets and diverse culinary practices expose individuals to unique foodborne hazards. Among these, the ingestion of fermented foods may pose unexpected health risks [Citation1].
The Gram-negative bacterium Burkholderia gladioli pv, cocovenenans proliferates in carbohydrate-rich substrates, particularly those undergoing fermentation. It is capable of producing an odorless, tasteless, heat-stable mitochondrial toxin known as bongkrekic acid (BA; Chemical Structure, ). Toxin production occurs in foods contaminated with the bacterium and requires warm temperatures (22 °C –30 °C), neutral pH, and the fungal organisms used for fermentation [Citation3].
Figure 1. Chemical structure of bongkrekic acid [Citation2].
![Figure 1. Chemical structure of bongkrekic acid [Citation2].](/cms/asset/3def746c-b0bc-4275-b8ba-3b6a70c700f6/ttxc_a_2377524_f0001_c.jpg)
Unlike more common mitochondrial toxins affecting the electron transport chain, BA inhibits adenine nucleotide translocase (ANT) [Citation1,Citation4]. Under physiologic conditions, ANT brings adenosine diphosphate (ADP) into the mitochondrial matrix to be converted to adenosine triphosphate (ATP) and transports ATP from the mitochondrial matrix into the intermembrane space. By inhibiting ANT, BA reduces ATP production and impairs its distribution for energy utilization. Consequently, the clinical effects are seen predominantly in the liver, brain, and kidneys [Citation4], and signs and symptoms include abdominal pain, vomiting, diarrhea, jaundice encephalopathy, lethargy, coma, and death [Citation5]. Laboratory findings include elevated transaminase levels, coagulopathy, and hyperglycemia, followed by hypoglycemia attributable to glycogen depletion.
BA poisonings are concentrated in Southeast Asia, particularly Indonesia [Citation4], China [Citation6,Citation7], and Mozambique [Citation3], where traditional food preservation methods involve the fermentation of grains, legumes, and coconuts. BA poisoning bears significant morbidity and mortality, with mortality rates up to 60% in past outbreaks [Citation4,Citation7]. Clusters have been traced back to communal consumption of contaminated products. However, the prevalence of BA poisoning is unknown due to underreporting, limited diagnostic capabilities, and inadequate surveillance. This report details a case of BA poisoning and death due to fulminant liver failure in a patient who ingested a homemade fermented cornmeal-based product.
Case report
A 67-year-old man with a past medical history of hypertension presented to an outside hospital with complaints of nausea, malaise, and fatigue two days following ingestion of home-fermented corn ogi, a Nigerian fermented cornmeal pudding. The patient’s spouse, who also consumed corn ogi but in a smaller amount than the patient, developed self-limited gastrointestinal symptoms and did not seek medical treatment. The patient died on hospital day 8. Consent for publication was provided by the patient’s spouse.
Clinical findings, timeline and diagnostic assessment
At the outside hospital, laboratory evaluation found AST 4425 U/L, ALT 7494 U/L, total bilirubin of 3.9 mg/dL, INR of 8.34 (), as well as metabolic acidosis. N-acetylcysteine and sodium bicarbonate infusions were initiated, and he was transferred to our institution the next day for liver transplant evaluation. His laboratories worsened to include AST 12091 U/L, ALT 15624 U/L, total bilirubin of 6 mg/dL, creatinine 4 mg/dL, and lactate 13.9 mmol/L. Workup for etiologies of liver injury, including viral hepatitis, acetaminophen toxicity, alcohol, Wilson’s disease, and autoimmune hepatitis, was negative. Despite a serum ammonia level of 620 mcmol/L, he had normal mentation. He was started on high-dose Continuous Renal Replacement Therapy (CRRT) to treat hyperammonemia, renal insufficiency, and metabolic acidosis. He received cryoprecipitate, fresh frozen plasma, and vitamin K. Abdominal ultrasound noted patent hepatic vasculature and normal hepatic echogenicity, and abdominal CT noted nonspecific heterogeneous attenuation of the liver.
Table 1. Laboratory Results. Reference values are listed below each test name.
On hospital day 3, he developed cyanosis with an oxygen saturation of 85% and elevated lactate while maintaining a PaO2 greater than 200 mmHg. His methemoglobin level was below diagnostic range, prompting the team to suspect a superimposed hemoglobinopathy. Glucose-6-phosphate dehydrogenase (G6PD) levels were in the low-normal range despite critical illness, raising concern for underlying G6PD deficiency, and prompting treatment with ascorbic acid and hyperoxia. Methemoglobin levels subsequently increased supporting a diagnosis of methemoglobinemia.
On hospital day 4, he developed grade II hepatic encephalopathy [Citation8] and was started on plasmapheresis. The hepatic encephalopathy worsened to grade IV on hospital day 5 despite ongoing CRRT. He received two rounds of plasmapheresis however developed distributive shock on hospital day 6. His clinical status worsened with the onset of atrial fibrillation and rapid ventricular rate, distributive shock refractory to vasopressors, and pancreatitis. He was not deemed to be a liver transplant candidate due to multisystem organ failure. Stool culture grew scant Bacillus cereus.
Post-mortem investigations
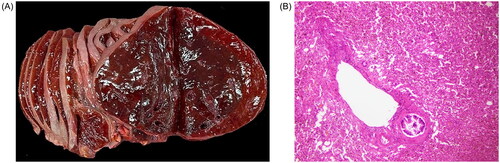
Autopsy demonstrated a liver with diffuse autolysis and parenchymal collapse, and a pancreas with hemorrhage and autolysis ().
Figure 2. Autopsy results. (A) Liver with diffuse softening, congestion and homogeneous dark red-brown cut surface and (B) confluent liver necrosis/autolysis with parenchymal collapse and mild fatty changes.
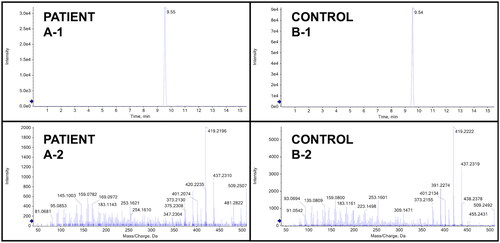
As no commercial assay was available, authors AJK, BKL, and SEW developed an assay using a method previously used on environmental samples by liquid chromatography quadrupole time-of-flight mass spectrometry. Blood collected on hospital day 2 returned a positive result in comparison to a standard reference material, with an estimated serum BA concentration of 1,000 ng/mL ().
Figure 3. Toxicology data from analytically confirmed bongkrekic acid ingestion. Panel A represents the data from the patient sample and panel B represents the data from a spiked control containing 2,000 ng/mL of bongkrekic acid. The top panes (-1) are extracted ion chromatograms and the bottom panes (-2) are high-resolution MS/MS fragmentation spectra, showing the data from the patient sample and the control are consistent.
Discussion
The history and symptoms observed in our patient are consistent with published cases of BA poisoning [Citation4]. However, the patient had a scantly positive stool culture for Bacillus cereus. B. cereus is known to produce cereulide, a mitochondrial toxin that can cause acute liver failure and microvesicular steatosis. It is produced under similar conditions to BA and can be found as a preformed toxin in similar foods [Citation9]. Prior reports of cereulide poisoning note that the cereulide-containing food had an unusual smell, and that the gastrointestinal symptoms appeared within minutes to hours [Citation10,Citation11]. In our patient, the lack of abnormal smell, late onset of gastrointestinal symptoms, and an estimated BA serum level multiple times higher than previously documented levels resulting in critical illness, all suggest that BA toxicity is the more likely diagnosis [Citation3].
No known antidote exists for BA toxicity; management is limited to symptomatic and supportive care. Death commonly occurs 1–20 h after symptom onset, with high mortality rates [Citation5]. The metabolism and toxicokinetics of BA are unknown, with little known regarding the toxic dose, metabolism, markers of likely mortality, or long-term effects in survivors [Citation4]. Our case holds significance as the first confirmed report of BA poisoning in North America and the first report outside of Asia or Africa. The home-fermented ogi was not available for testing but remains the likely source of the patient’s exposure. There is a need for increased awareness of the potential hazards associated with home fermentation of corn products, where conditions can facilitate the proliferation of B. cocovenenans. PCR testing for this bacteria, while potentially helpful, was not available in our case. Limited knowledge and diagnostic capabilities contribute to a missed diagnosis, and efforts should be directed toward expanded access to diagnostics and potential treatment options.
Conclusions
This case report highlights the importance of considering BA poisoning in patients with acute liver failure following exposure to fermented corn or coconut products, as well as the crucial connection between toxicology and public health. Increasing awareness among medical professionals and the general public is essential. Further efforts are needed to make testing and diagnosis more accessible, enhance the understanding of toxicokinetics and to develop potential treatment options.
Author contributions
LERB, DK, NT, ZH, ND, and JEC conceived and designed the study.
LERB, DK, NT, BF, DR, ZH, ND, AJK, BL, SW, IL, and MY prepared the first draft of the manuscript.
LERB, BF, DR, ZH, AJK, BL, SW, IL, MY and JEC conducted data curation, analysis, and interpretation.
All authors participated in critical revision of the manuscript for intellectual content, approved the final version for publication, and agree to be accountable for all aspects of the work.
Prior presentations
None.
Acknowledgements
The authors are grateful to Ram Subramanian MD, Stephanie Pouch MD, and Sandra Gjorgova MD, all of whom contributed to this report.
Disclosure statement
No potential conflict of interest was reported by the author(s)
Data availability statement
The data that support the findings of this study are available from the corresponding author, JEC, upon reasonable request.
Additional information
Funding
References
- Dimidi E, Cox SR, Rossi M, et al. Fermented foods: definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients. 2019;11(8):1806. doi: 10.3390/nu11081806.
- National Center for Biotechnology Information. PubChem Compound Summary for CID 6433556, Bongkrekic acid. 2024 Retrieved June 7, 2024 fromhttps://pubchem.ncbi.nlm.nih.gov/compound/Bongkrekic-acid.
- Gudo ES, Cook K, Kasper AM, Chitima Investigation Group., et al. Description of a mass poisoning in a rural district in Mozambique: the first documented bongkrekic acid poisoning in Africa. Clin Infect Dis. 2018;66(9):1400–1406. doi: 10.1093/cid/cix1005.
- Anwar M, Kasper A, Steck AR, et al. Bongkrekic acid-a review of a lesser-known mitochondrial toxin. J Med Toxicol. 2017;13(2):173–179. doi: 10.1007/s13181-016-0577-1.
- Shi R, Long C, Dai Y, et al. Bongkrekic acid poisoning: severe liver function damage combined with multiple organ failure caused by eating spoiled food. Leg Med (Tokyo). 2019;41:101622. doi: 10.1016/j.legalmed.2019.07.010.
- Zhang H, Guo Y, Chen L, et al. Epidemiology of foodborne bongkrekic acid poisoning outbreaks in China, 2010 to 2020. PLoS One. 2023;18(1):e0279957. doi: 10.1371/journal.pone.0279957.
- Yuan Y, Gao R, Liang Q, et al. A foodborne bongkrekic acid poisoning incident - Heilongjiang Province, 2020. China CDC Wkly. 2020;2(51):975–978. doi: 10.46234/ccdcw2020.264.
- Patton H, Misel M, Gish RG. Acute liver failure in adults: an evidence-based management protocol for clinicians. Gastroenterol Hepatol (N Y). 2012;8(3):161–212.
- Kim SA, Park HJ, Cho TJ, et al. Toxic potential of Bacillus cereus isolated from fermented alcoholic beverages. Food Res Int. 2020;137:109361. doi: 10.1016/j.foodres.2020.109361.
- Dierick K, Van Coillie E, Swiecicka I, et al. Fatal family outbreak of Bacillus cereus-associated food poisoning. J Clin Microbiol. 2005;43(8):4277–4279. doi: 10.1128/JCM.43.8.4277-4279.2005.
- Mahler H, Pasi A, Kramer JM, et al. Fulminant liver failure in association with the emetic toxin of Bacillus cereus. N Engl J Med. 1997;336(16):1142–1148. doi: 10.1056/NEJM199704173361604.
