Abstract
Dementia is related to disturbances in the sleep-wake pattern, behavioural and psychological symptoms of dementia (BPSD). These phenomena are the main reason for institutionalization. Assistive light technology is relevant to study, as mitigation of BPSD may allow for the improvement of quality of life for both people with dementia and their caregivers. Studies of dynamic light exposure in home-dwelling populations are scarce. In this single-case experimental design study, we evaluated the effects of exposure to dynamic light on the sleep-wake pattern and symptoms of depression, agitation, and anxiety in 11 home-dwelling people with dementia. A four-phase light-exposure therapy oscillating between the control and intervention waves was offered. Objective and questionnaire data were analysed and discussed. The results show that the used dynamic light system did not significantly affect the sleep variables. The severity of BPSD fluctuated in the expected pattern, reducing in intensity with increased light exposure. This pattern was significant for depression and agitation. This longitudinal study included an exploration of a low-cost assistive light intervention within a hard-to-study home-dwelling dementia population. The lessons learned are discussed and recommendations are made for future studies, as this design seems suitable for studying lifestyle interventions to support home-dwelling people with dementia.
Introduction
Behavioural and psychological symptoms of dementia (BPSD) and sleep disturbances have a significant impact on the quality of life of individuals with dementia and their caregivers (Barbe, Jolly, and Morrone Citation2018). This population could benefit from suitable and applicable lifestyle interventions to support them. Strong hopes have been attached to assistive technology, such as dynamic light exposure. This carefully timed light exposure, varying in intensity and/or spectral power distribution over time, resulting in modified activation of the biological clock (Kompier, Smolders, and de Kort Citation2020) has shown promise to help regulate sleep-wake patterns and reduce BPSD, although mainly studied in institutional contexts (Lieshout-van Dal, Snaphaan, and Bongers Citation2019). Similar effects were also reported for constant, higher light levels or short-wavelength enriched lighting in this demographic (Lieverse et al. Citation2011; CitationFigueiro et al. 2014). This study focuses on the potential benefit of improving the sleep pattern and reducing BPSD in people with dementia by a transportable dynamic light system.
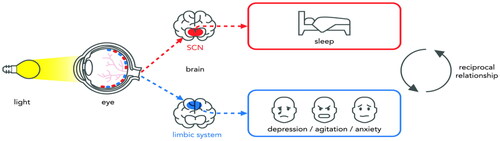
Multiple mechanisms may explain the effect of light on sleep and BPSD. Light information is projected directly to the biological clock, located in the suprachiasmatic nuclei (SCN) of the brain (Sollars and Pickard Citation2015). Disruption of the biological clock contributes to a range of health problems such as sleep disturbances, mood disorders, and even neuropsychiatric disorders such as dementia (Videnovic and Zee Citation2015).
Light may also have direct effects on mood and mental health via other retinal projections as shown in (Konjarski et al. Citation2018). Specifically, pathways to the brain structures of the limbic system have been identified, centres that are involved in regulating emotions and behaviour, mediating the effects of light on symptoms of depression, anxiety, and agitation (Fernandez et al. Citation2018; Vandewalle et al. Citation2011).
Figure 1. The influence of light on sleep, mood, and mental health via different pathways (adapted from Fernandez et al. Citation2018).
Sleep-wake pattern and light
Dementia can disturb the functioning of the biological clock more strongly than normal ageing can, leading to nightly wandering, daytime napping and sundowning, a phenomenon known as early-evening confusion and agitation (Hood and Amir Citation2017). In addition, people with dementia tend to spend less time outside. With less time spent outside, the biological clock is barely stimulated by natural daylight (Konis, Mack, and Schneider Citation2018).
A promising intervention to stimulate the biological clock is light exposure with the right specifications for light level, spectrum, timing and duration. The biological clock is maximally sensitive to short (blue) wavelengths (460–480 nm) and the light level required thus depends on the exact spectrum, but is substantially higher than is commonly installed in private housing for seniors (Aarts and Westerlaken Citation2005). Because of the increased sensitivity of the ageing eye to discomfort glare and blinding by light, standard light therapy methods are not suitable for older adults (Konis, Mack, and Schneider Citation2018). Light exposure adapted to the needs and preferences of older adults, is therefore a suitable alternative (Figueiro et al. Citation2015). In a recent systematic literature review, Kompier, Smolders, and de Kort (Citation2020) concluded that only a few studies on the effects of dynamic light scenarios have been conducted.
BPSD and light
BPSD is estimated to be prevalent in 90% of all patients with dementia over the course of their illness. BPSD is associated with distress among people with dementia and their caregivers, early institutionalization, and the misuse of medication (Magierski et al. Citation2020).
Clinically, BPSD can be classified into five symptom domains: cognitive, motor, verbal, vegetative, and emotional (Gerlach and Kales Citation2020). Our study focussed on the impact of dynamic light exposure on the emotional domain, specifically, symptoms of depression, agitation, and anxiety.
The prevalence of depression in people with dementia is estimated to be 30–40% (Kitching Citation2015). Based on a systematic review, Mitolo et al. (Citation2018) stated that the effects of light exposure on symptoms of depression in people with dementia show a general trend towards a positive effect, even in people with dementia still living at home.
The prevalence of agitation ranged from 68% in home-dwelling people with dementia to 80% in people with dementia living in a nursing home. Agitation is strongly associated with lower quality of life and increased medication use (Schmüdderich, Holle, and Ströbel Citation2021). A study by Onega, Pierce, and Epperly (Citation2016) showed promising results for light exposure on agitated behaviour in institutionalized people with dementia. To the best of our knowledge, no study has investigated the impact of light exposure on agitated behaviour in home-dwelling people with dementia.
The prevalence of anxiety varies from 8% to 81%. The large variance in these estimates may be due to the difficulty in operationalizing anxiety separately from symptoms such as depression and agitation in dementia (Kaiser et al. Citation2014). Kolberg et al. (Citation2021) found in a recent randomized controlled trial (RCT) that anxiety symptoms in people with dementia in a nursing home significantly improved after light exposure.
In conclusion, assistive light technology is considered a promising intervention for affecting the sleep-wake pattern and symptoms of depression, agitation, and anxiety in people with dementia. However, there is no standard solution available, and previous studies have shown large heterogeneity (Mitolo et al. Citation2018). Most studies lack a complete description and motivation of the light scenario, the study design and analysis of the results (Mitolo et al. Citation2018; Kolberg et al. Citation2021). Moreover, most previous studies were conducted in nursing homes. Despite their potential efficacy, only few studies were conducted at home (Figueiro et al. Citation2015; Lieshout-van Dal et al. Citation2021).
The purpose of this study was to investigate the effects of a transportable dynamic light system, offering a scenario simulating a daylight curve adapted to the needs and preferences of older adults, on the sleep-wake pattern and BPSD in a home-dwelling population of people with dementia. Positive effects are expected to be found in the intervention phases when people are exposed to dynamic light. These effects are expected for both the sleep-wake pattern and the symptoms of depression, agitation, and anxiety.
Material and methods
Participants
Participants were recruited using social media from September 2019 to June 2020. The inclusion criteria, assessed by a professional caregiver (i.e. geriatrist or psychiatrist), were (1) a primary diagnosis of dementia, based on Diagnostic and Statistical Manual-V (American Psychiatric Association Citation2013) criteria; (2) home-dwelling; (3) assessed sleeping problems; (4) a score >22 on the Mini Mental State Exam (MMSE), being mentally competent to decide for themselves to participate; (5) no visual disabilities and physical independence; and (6) an actively involved informal caregiver. We excluded patients if serious eye disease was diagnosed. No restrictions were imposed on medication use. All the participants used medication at the start of the study. Medication did not change during participation.
Thirteen participants and their informal caregivers received information and were willing to participate. Both signed a written informed consent form in accordance with the Declaration of Helsinki (Seoul Revision 2008) and General Data Protection Regulation (AVG). The rationale for including informal caregivers was to assist participants during the study in using wearables and devices. Two dyads decided to stop before data collection started because of personal circumstances. We obtained complete data for the four phases of 10 participants. One participant completed two out of the four phases and decided not to continue. presents the participants’ descriptions.
Table 1. Participant demographic variables at baseline (N = 11).
The study protocol was approved (23 April 2015) by the institutional review board of the Mental Health Care Institute Eindhoven (GGzE) and the Medical Ethics Review Committee (MERC, METC in Dutch) of Noord-Brabant (29 August 2015 P1826), Netherlands.
Design and procedure
In the current study, a single-case experimental design (SCED) was chosen in which the results were analysed by randomization testing, aiming to combine the advantages of an RCT with those of a real-life field study. In SCEDs, a small number of patients undergo repeated measurements during the control and intervention phases (Smith Citation2012). Although the external validity of the SCED is low, it combines high ecological and internal validity.
A four-phase reversal control-intervention setup (A1(regular_light) B1(intervention_light) A2(regular_light) B2(intervention_light)) was used to evaluate the effect of dynamic light exposure on sleep-wake variables and symptoms of depression, agitation, and anxiety (A1 vs. B1; A2 vs. B2), and the reversal effect of the removal of the dynamic light system (B1 vs. A2).
Participants started in the control phase A1 and received only natural daylight or light from their own light systems. Every phase had a duration of four weeks, because this time is needed to adjust the biological clock in people with dementia, although Sekiguchi, Iritani, and Fujita (Citation2017) showed effects within two weeks. To minimize carry-over effects between phases and minimize participant burden, only the last week of each phase was used for the sleep and light data collection. Similar exposure periods and measurement protocols were successfully implemented by for instance Figueiro et al. (Citation2014, Citation2019, or see Jao et al. Citation2022 for a review).
An off-the-shelf transportable dynamic lighting system was used. The lighting system followed a daylight curve in terms of timing, duration, level and spectrum. In our previous study, we found that this lighting system was effective in delivering significantly higher light intensities and correlated colour temperatures in both exposure phases than in the second baseline lighting phase (Lieshout-van Dal et al. Citation2021).
A timer switch was connected to all three light systems with a program tailored to the personal preferred day rhythm of each participant. Participants received a wearable light sensor button (LYS) that was connected to an app and placed as close to the eyes as possible, usually on the collar. The researcher installed the app on a smart device, and participants received instructions on how to use the app. During the last week of each phase (control or intervention), the participants wore the light sensor button from the moment they woke up until the moment they went to bed. During the study, a help desk was offered to resolve technical problems.
Testing measures
Dynamic light systems for home use
In this study, a Waldmann visual timing light lamp (VTL-lamp) and a LIFX-A60 light bulb, shown in , were used. The VTL-lamps were placed in the kitchen and living room near the seats where the participants spent most of the daytime. These luminaires provided both direct and indirect lighting.
Figure 2. The used lighting system: on the left the Waldmann VTL-lamp, on the right the LIFX dynamic light bulb.

The illuminance level and colour temperature of light varied dynamically throughout the day. The exact light levels on the eye depend on the exact seating position of the person, ceiling height, colouring, furniture shape, and texture, and are estimated in detail in Appendix Table A1. From 7 to 7:30 AM, the light level increased until its peak value of 2500 lx. It remained until 3:30 PM, when it gradually started to decline, reaching 0 lx at 9 PM. The correlated colour temperature (CCT) started high in the morning at 6000K and then gradually decreased to normal white light (4000K) and remained there until 4 PM. In the late afternoon and early evening, it slowly lowered to a warm, yellowish light (2500K). Measurements were taken horizontally, approximately at the height of the lap of a seated individual, and vertically, approximately at the height of the eyes of a seated individual. As light may have both visual and non-visual effects on people, pathways of which both start in the eye, but are driven by different photoreceptors in the retina (Kort and Veitch Citation2014), we report traditional light level (lx) and CCT (the colour appearance of white LEDs). Whereas visual experiences are driven mainly by the classical cone and rod receptors, the primary drivers of so-called non-visual effects are the ipRGCs, representing a photoreceptor class that was discovered some 20 years ago only and has a different sensitivity curve than the classical receptors (Berson, Dunn, and Takao Citation2002). Where full spectral data were available, we also therefore also report the melanopic equivalent daylight intensity (EDImel), as this captures the effective irradiance of the light (that is, including also the effect of the changing wavelengths in the light spectrum) for the biological clock most accurately according to the International Commission of Illumination (CIE) (CIE Citation2019).
The LIFX-A60 light bulb was placed on a bedside table in the bedroom and offered a 30 min wake-up scenario of exposure to 770 lumen and 7500K, corresponding to very cool light to boost the circadian rhythm.
Baseline light measurements
In both the control and intervention phases, in each individual’s home, light level, CCT, and EDImel were measured vertically at eye level in a baseline measurement in the morning between 9 AM and 12 AM using a Sekonic C-700 spectrometer. Measurements also included the contributions of daylight and additional lighting routinely used in homes. The results are presented in Table A2 in the Appendix. In all the rooms, the amount of light was significantly higher during the intervention phase. There were no significant differences in CCT between the two phases.
Personal light measurements
The LYS button, app, and data services were used to objectively measure the light level received by each individual participant. The button, shown in , uses a Bluetooth connection to connect to a smart device. It also contains an accelerometer as an indicator for movement. Data were sampled every 15 s.
The buttons were calibrated and tested before use. Lux data were 10-log transformed to correct for skewness. We assumed illuminance values below 10, while participants were moving, to be invalid. For situations in which people were not moving, illuminance below 5 was assumed to be invalid.
Individual light data from the light buttons were compared at individual and group levels between the phases. These data include light level (lx) and estimated CCT, based on red, green, blue (RGB) data (Lieshout-van Dal et al. 2021, supplementary material).
Our previous study (Lieshout-van Dal et al. 2021) demonstrated that the participants received significantly more light in the intervention phase than in the control phase. Unfortunately, as full spectral data could not be acquired with these wearable sensors, similar analyses could not be performed for EDImel. Furthermore, participants received light with higher colour temperature values in the intervention phase than in the second control phase.
Outcome measurements
Sleep-wake pattern
The sleep-wake pattern was measured using McRoberts MoveMonitor. The device was worn on an elastic strap on the lower back and objectively measures sleep movement, body posture, and physical activity during day and night. The move monitor has been validated (Gloeckl et al. Citation2015).
Data from seven consecutive days and nights during the last week of each phase were used. The primary measure was the number of minutes a participant had night rest. Night rest was recorded as the longest period of three hours or more, not interrupted for more than 30 min by another activity, such as a toilet visit or awakening. This was computed as the total number of minutes lying minus the total number of minutes of movement. Because the data were skewed, we used the median in the randomization tests. Secondary sleep measurements are transitions, times and duration out of bed, duration upright and movement.
Depression, anxiety, and agitation
Participants, along with professional caregivers, completed questionnaires on depression and anxiety. The informal caregivers completed a questionnaire on agitation.
Geriatric Depression Scale (GDS-15). The GDS-15 is a short 15-item instrument specifically designed to assess depression in geriatric populations (Yesavage, Brink, and Rose Citation1982).
Hospital Anxiety and Depression Scale, Anxiety Subscale (HADS-A). The HADS-A is a 7-item scale, frequently used for individuals with dementia (Zigmond and Snaith Citation1983).
Cohen-Mansfield Agitation Inventory (CMAI). The CMAI is a 29-item scale developed to assess agitation in institutionalized older adults (Cohen-Mansfield Citation1989).
Sum scores on each of the three scales were employed as indicators of BPSD: BPSDDepression, BPSDAnxiety, and BPSDAgitation.
Statistical analyses
As the number of participants was small, distributional assumptions of the parametric analysis were not warranted. Therefore, randomization tests were used to compare the phases. For the primary and secondary sleep-wake variables, we observed seven observations within each phase. The power to find a significant effect for just one individual was low because the number of measurements within each phase was relatively small. Therefore, we used a more lenient type-1 criterion of .1 to reject the null hypothesis at the individual level. In the randomization test, the median difference between the two phases was compared to a randomization distribution of median differences formed by random resampling, without replacement; all measurements were observed within the two phases for one participant. The p-value was then calculated by dividing the number of median differences from the randomization distribution that were equal or larger (or smaller when the observed median difference was negative) than the observed mean difference (Bouwmeester and Jongerling Citation2020).
Each p-value indicates whether a significant effect was found for each individual. By combining the results at the individual level, the meta-effects for all participants can be evaluated using a replicated single-case design.
For the BPSD variables, we used a between-subject randomization test to test the differences between phases and tested whether the expected trend was significant. The expected pattern showed a positive trend from phase A1 to phase B1, a negative trend from phase B1 to phase A2, and a positive trend when the light system was reintroduced in phase B2.
For each BPSD variable, we randomly resampled all observations of all phases for all participants to create a randomization distribution. The observed mean differences between the phases T0 and A1, A1 and B1, B1 and A2, and A2 and B2 were compared to the randomization distribution, and the p-value for each comparison was calculated by dividing the number of random mean differences that were equal to or larger than the observed mean difference. A type one-error rate of .05 was used as the criterion to reject the null hypothesis.
Results
Sleep duration and sleep disturbance
Primary and secondary measures were distinguished to investigate the effect of light intervention on sleep-wake patterns. Boxplots of every sleep variable are presented in Appendix A3.
shows the results of the randomization test for primary sleep variable minutes of night rest, shows a boxplot of the results. Although it was hypothesized that the minutes of night rest would increase during phase B1, this effect was not observed for most participants. The overall p-value was not significant (Sobs = 5.37, p = .447), indicating that the minutes of night rest did not differ between phases A1 and B1. Only one participant showed a significant increase in individual analyses. The expected decrease in minutes of night rest was also not found from phases B1 to A2 (Sobs = 4.88, p = .450), although there were significant differences for participants 3, 4, and 12 in the expected direction. Finally, no significant increase in the minutes of night rest was found from phases A2 to B2 (Sobs = 4.86, p = .440). None of the participants showed an expected significant increase in the number of minutes of night rest.
Figure 4. Boxplots of the median minutes of night rest for the four phases aggregated over participants.
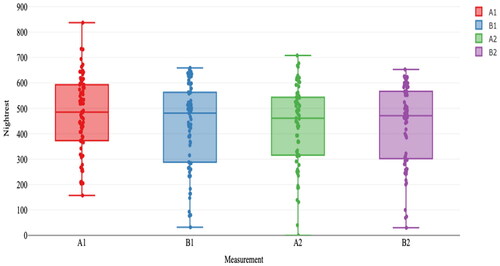
Table 2. Median minutes of night rest per phase per participant and the p-values from the randomization tests.
None of the secondary sleep variables at the group level showed significant differences between the phases (results in Appendix A4).
Depression, anxiety, and agitation
shows the results of the randomization test (mean and standard deviation results in Appendix A5). The randomization tests showed no significant differences between the phases, except for the CMAI scores between phases A1 and B1 (mean difference = 6.91, p = .02).
Table 3. Results of the Randomization Tests for Psychological Wellbeing Measures.
Many mean differences had the expected direction. For GDS-15, HADS-A, and CMAI, lower means were expected in B1 and B2 than in A1 and A2. These results showed a significant difference in the direction of the means of depression (GDS-15) and agitation (CMAI).
Discussion
General discussion
The purpose of this study was to investigate the effects of a transportable dynamic light system on sleep-wake patterns and BPSD in a home-dwelling population. Light studies focussing on this complex and vulnerable population are scarce or lack a complete description and analysis of the light scenario and results (Kompier, Smolders, and de Kort Citation2020).
In an earlier study, we demonstrated that despite the complexity of the study population and different individual circumstances, the light system was effective in delivering more light during the intervention phases. It was also demonstrated that the study design was suitable and applicable to this hard-to-study population (Lieshout-van Dal et al. Citation2021).
Findings on sleep
The sleep-wake pattern did not improve in the current study. Neither the primary measure duration of night rest nor the secondary measures differed between the control and intervention phases. This was not what we expected based on the results of previous studies. However, most of these studies were performed in care facilities (Figueiro et al. Citation2019) and not in real-life fields. Figueiro et al. (Citation2015) demonstrated the effects of lighting intervention in people with dementia living at home. In their field study, effects were shown on sleep and symptoms of depression. Similar to our study, seasonal effects had an impact on their results, and electric light could not compete with daylight. A possible, albeit partial, explanation could be that the participants in our study spent more time outside than inside during the first control phase. Lieshout-van Dal et al. (Citation2021) reported that the lighting system did result in more light in the homes of the participants, as well as higher actual individual light exposures; however, the latter increased modestly and not consistently across all participants, seasons, and times of day. Although this resulted in a significant increase in light dosage, the increase may have been too modest to induce meaningful effects on the sleep-wake rhythm. This implies that it may be useful to focus on how to get more light exposure to the eyes of people with dementia. This can be done by offering more intense lighting or by encouraging participants to spend more time sitting under the light system. It could also be done by giving lifestyle advice to participants, for example, leave the curtains open, take a morning walk outside, or sit by the window as much as possible.
Although we were unable to demonstrate the effects of dynamic light exposure on the sleep-wake pattern, one should also consider that there may be multiple causes for a reduced capability to achieve sufficient sleep, such as illness, life changes, environmental circumstances, and nutrition (Neikrug and Ancoli-Israel Citation2010). For example, in our study, one participant was sick during the intervention phase and spent considerable time in bed. Finally, it is possible that the sleep measurements in this study did not reliably reflect reality. Perhaps people slept more comfortably, but this may not be reflected by the way sleep quality was measured by the movemonitor belt. Methods to objectively measure sleep do not have the accuracy and reliability of polysomnography, used in laboratory sleep research. However, this is not a suitable measurement technique for field studies in this population.
Findings on BPSD-symptoms
An effect on BPSD symptoms was partly demonstrated in this study. Differences between the phases did not reach significance for the separate BPSD variables, except for agitation in the first intervention phase. However, the overall pattern between phases showed changes in the expected direction in every phase for all studied BPSD symptoms. This pattern was significant for symptoms of depression and agitation. This is an important finding as depression and agitation are known to have a severe impact on quality of life and caregiver burden (Schmüdderich, Holle, and Ströbel Citation2021; Barbe, Jolly, and Morrone Citation2018).
It may be somewhat striking that improvements in BPSD symptoms emerged without parallel improvements in sleep. However, recent findings, particularly in rodent-based research may shed light on this. For instance, LeGates, Fernandez, and Hattar (Citation2014) and Fernandez et al. (Citation2018) have reported that light influences emotion regulation directly via pathways starting in the ipRGCs, but projecting to regions other than the internal biological clock, targeting for example the lateral habenula (LHb), a region implicated in emotion regulation. What’s more, activity in the LHb was accompanied by changes in depression-like behaviours (LeGates, Fernandez, and Hattar Citation2014), and may even be necessary for the antidepressant effects of light (Huang et al. Citation2019). These mechanisms have to still be confirmed in human-based research however, but may explain also the findings in the current study.
The fact that not all symptoms showed significant improvement may be because BPSD-symptoms are difficult to influence over a short period of time. The treatment of these symptoms in dementia may require a long-term multiple treatment approach, such as light therapy with longer exposure periods, for example 8 weeks (Onega, Pierce, and Epperly Citation2016), combined with cognitive behavioural therapy (Maanen et al. Citation2016).
Strengths, limitations, and directions for future research
Our study sample was heterogeneous, similar to the population of older adults with dementia, implicating inter-individual variability such as lifestyle and type of dementia. An important strength of this study was the use of the SCED and its longitudinal setup. This design was chosen because it is suitable for the population of people with dementia and its ecological validity is considered high. It controls for individual non-specific treatment effects. Of course, not every non-specific effect can be controlled. For example, seasonality plays an important role. Despite the natural behaviour of spending time outside when the weather conditions are pleasant, and the fact that inside illuminance values do not equal the outside values, we were still able to find significant differences between the regular and intervention phases.
Several studies have demonstrated that light is a promising intervention for improving the sleep-wake pattern of older adults with dementia (Goodman et al. Citation2019). However, most studies were unable to demonstrate a significant positive effect of light exposure (Forbes et al. Citation2014; Sloane et al. Citation2015). These studies hypothesized that the light sources used did not have a sufficiently high light output to stimulate circadian entrainment. This suggests that people with dementia can be exposed to light systems with a greater light intensity. Alternatively, we can encourage people to spend more time using a light system. Additionally, the symptoms of dementia may have deteriorated during the study period, affecting the studied symptoms. In addition, life events can have an impact independent of the exposure phase. This emphasizes the importance of lifestyle recommendations. Recommendations as take a daily morning walk outside, place seating furniture close to the window, eat and drink healthy, use relaxation techniques, and meet other people.
Furthermore, our study was conducted during the COVID-19 pandemic. The impact of the pandemic on the results is unclear, but negative behavioural effects were expected, as people are forced to spend more time indoors. Future studies should consider the impact of seasonality on their designs. In addition, in this study, measurements were collected during the last seven days of each phase. More measurements in each phase can lead to a more complete dataset. Certain personal circumstances may have strongly influenced the dataset.
Conclusions
This paper contributes to the understanding of the impact of light exposure on people with dementia. The method adopted was effective in delivering more light during the intervention phases. Depression and agitation were observed to reduce in intensity in line with increased light exposure. Furthermore, this paper contributes to the understanding of designing for health interventions in real-life situations. The design of dynamic lighting scenarios aimed at enhancing vitality requires tailoring to the individual instead of the general population to create visually comfortable environments, as effects of for instance light level on visual comfort vary widely across individuals (Kompier, Smolders, and de Kort Citation2021). This implies that more studies on this heterogeneous sample can result in the ability to identify inter-individual variability and the development and testing of more personalized lighting scenarios. This could be a potentially valuable direction for future studies on the effects of dynamic light exposure on other symptoms of dementia. The design used demonstrated to be suitable for this purpose and could also be suitable for future studies on the impact of lifestyle interventions in this population.
Supplemental Material
Download MS Word (180.3 KB)Acknowledgements
We would like to thank all the people with dementia and their caregivers who took part in the study. We also like to thank Waldmann, LYS-technologies and McRoberts who made their products available for our study and for their non-committal technical and practical assistance during this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Notes on contributors
E. E. (Ellen) van Lieshout-van Dal
E. E. (Ellen) van Lieshout-van Dal is a clinical psychologist and family therapist and works at the Mental Health Care Organization Eindhoven (GGzE). She is a lecturer for postdoctoral study programs and PhD candidate at Tilburg University on the project Dynamic light and Dementia.
L. J. A. E. (Liselore) Snaphaan
L. J. A. E. (Liselore) Snaphaan is a clinical and experimental neuroscientist and works as a senior researcher at the mental Health Care Organization Eindhoven (GGzE). She is leading the program Light, Lifestyle and Green Mental Health of GGzE in which researchers collaborate with different stakeholders to enhance user-based innovative solutions for mental health care.
Y. A. W. D. (Yvonne) de Kort
Y. A. W. D. (Yvonne) de Kort is a professor in Environmental Psychology. With her group, she investigates the effects of daylight and electric light on human functioning, focussing mainly on alertness, performance, health and mental wellbeing.
S. (Samantha) Bouwmeester
S. (Samantha) Bouwmeester is a researcher in methodology and statistics. She works at Tilburg University and is consultant in statistical analysis and design. She specialized in single case experimental designs and created an application for the analyses of these designs.
I. M. B. (Inge) Bongers
I. M. B. (Inge) Bongers is a professor in Technological and Social Innovation for Mental Health. Her research area is on implementation and embedding of innovation in daily life and practice with a focus on human-centred design and multi-stakeholder collaboration.
References
- Aarts, M. P. J., and A. C. Westerlaken. 2005. “Field Study of Visual and Biological Light Conditions of Independently-Living Elderly People.” Gerontechnology 4 (3): 141–152. doi:10.4017/gt.2005.04.03.004.00.
- American Psychiatric Association. 2013. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: APA. doi:10.1176/appi.books.9780890425596.
- Barbe, C., D. Jolly, and I. Morrone. 2018. “Factors Associated with Quality of Life in Patients with Alzheimer’s Disease.” BMC Geriatrics 18: 159. doi:10.1186/s12877-018-0855-7.
- Berson, D. M., F. A. Dunn, and M. Takao. 2002. “Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock.” Science 295 (5557): 1070–1073. doi:10.1126/science.1067262.
- Bouwmeester, S., and J. Jongerling. 2020. “Power of a Randomization Test in a Single Case Multiple Baseline AB Design.” PLoS One.15 (2): e0228355. doi:10.1371/journal.pone.0228355.
- CIE. 2019. “Position Statement on Non-Visual Effects of Light – Recommending Proper Light at the Proper Time.” https://cie.co.at/publications/position-statement-non-visual-effects-light-recommending-proper-light-proper-time-2nd.
- Cohen-Mansfield, J. 1989. “Agitation in the Elderly.” Advances in Psychosomatic Medicine 19: 101–113. doi:10.1159/000417403.
- Fernandez, D. C., P. M. Fogerson, L. Lazzerini Ospri, M. B. Thomsen, R. M. Layne, D. Severin, and J. Zhan. 2018. “Light Affects Mood and Learning through Distinct Retina-Brain Pathways.” Cell 175 (1): 71–84. e18. doi:10.1016/j.cell.2018.08.004.
- Figueiro, M. G., C. M. Hunter, P. Higgins, T. Hornick, G. E. Jones, B. Plitnick, J. Brons, and M. S. Rea. 2015. “Tailored Lighting Intervention for Persons with Dementia and Caregivers Living at Home.” Sleep Health 1 (4): 322–330. doi:10.1016/j.sleh.2015.09.003.
- Figueiro, M. G., B. A. Plitnick, A. Lok, G. E. Jones, P. Higgins, and T. R. Hornick. 2014. “Tailored Lighting Intervention Improves Measures of Sleep, Depression, and Agitation in Persons with Alzheimer’s Disease and Related Dementia Living in Long-Term Care Facilities.” Clinical Interventions in Aging 9: 1527–1537. doi:10.2147/CIA.S68557.
- Figueiro, M. G., B. Plitnick, C. Roohan, L. Sahin, M. Kalsher, and M. S. Rea. 2019. “Effects of a Tailored Lighting Intervention on Sleep Quality, Rest-Activity, Mood, and Behavior in Older Adults with Alzheimer Disease and Related Dementias: A Randomized Clinical Trial.” Journal of Clinical Sleep Medicine 15 (12): 1757–1767. doi:10.5664/jcsm.8078.
- Forbes, D., C. M. Blake, E. J. Thiessen, S. Peacock, and P. Hawranik. 2014. “Light Therapy for Improving Cognition, Activities of Daily Living, Sleep, Challenging Behaviour, and Psychiatric Disturbances in Dementia.” The Cochrane Database of Systematic Reviews 2: CD003946. doi:10.1002/14651858.CD003946.pub4.
- Gerlach, L., and H. Kales. 2020. “Managing Behavioral and Psychological Symptoms of Dementia.” Clinics in Geriatric Medicine 36: 315–327.
- Gloeckl, R., T. Damisch, J. Prinzen, R. van Lummel, E. Pengel, U. Schoenheit-Kenn, and K. Kenn. 2015. “Validation of an Activity Monitor during Sleep in Patients with Chronic Respiratory Disorders.” Respiratory Medicine 109 (2): 286–288.
- Goodman, E., A. Milione, C. Mikus, E. Jacobs, A. Torres, V. Vu, O. Kaiser, and O. Potvin. 2019. “A Systematic Review: Light Therapy for Individuals with Dementia and Implications for Practice.” https://jdc.jefferson.edu/student_papers/35
- Hood, S., and S. Amir. 2017. “Neurodegeneration and the Circadian Clock.” Frontiers in Aging Neuroscience 9: 170. doi:10.3389/fnagi.2017.00170.
- Huang, L., Y. Xi, Y. Peng, Y. Yang, X. Huang, Y. Fu, Q. Tao, et al. 2019. “A Visual Circuit Related to Habenula Underlies the Antidepressive Effects of Light Therapy.” Neuron 102 (1): 128–142.
- Jao, Y. L., J. Wang, Y. J. Liao, J. Parajuli, D. Berish, M. Boltz, K. Van Haitsma, et al. 2022. “Effect of Ambient Bright Light on Behavioral and Psychological Symptoms in People with Dementia: A Systematic Review.” Innovation in Aging 6 (3): igac018. doi:10.1093/geroni/igac018.
- Kaiser, N. C., L. J. Liang, R. J. Melrose, S. S. Wilkins, D. L. Sultzer, and M. F. Mendez. 2014. “Differences in Anxiety among Patients with Early- versus Late-Onset Alzheimer’s Disease.” Journal of Neuropsychiatry and Clinical Neurosciences 26 (1): 73–80. doi:10.1176/appi.neuropsych.12100240.
- Kitching, D. 2015. “Depression in Dementia.” Australian Prescriber 38 (6): 209–2011. doi:10.1097/YCO.0b013e32834bb9d4.
- Kolberg, E., G. J. Hjetland, E. Thun, S. Pallesen, I. H. Nordhus, B. S. Husebo, and E. Flo-Groeneboom. 2021. “The Effects of Bright Light Treatment on Affective Symptoms in People with Dementia: A 24-Week Cluster Randomized Controlled Trial.” BMC Psychiatry 21: 377. doi:10.1186/s12888-021-03376-y.
- Kompier, M., K. Smolders, and Y. de Kort. 2020. “A Systematic Literature Review on the Rationale for and Effects of Dynamic Light Scenarios.” Building and Environment 186, 107326. doi:10.1016/j.buildenv.2020.107326.
- Kompier, M., K. Smolders, and Y. de Kort. 2021. “Abrupt Light Transitions in Illuminance and Correlated Color Temperature Result in Different Temporal Dynamics and Interindividual Variability for Sensation, Comfort and Alertness.” PLoS ONE 6 (3): e0243259. doi:10.1371/journal.pone.0243259.
- Konis, K., W. J. Mack, and E. L. Schneider. 2018. “Pilot Study to Examine the Effects of Indoor Daylight Exposure on Depression and Other Neuropsychiatric Symptoms in People Living with Dementia in Long-Term Care Communities.” Clinical Interventions in Aging 13: 1071–1077. doi:10.2147/CIA.S165224.
- Konjarski, M., G. Murray, V. V. Lee, and M. L. Jackson. 2018. “Reciprocal Relationships between Daily Sleep and Mood: A Systematic Review of Naturalistic Prospective Studies.” Sleep Medicine Review 42: 47–58. doi:10.1016/j.smrv.2018.05.005.
- Kort, Y., and J. Veitch. 2014. “From Blind Spot into the Spotlight.” Journal of Environmental Psychology 39: 1–4. doi:10.1016/j.jenvp.2014.06.005.
- LeGates, T. A., D. C. Fernandez, and S. Hattar. 2014. “Light as a Central Modulator of Circadian Rhythms, Sleep and Affect.” Nature Reviews Neuroscience 15 (7): 443–454.
- Lieshout-van Dal, E. E., L. J. A. E. Snaphaan, and I. M. B. Bongers. 2019. “Biodynamic Lighting Effects on the Sleep Pattern of People with Dementia.” Building and Environment 150: 245–253. doi:10.1016/j.buildenv.2019.01.010.
- Lieshout-van Dal, E. E., L. J. A. E. Snaphaan, S. Bouwmeester, Y. A. W. de Kort, and I. M. B. Bongers. 2021. “Testing a Single-Case Experimental Design to Study Dynamic Light Exposure in People with Dementia Living at Home.” Applied Sciences 11 (21): 10221. doi:10.3390/app112110221.
- Lieverse, R., E. J. van Someren, M. M. Nielen, B. M. Uitdehaag, J. Smit, and W. J. Hoogendijk. 2011. “Bright Light Treatment in Elderly Patients with Nonseasonal Major Depressive Disorder: A Randomized Placebo-Controlled Trial.” Archives of General Psychiatry 68 (1): 61–70. doi:10.1001/archgenpsychiatry.2010.183.
- Maanen, A., A. Meijer, K. B. van der Heijden, and F. J. Oort. 2016. “The Effects of Light Therapy on Sleep Problems: A Systematic Review and Meta-Analysis.” Sleep Medicine Reviews 29: 52–62. doi:10.1016/j.smrv.2015.08.009.
- Magierski, R., T. Sobow, E. Schwertner, and D. Religa. 2020. “Pharmacotherapy of Behavioral and Psychological Symptoms of Dementia: State of the Art and Future Progress.” Frontiers in Pharmacology 11: 1168. doi:10.3389/fphar.2020.01168.
- Mitolo, M., C. Tonon, C. La Morgia, C. Testa, V. Carelli, and R. Lodi. 2018. “Effects of Light Treatment on Sleep, Cognition, Mood, and Behavior in Alzheimer’s Disease: A Systematic Review.” Dementia and Geriatric Cognitive Disorders 46: 371–384.
- Neikrug, A., and S. Ancoli-Israel. 2010. “Sleep Disorders in the Older Adult: A Mini-Review.” Gerontology 56: 81–189.
- Onega, L. L., T. W. Pierce, and L. Epperly. 2016. “Effect of Bright Light Exposure on Depression and Agitation in Older Adults with Dementia.” Issues in Mental Health Nursing 37 (9): 660–667. doi:10.1080/01612840.2016.1183736.
- Schmüdderich, K., D. Holle, and A. Ströbel. 2021. “Relationship between the Severity of Agitation and Quality of Life in Residents with Dementia Living in German Nursing Homes – A Secondary Data Analysis.” BMC Psychiatry 21: 191. doi:10.1186/s12888-021-03167-5.
- Sekiguchi, H., S. Iritani, and K. Fujita. 2017. “Bright Light Therapy for Sleep Disturbance in Dementia is Most Effective for Mild to Moderate Alzheimer’s Type Dementia: A Case Series.” Psychogeriatrics: The Official Journal of the Japanese Psychogeriatric Society 17 (5): 275–281. doi:10.1111/psyg.12233.
- Sloane, P. D., M. Figueiro, S. Garg, L. W. Cohen, D. Reed, C. S. Williams, J. Preisser, and S. Zimmerman. 2015. “Effect of Home-Based Light Treatment on Persons with Dementia and Their Caregivers.” Lighting Research & Technology 47 (2): 161–176. doi:10.1177/1477153513517255.
- Smith, J. 2012. “Single-Case Experimental Designs: A Systematic Review of Published Research and Current Standards.” Psychological Methods 17 (4): 510–550. doi:10.1037/a0029312.
- Sollars, P. J., and G. E. Pickard. 2015. “The Neurobiology of Circadian Rhythms.” Psychiatric Clinics of North America 33: 395–401. doi:10.1038/nbt.3121.ChIP-nexus.
- Vandewalle, G., M. Hébert, C. Beaulieu, L. Richard, V. Daneault, M. L. Garon, and J. Leblanc. 2011. “Abnormal Hypothalamic Response to Light in Seasonal Affective Disorder.” Biological Psychiatry 70 (10): 954–961. doi:10.1016/j.biopsych.2011.06.022.
- Videnovic, A., and P. Zee. 2015. “Consequences of Circadian Disruption on Neurologic Health.” Sleep Medicine Clinics 10 (4): 469–480. doi:10.1016/j.jsmc.201.5.08.004.
- World Medical Association Declaration of Helsinki. 2001. “Ethical Principles for Medical Research Involving Human Subjects.” Bulletin of the World Health Organization 79 (4): 373–374. https://apps.who.int/iris/handle/10665/268312.
- Yesavage, J. A., T. L. Brink, and T. Rose. 1982. “Development and Validation of a Geriatric Depression Screening Scale: A Preliminary Report.” Journal of Psychiatric Research 17 (1): 37–49. doi:10.1016/0022-3956(82)90033-4.
- Zigmond, A. S., and R. P. Snaith. 1983. “The Hospital Anxiety and Depression Scale.” Acta Psychiatry Scandinavia 67 (6): 361–370. doi:10.1111/j.1600-0447.1983.tb09716.