ABSTRACT
Background: Reviews in pediatric chronic pain often focus on only one intervention or population, making it difficult for policymakers and decision makers to quickly synthesize knowledge to inform larger-scale policy and funding priorities.
Aims: The aims of this study were to (1) create an evidence and gap map of interventions for pediatric chronic pain and (2) identify gaps between existing evidence and recently identified patient-oriented research priorities.
Methods: We performed a systematic review of English-language peer-reviewed systematic reviews or clinical practice guidelines of pediatric chronic pain intervention published in the past 20 years. Database searches of Medline, Embase, PsycINFO, Web of Science, CINAHL, and SCOPUS were conducted inclusive to June 3, 2019. Review quality was assessed using the AMSTAR-2.
Results: Of 4168 unique abstracts, 50 systematic reviews (including 2 clinical practice guidelines) crossing diverse pediatric chronic pain populations and intervention settings were included. One third were rated high quality, with half rated low to critically low quality. The largest proportion of reviews addressed psychological and pharmacological interventions, followed by interdisciplinary, other (e.g., dietary), and physical interventions. Most common outcomes included pain, physical, emotional, and role functioning and quality of life. Treatment satisfaction and adverse events were less common, with minimal report of sleep or economic factors. Most patient-oriented research priorities had not been investigated.
Conclusions: Sufficient quality evidence is available to guide evidence-informed policies in pediatric chronic pain, most notably regarding psychological and pharmacological interventions. Numerous evidence gaps in patient-oriented research priorities and treatment outcomes should guide prioritization of research funds, as well as study aims and design.
RÉSUMÉ
Contexte: Les études sur la douleur chronique chez les enfants se concentrent souvent sur une seule intervention ou sur une seule population, de sorte qu’il est difficile pour les responsables politiques et les décideurs de synthétiser rapidement les connaissances afin d'éclairer les priorités de financement et les politiques à plus grande échelle.
Objectifs: Les objectifs de cette étude étaient (1) de créer une carte présentant les lacunes dans les données probantes en ce qui concerne les interventions pour la douleur chronique chez les enfants et (2) de répertorier les lacunes dans les données probantes existantes par rapport aux priorités de recherche axées sur le patient récemment déterminées.
Méthodes: Nous avons effectué une revue systématique des revues systématiques évaluées par des pairs ou des guides de pratique clinique portant sur les interventions en matière de douleur chronique pédiatrique publiés en langue anglaise au cours des 20 dernières années. Des recherches ont été menées dans les bases de données Medline, Embase, PsycINFO, Web of Science, CINAHL et SCOPUS jusqu’au 3 juin 2019 inclusivement. La qualité des revues a été évaluée à l’aide d’AMSTAR-2.
Résultats: Sur 4168 résumés uniques, 50 revues systématiques (dont deux guides de pratique clinique) portant sur diverses populations pédiatriques souffrant de douleur chronique et divers milieux d'intervention ont été incluses. Un tiers d’entre elles ont été jugées de haute qualité, tandis que la moitié était jugée de basse à très basse qualité. La majeure partie des revues portait sur des interventions psychologiques et pharmacologiques, tandis que les autres portaient sur des interventions interdisciplinaires, d’autres types d’interventions (ex.: nutritionnelles) et des interventions physiques. Les issues les plus courantes comprenaient la douleur, le fonctionnement physique, émotionnel et de rôle, ainsi que la qualité de vie. La satisfaction à l'égard du traitement et les effets indésirables étaient moins souvent abordés, tandis que le sommeil et les facteurs économiques étaient peu mentionnés. La majeure partie des priorités de recherche axées sur les patients n'avait pas fait l’objet d’études.
Conclusions: Il existe suffisamment de données probantes de qualité pour guider les politiques fondées sur des données probantes en matière de douleur chronique pédiatrique, surtout en ce qui concerne les interventions psychologiques et pharmacologiques. L’existence de nombreuses lacunes dans les données probantes concernant les priorités de recherche axées sur le patient et les issues de traitement devrait guider la hiérarchisation du financement de la recherche, ainsi que les objectifs et la conception des études.
Chronic pain is a leading cause of disability and morbidity for children and adults.Citation1 Despite this, chronic pain was only recently recognized as its own disease through the inclusion of new chronic pain diagnostic codes in the World Health Organization’s International Classification of Diseases, 11th revision, in 2018.Citation2 The classification of chronic primary pain as a disease in its own right facilitates the progress of large-scale national policies to improve chronic pain management across the life span, many of which are underway in countries around the world.Citation3–5 Furthermore, an evidence-based policy focus on improved chronic pain management is critical alongside policy efforts to address the opioid epidemic.Citation6
The development of effective health policies is informed by both scientific evidence and stakeholder experience to ensure relevance and tailored implementation to the local context. Our group recently completed a national priority-setting partnership that engaged people with lived experience with pediatric chronic pain, family members, and treating health care providers across Canada to identify the top priorities for pediatric chronic pain research and care.Citation7 The final top ten identified patient-oriented priorities direct the need for more evidence on prevention and treatment, as well as an improved understanding of the impact of pediatric chronic pain, delivery, access to care, and coordination of care. On its own, this priority-setting work provides a guided call to action for policymakers, decision makers, and researchers—from basic science to clinical research to health systems design—to address identified patient-oriented priorities; however, the uptake of these priorities may be limited by a lack of information about what research already exists in these areas. Current systematic reviews in pediatric chronic pain are often very niche and focus on one type of intervention.Citation8–10 This makes it difficult for policymakers and decision makers to quickly synthesize knowledge across a variety of intervention modalities to inform clinical practice policy and research funding priorities. This is problematic given the current recommendation for multimodal care to thoroughly address biopsychosocial contributors to pediatric chronic pain.Citation11
Although pediatric pain research is growing rapidly,Citation12 there remains a disconnect between existing scientific evidence and current clinical practice, a further challenge to developing pediatric chronic pain policy.Citation13 Estimates suggest that it can take up to 17 years for research to impact patient care,Citation14 and many children and adolescents with chronic pain struggle to access evidence-based treatment.Citation15–17 There is an identified need for more effectual and efficient knowledge mobilization in pediatric pain.Citation13 The availability of high-quality evidence synthesis is a key step in the process of moving generated scientific knowledge into sustainable action, as outlined in the knowledge-to-action framework.Citation18 Evidence and gap maps have emerged as an effective knowledge translation evidence synthesis tool to inform evidence-informed policymaking and the development of strategic research agendas.Citation19,Citation20 Like other evidence synthesis methods, such as a Cochrane reviews,Citation21 evidence and gap maps are rigorous in their search for and assessment of research evidenceCitation19,Citation22; however, they differ in their primary goal, which is to review the breadth and quality of available evidence compared to determining the efficacy of single specific interventions. This shift in focus facilitates the strategic identification of key gaps where little or no evidence exists or areas currently with only poor quality research.Citation19,Citation22 The use of schematic visual representation of findings in evidence and gap maps also makes research evidence more easily accessible and usable to researchers and decision makers.Citation19,Citation22
Our primary goal was to create a contemporary evidence and gap map of systematic reviews of all interventions for pediatric chronic pain. Our secondary aim was to identify gaps between existing evidence and recently identified patient-oriented research priorities for pediatric chronic pain.Citation7 Given that many pediatric chronic pain interventions have limited evidence,Citation9,Citation23 we expected many priority areas to be lacking high-quality research evidence.
Methods
Protocol and Registration
This review adheres to PRISMA reporting guidelines for systematic reviews.Citation24 A protocol was registered for this review in February 2018 on PROSPERO: CRD42018086817. The current review presents a minor modification from our original review protocol that outlines our initial intent to conduct a traditional overview of systematic reviews.Citation25 The decision to modify the current review to an evidence and gap map was made following consultation with an international expert in evidence-informed health policy (Dr. John Lavis, personal communication, April 17, 2019) and in response to emerging national chronic pain policy efforts through the development of the Government of Canada’s Canadian Pain Task Force.Citation3 It was felt that an evidence and gap map would better achieve our primary goal of uptake of evidence and patient priorities by policymakers and decision makers and identify clear gaps to guide research efforts in key areas identified by patients and clinicians as priorities.
Evidence and gap maps provide an overview of the availability and quality of evidence of a sector, in this case interventions for pediatric chronic pain.Citation19,Citation20,Citation22,Citation26 Recommended evidence and gap map methodology includes completion of six primary steps: development of scope, inclusion criteria, systematic review of the literature, data extraction, analysis, and visualization.Citation19,Citation22,Citation27 Evidence and gap maps are underpinned by a rigor similar to that of other systematic review methodology.Citation28 As such, reporting of the evidence and gap map methods in this article adhere to the PRISMA statement for reporting of systematic reviews and meta-analyses.Citation24 The current review adheres to our original protocol with regards to the stated review question, search strategy, type of study, participants/population, interventions, risk of bias/quality assessment, narrative synthesis, and report by type of intervention modality. The current review diverges only from that outlined in our original protocol in that the current review now is restricted to publications within the past 20 years (since 1999), no longer extracts specific efficacy findings for intervention outcomes, and does not conduct subgroup analyses by type of chronic pain condition; additionally, the review now includes an evidence and gap map.
A completed evidence and gap map provides a simple and accessible visual summary of existing systematic review evidence for various types of interventions in pediatric chronic pain across selected outcome domains.Citation19,Citation20,Citation22,Citation26 The rows of the evidence and gap map list the types of interventions and the columns list the outcome domains. Each cell shows the number and quality of systematic reviews that contain evidence on that combination of intervention and outcome domain. In doing so, evidence and gap maps identify areas where little or no evidence exists (“absolute gaps”) and areas where there is systematic review evidence is available but is either out of date and/or of poor quality (“synthesis gaps”).Citation19,Citation20,Citation22,Citation26 Evidence and gap maps can be used to inform strategic research investment by highlighting intervention and outcome areas where new primary studies and/or systematic reviews can add value. They can also be used to inform decision making by capturing best available evidence that can then be compared against existing policy or programming to inform discussions about areas of prioritizing future research, policy, or investment.Citation19,Citation20,Citation22,Citation26
Eligibility Criteria
Papers were eligible for inclusion if they
were peer-reviewed published systematic review or clinical practice guidelines;
were published in English;
included at least 50% or more reviewed studies focused on children or adolescents ≤18 years old or reported findings from pediatric studies separately;
included randomized and nonrandomized studies focused on any intervention for any type of chronic pain (defined as pain lasting at least 3 months or longer and/or pain described as “chronic,” “recurrent,” or “persistent”); and
reported on at least one primary or secondary outcome included in PedIMMPACT recommended for clinical trials in pediatric chronic pain (that is, pain intensity, physical functioning, emotional functioning, role functioning, quality of life, sleep, global treatment satisfaction, economic factors, and/or adverse events).Citation29
Systematic reviews were excluded if they focused exclusively on chronic pain diagnosis or assessment and/or only reported prevalence. Prior iterations of eligible reviews were also excluded, as well as reviews/clinical practice guidelines published >20 years ago, given the availability of more current up-to-date evidence. Reviews including any type of intervention study design were included given recognized difficulty in conducting randomized controlled trials (RCTs) for some intervention modalities in pediatric populations (e.g., pharmacological) and given that interventions needed to address identified patient-oriented research priorities may not lend themselves easily to randomized study designs (e.g., school-based interventions).
Given variability in evidence synthesis methodology, requirements for being defined as a systematic review and/or clinical practice guideline were drawn from those used by the James Lind Alliance.Citation30 Thus, a systematic review was defined as a review that attempts to identify, appraise, and synthesize all of the empirical evidence that meets prespecified eligibility criteria to answer a given research question. Therefore, a systematic review typically states/identifies a research question, provides search terms, searches multiple scientific databases, and reviews titles, abstracts, and full-text publications against some identified inclusion criteria. Clinical practice guidelines are clearly defined as such and include a systematic review to inform development of the guidelines. If multiple iterations or updates of the same systematic review or clinical practice guideline were found and identified as such, only the most recently published version of the systematic review or clinical practice guideline meeting the eligibility criteria was reviewed to reflect the most up-to-date evidence.
Search Strategy and Conduct
Searches were conducted in Medline, Embase, PsycINFO, Web of Science, CINAHL, and SCOPUS from database inception to June 3, 2019. Database search strategies were developed in collaboration with a pediatric medical librarian and experts in pediatric chronic pain care and research. A sample comprehensive search strategy for Medline is available in Supplementary Online Material 1.
Study Selection
Database search results were imported into CovidenceCitation31 for study selection. Initial abstract screening was conducted independently by two review authors (K.A.B. and T.D.A.), and full-text screening was independently performed by two review authors (C.O. and T.D.A.), with conflicts resolved by a third author (K.A.B.).
Data Extraction and Quality Ratings
Data were independently extracted by two review authors (K.A.B. and T.D.A.). Extracted data items included review sponsorship, country, author, primary review objective, inclusion/exclusion criteria, date of literature search, inclusion of meta-analysis, types of reviewed studies (RCT or nonrandomized study [NRS]), total number of reviewed studies, number of reviewed studies focused on pediatric chronic pain intervention, population of reviewed studies (type of chronic pain/disease), setting of reviewed studies (e.g., outpatient, inpatient, emergency, etc.), type of intervention (pharmacological, psychological, physical, interdisciplinary, other), comparator groups, inclusion of quality of evidence rating, inclusion of PedIMMPACTCitation29 recommended outcomes (pain intensity, physical functioning [e.g., mobility, disability], emotional functioning [e.g., anxiety, depression], role functioning [e.g., school attendance], quality of life, sleep, global treatment satisfaction, economic factors [e.g., cost, health care utilization, parent missed workdays], and/or adverse events), and time of outcome assessments.
When eligible systematic reviews included nonrelevant data (that is, pertaining to adults and/or nonchronic pain pediatric samples), only data relevant to reviewed studies focused on pediatric chronic pain were extracted. Supplementary material was accessed to inform data extraction and quality assessment if cited in published eligible systematic reviews. Separately reported systematic reviews that informed eligible clinical practice guidelines were accessed online (published and unpublished) and informed data extraction and quality assessment.
Risk of bias/quality of assessment of all eligible systematic reviews was conducted using the AMSTAR-2.Citation32 The AMSTAR-2 critical appraisal tool includes 16 items that are rated to assess the quality of systematic reviews that include randomized or nonrandomized studies of health care interventions or both. Items address the review’s reporting of review criteria including elements of PICO (Population, Intervention, Comparator group, and Outcome), a priori review protocol registration, justification of study design selection, adequacy of literature search, study selection and data extraction in duplicate, justification for excluding individual studies, adequate description of included studies, risk of bias from individual studies included in the review, report of funding of included studies, appropriateness of meta-analytical methods (if applicable), consideration of risk of bias when interpreting the review results, and assessment and likely impact of publication bias (italics denote critical domains). Quality assessments for all eligible systematic reviews were rated independently by two authors (K.A.B. and C.O.), with disagreements resolved by consensus. Each systematic review was summarized by an single overall quality rating reflecting confidence in the results of review.Citation32 Overall quality ratings are described as follows:
High: No or one noncritical weakness; the systematic review provides an accurate and comprehensive summary of the results of the available studies that address the question of interest.
Moderate: More than one noncritical weakness; the systematic review has more than one weakness but no critical flaws. It may provide an accurate summary of the results of the available studies that were included in the review.
Low: One critical flaw with or without noncritical weaknesses; the systematic review has a critical flaw and may not provide an accurate and comprehensive summary of the available studies that address the question of interest.
Critically Low: More than one critical flaw with or without noncritical weaknesses; the systematic review has more than one critical flaw and should not be relied on to provide an accurate and comprehensive summary of the available studies.
Eligible systematic reviews and clinical practice guidelines were also independently coded by two authors (K.A.B. and C.O.) for relevance to each of the top ten patient-oriented research priorities for pediatric chronic pain identified in the Partnering For Pain priority-setting partnership.Citation7 In brief, Partnering For Pain engaged hundreds of diverse Canadians with lived experience with pediatric chronic pain, family members, and multidisciplinary health care providers across four priority setting phases using the James Lind Alliance Priority Setting Partnership methodology. The James Lind Alliance methodology is recognized as being robust, strategic, objectively based and inclusive, and promoting equity in patient voices.Citation33 In phase 1, 215 Canadians (86 patients [40.0%], 56 family members [26.0%], and 73 health care providers [34.0%]) submitted 540 potential priorities that were developed into 112 unique research questions (phase 2). Of the 112 questions, 63 were rated for importance by 57 participants (19 patients [33%], 17 family members [30%], and 21 health care providers [37%]) in phase 3. In phase 4, 20 participants (6 patients [30%], 6 family members [30%], and 8 health care providers [40%]) discussed the 25 most highly rated questions and reached consensus on the final top ten.Citation7 The participant group was diverse with regards to age, sex, ethnicity, geographic location, chronic pain condition, care setting, and health care profession. A thorough discussion about the rationale, methodology, findings, and limitations of the Partnering For Pain priority-setting partnership is available in our previous peer-reviewed publication.Citation7
Results
Study Selection
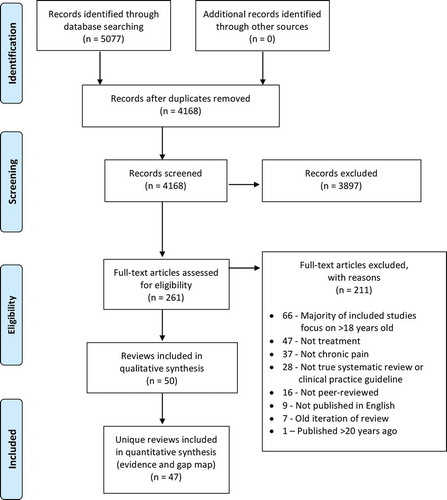
Database searches identified 5077 records. After duplicates were removed, 4168 unique abstracts remained for review. Of these, 3897 were deemed not eligible. A total of 261 full texts were reviewed and 211 were excluded. Fifty full texts met inclusion criteria. See for the PRISMA review flowchart, including reasons for full-text exclusion.
Study Characteristics
Of the 50 full texts meeting review inclusion criteria, 2 reported a related systematic review and clinical practice guideline for pediatric chronic abdominal painCitation34,Citation35 and 2 reported a related systematic review and clinical practice guideline for pediatric chronic widespread pain.Citation36,Citation37 Data extraction and quality assessment were combined for each pair of related systematic reviews and clinical practice guidelines. One additional paperCitation38 was a summary of 3 other included systematic reviews.Citation10,Citation39,Citation40 Data extraction and quality ratings were not conducted for the summary review because information was obtained from the original included systematic reviews. Thus, data and quality ratings are reported for 47 unique reviews/clinical practice guidelines. Of these, 24 (51.6%) included meta-analyses. Almost half of the reviews reported no funding or sponsorship (n = 22; 46.8%). See for characteristics and outcomes for each included review.
Table 1. Characteristics of included reviews
Table 2. Interventions and outcomes of included reviews
Types of Populations
Most reviews (n = 19; 40.4%) included variations of mixed chronic pain populations (e.g., abdominal pain, headaches or migraines, widespread pain/fibromyalgia, complex regional pain syndrome, neuropathic pain, sickle cell disease, cancer pain, back pain, and/or pelvic pain).Citation8,Citation37,Citation41–58 Reviews focused on single populations most frequently examined abdominal pain (n = 10; 21.3%),Citation10,Citation35,Citation39,Citation40,Citation57,Citation59–61,Citation77,Citation78 headaches or migraines (n = 5; 10.6%),Citation62–66 rheumatological conditions (e.g., juvenile idiopathic arthritis, lupus; n = 4; 8.5%),Citation67–70 cancer-related pain (n = 3; 6.4%),Citation71–73 or sickle cell disease (n = 2; 4.3%).Citation74,Citation75 Single reviews focused on patellar tendon pain/Osgood-Schlatter’s (n = 1; 2.1%),Citation76 cerebral palsy (n = 1; 2.1%),Citation80 endometriosis (n = 1; 2.1%),Citation79 or joint hypermobility/Ehlers-Danlos/osteogenesis imperfecta (n = 1; 2.1%).Citation81
Reviews included children 2–18 years old. Most reviews included studies crossing childhood and adolescence (n = 45; 95.7%), with two reviews (4.3%) focused on adolescents (>12–18 years old).Citation41,Citation75 Five reviews also included studies with adults (>18 years old).Citation48,Citation50,Citation70,Citation74,Citation75
Types of Settings
Reviews included studies conducted in a variety of settings, including primarily tertiary care or hospital settings (inpatient, day treatment, outpatient clinics, and emergency departments), followed by primary care or community-based clinics and, rarely, schools. Three reviews (6.4%) focused exclusively on “e-health” or remotely delivered interventions.Citation47,Citation56,Citation75 The setting was not clearly reported in 12 (25.5%) reviews.
Types of Studies Included
The majority of reviews exclusively included RCTs or reviews of RCTs (n = 26; 55.3%). The remaining reviews included a variety of study designs, including nonrandomized intervention studies, cohort or observational studies, retrospective chart reviews, and case studies or case series (n = 21; 44.6%). Most reviews included at least one study with a comparator group (n = 41; 87.2%). Comparator groups included usual/standard medical care, waitlist controls, placebo or sham interventions, or other active interventions.
Types of Interventions
Though some reviews focused on singular types of intervention, others focused on varied types of treatment for a particular pain population or setting. Almost half of the reviews examined psychological interventions (n = 23; 48.9%), with 19 (40.4%) reviewing pharmacological interventions, 12 (25.5%) reviewing interdisciplinary interventions, 11 (23.4%) reviewing “other” interventions, and 7 (14.9%) reviewing physical interventions. The “other” types of treatments reviewed were primarily dietary (e.g., fiber, lactose avoidance), botanicals (e.g., peppermint oil, herbal therapy), and surgical interventions.
Types of Outcomes
Three reviews of pharmacological interventions found no eligible studies for inclusionCitation8,Citation72,Citation73; as such, extraction of assessed outcomes was not possible for those reviews. Of the remaining 44 reviews, all (100%) reported on pain intensity, 27 (61.3%) reported on physical functioning, 20 (45.5%) reported on emotional functioning, 20 (45.5%) reported on role functioning, 21 (47.7%) reported on quality of life, 8 (18.2%) reported on sleep, 5 (11.4%) reported on economic factors, 13 (29.5%) reported on treatment satisfaction, and 20 (45.5%) reported on adverse events. Time points for outcome reporting ranged from immediately postintervention to hours, days, weeks, months, or up to 5 or more years later. Most reviews included some sort of risk of bias or quality ratings of included studies (n = 32; 72.7%).
Quality of Systematic Reviews
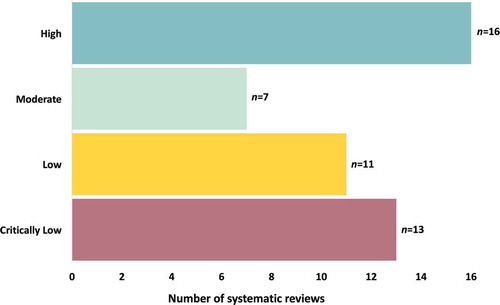
See for a summary of the AMSTAR-2 quality ratings for the included reviews. Of the 47 reviews, the greatest number were rated as high quality (n = 16; 34.0%), followed by critically low quality (n = 13; 27.7%) and low quality (n = 11; 23.4%), with the fewest rated as moderate quality (n = 7; 14.9%). Reviews were primarily downgraded in quality for failing to register a review protocol or demonstrate clear evidence of review methods established a priori or failing to provide a list of excluded studies with justification, with fewer studies failing to use a comprehensive literature strategy, failing to include a satisfactory technique for assessing risk of bias, or failing to account for risk of bias in the interpretation of review results.
Synthesis of Results
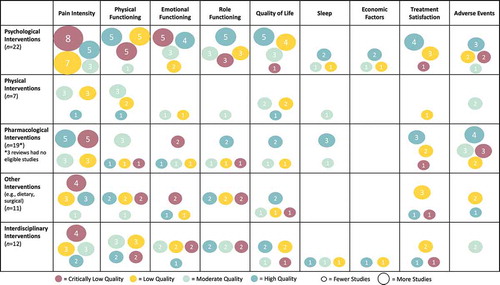
See for the evidence and gap map summarizing the quality and number of included reviews relevant to each extracted treatment outcome of interest.
Figure 3. Evidence and gap map of interventions for pediatric chronic pain. The figure rows list the types of interventions and the columns list the PedIMMPACT outcome domains. Each cell shows the number and quality of included systematic reviews as assessed using AMSTAR-2 that contain evidence on that combination of type of intervention for pediatric chronic pain and outcome domain
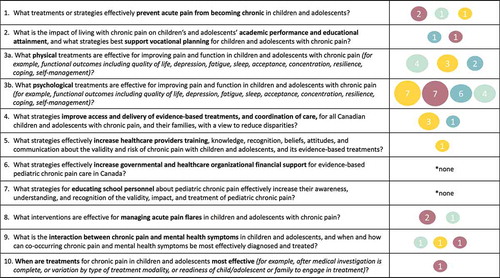
Additional Analyses: Mapping to the Top Ten Patient-Oriented Research Priorities
See for a summary of the quality and number of included reviews relevant to each of the top ten patient-oriented research priorities for pediatric chronic pain. All but two priorities had at least one relevant review and/or clinical practice guideline. Priority 3 (physical and psychological interventions) had the greatest number of relevant reviews (n = 9; 19.1% and n = 24; 51.1%, respectively), albeit primarily from reviews of low and critically low quality. Priority 1 (prevention of chronic pain) and priority 4 (improved access and delivery) were addressed by four reviews each (8.5%), and priority 2 (impact on education and vocational planning), priority 8 (managing acute pain flares), and priority 9 (treatment of co-occurring mental health symptoms) were addressed by only two to three reviews each (4.3–6.4%). Priority 5 (increase health care providers’ knowledge) and priority 10 (timing of interventions) had only one relevant review each (2.1%), and priority 6 (increase government and organization financial support) and priority 7 (educating school personnel) had no relevant reviews. Almost one third of included reviews and clinical practice guidelines did not address any of the patient-oriented research priorities (n = 15; 31.9%).
Discussion
Summary of Evidence
This systematic review offers a rigorous synthesis of available systematic reviews and clinical practice guidelines of interventions of any modality for pediatric chronic pain. The resulting evidence and gap map offers a succinct but thorough data visualization to effectively convey the current state of the evidence for use by key stakeholders, including members of the public, policymakers and decision makers, health care providers, and researchers alike. The broad scope of this review across intervention modalities and pediatric chronic pain populations, as well as its evidence and gap map methodology, uniquely positions its findings to be quickly and easily utilized.
This review reveals much about the contemporary state of synthesized evidence of interventions for pediatric chronic pain. It is promising for policymakers that many high-quality reviews exist to guide decisions (most of which were Cochrane reviews); however, more than half (55%) of included reviews were rated to be of low or critically low quality. It was surprising that only two clinical practice guidelines were identified. Most systematic reviews examine psychological interventions only, followed closely by pharmacological interventions. The sizable study of psychological interventions for pediatric chronic pain is promising given its prioritization among patients, family members, and treating health care providersCitation7 but stands in stark contrast to the generally poor access to specialized multidisciplinary pediatric chronic pain interventionCitation17 or mental health treatment.Citation82 Three reviews focused on the remote or computerized delivery of psychological interventions.Citation47,Citation56,Citation75 Far fewer systematic reviews examined interdisciplinary interventions despite this being the recommended approach to chronic pain management,Citation83 followed by reviews of other interventions such as alternative diets, herbal supplements, and surgeries. The fewest reviews examined physical interventions, which highlights this as a key area for further research given its prioritization by patients and families,Citation7 as well as the evidence for multimodal interventions, of which physical interventions are included. Possible contributing factors for less evidence in these areas could be their greater difficulty in studying with traditional clinical trial methodologies and fewer professionals in areas outside of medicine and psychology with advanced training to conduct research.
The largest proportion of reviews included diverse pediatric chronic pain populations. This suggests the applicability of many interventions across types of chronic pain and aligns with an all-encompassing primary chronic pain diagnosis.Citation2 Reviews with medically complex children and adolescents were largely absent, with the exception of cerebral palsy.Citation80 No reviews obviously addressed interventions for children with cognitive or intellectual disabilities or those who are nonverbal, which is of concern given their greater risk for undertreated and poorly recognized pain.Citation84 When reviews focused on single patient groups, headaches and migraines or abdominal pain were the most common, possibly reflecting their higher prevalence rates.Citation85 Reviews of interventions for pediatric migraines and headaches offered unique contributions and alignment with patient-oriented priorities not well addressed by other evidence, including a focus on prevention (prophylaxis) and management of acute pain flares. Only one review focused on interventions in the emergency department.Citation63 This is of great relevance given the high frequency with which children with chronic pain seek care in the emergency setting,Citation63 its high economic cost, the use of opioids to treat acute pain, and the potential for interdisciplinary care to reduce utilization of emergency care.Citation86,Citation87 Other reviews largely addressed interventions in outpatient or community clinics or within tertiary care centers.
With regards to intervention impact, all reviews addressed the PedIMMPACTCitation29 recommended outcome of pain intensity, with fewer reporting on outcomes related to physical (disability, mobility), emotional (anxiety, depression), and role functioning (school attendance) or quality of life. Fewer still reported outcomes of treatment satisfaction or adverse events, with very little about sleep or economic factors. This reflects a neglect of outcomes identified as relevant by patients, family members, and treating health care providers, such as self-efficacy, participation in meaningful activities, social roles and relationships, vocational planning, concentration, acceptance, and resilience.Citation7,Citation88 Although almost half of reviews addressed emotional functioning, many excluded children with co-occurring primary mental health disorders. Thus, these reviews effectively omitted a large proportion of children with chronic pain with mental health concernsCitation89 and decreased the relevance of available evidence to the identified patient-oriented priority about how co-occurring chronic pain and mental health can be effectively addressed.Citation7 Given that the estimated annual incremental costs of treating an individual with chronic pain are CA$1742 per person, costing billions to society overall,Citation86 there is a clear need to better demonstrate the economic benefit of evidence-based interventions to guide policymakers and decision makers. Though this review focused on previously recommended key outcome categories for clinical trials of interventions for pediatric chronic pain,Citation29 we note that this approach is likely to miss all outcomes included in the systematic reviews, clinical practice guidelines, or the original studies they include. Other than physical and psychological interventions, less than 10% of included reviews addressed any of the other top ten patient-oriented priorities. The movement toward patient engagement and partnership in health research offers a great opportunity to lessen the divide between existing intervention studies and outcomes and that of patient priorities.Citation13,Citation90,Citation91 Effectiveness-implementation hybrid research designs are gaining traction to enhance public health impact through efficient, feasible, sustainable, and widespread adoption of studied treatments.Citation13,Citation92,Citation93
Limitations
Several limitations warrant mention in considering the above presented evidence. First, this review and evidence and gap map included published systematic reviews and clinical practice guidelines only. A comprehensive review of all original intervention studies in pediatric chronic pain would be a phenomenal undertaking and beyond the scope and resources available. However, it is possible, if not likely, that additional original studies exist with relevance to identified patient-oriented research priorities that are not captured here (see interventions to educate teachersCitation94 and health care providersCitation95 about pediatric chronic pain, for example). This suggests that the current review overlooks areas or priorities where systematic reviews have not yet been conducted and/or in research areas less likely to rely on randomized controlled trials or other traditional treatment study designs. The patient-oriented priorities with minimal systematic review evidence shown here would likely benefit from quality systematic reviews of original studies.
Conclusions
This systematic review reveals the great amount of contemporary evidence synthesis that has been conducted to identify effective multimodal interventions for pediatric chronic pain to date. Creation of an evidence and gap map identifies the availability of sufficient quality evidence to guide the development of evidence-informed policies and additional practice guidelines, most notably regarding psychological and pharmacological interventions to improve children’s pain and quality of life and across physical, emotional, and role functioning domains. Despite this success, the numerous obvious evidence gaps in the top patient-oriented research priorities and treatment outcomes in pediatric chronic pain should be noted by health research funders and researchers to guide prioritization of funds, as well as study aims and design.
Supplemental Material
Download MS Word (27.2 KB)Acknowledgments
The authors express sincere gratitude to other Partnering For Pain team members, including Dr. Krista Baerg, Dr. Fiona Campbell, Dr. Jill Chorney, Katherine Dib, Mary Anne Dib, Esther Fleurimond, Dr. Paula Forgeron, Isabel Jordan, Dr. Christine Lamontagne, Justina Marianayagam, Kimberly Nelson, Dr. Melanie Noel, Dolores Pahtayken, Dr. Patricia Poulin, and Adam Val Bonzil.
Disclosure statement
Kathryn A. Birnie does not have any conflicts of interest. Carley Ouellette does not have any conflicts of interest. Tamara Do Amaral does not have any conflicts of interest. Jennifer N. Stinson does not have any conflicts of interest.
Supplementary material
Supplemental material for this article can be accessed publisher’s website.
Additional information
Funding
References
- Rice ASC, Smith BH, Blyth FM. Pain and the global burden of disease. PAIN. 2016;157(4):791–96. doi:10.1097/j.pain.0000000000000454.
- Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, Cohen M, Evers S, Finnerup NB, First MB. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). PAIN. 2019;160(1):19–27. doi:10.1097/j.pain.0000000000001384.
- Canadian Pain Task Force. Chronic pain in Canada: laying a foundation for action. Government of Canada; June 2019 https://www.canada.ca/en/health-canada/corporate/about-health-canada/public-engagement/external-advisory-bodies/canadian-pain-task-force/report-2019.html#pre.
- NIH Interagency Pain Research Coordinating Committee. National pain strategy: a comprehensive population health-level strategy for pain. Bethesda (Maryland): National Institutes of Health; 2016. https://www.iprcc.nih.gov/sites/default/files/HHSNational_Pain_Strategy_508C.pdf.
- Pain Australia. National strategic action plan for pain management. Deakin (Australia): Department of Health, Australian Government; 2019. https://www.painaustralia.org.au/improving-policy/national-action-plan.
- Martin SR, Zeltzer LK. Prioritizing pediatric chronic pain and comprehensive pain treatment in the context of the opioid epidemic. Pain Manag. 2018;8(2):67–70. doi:10.2217/pmt-2017-0072.
- Birnie KA, Dib K, Ouellette C, Dib MA, Nelson K, Pahtayken D, Baerg K, Chorney J, Forgeron P, Lamontagne C. Partnering for pain: a priority setting partnership to identify patient-oriented research priorities for pediatric chronic pain in Canada. CMAJ Open. 2019;7(4):E654–E664. doi:10.9778/cmajo.20190060.
- Cooper TE, Fisher E, Gray AL, Krane E, Sethna N, van Tilburg, MA, Zernikow B, Wiffen PJ. Opioids for chronic non-cancer pain in children and adolescents. Cochrane Database Syst Rev. 2017;(2). doi:10.1002/14651858.CD012538.pub2.
- Eccleston C, Fisher E, Cooper TE, Grégoire M-C, Heathcote LC, Krane E, Lord SM, Sethna NF, Anderson A-K, Anderson B. Pharmacological interventions for chronic pain in children: an overview of systematic reviews. PAIN. 2019;160(8):1698–707. doi:10.1097/j.pain.0000000000001609.
- Newlove-Delgado TV, Martin AE, Abbott RA, Bethel A, Thompson-Coon J, Whear R, Logan S. Dietary interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev. 2017;(3). doi:10.1002/14651858.CD010972.pub2.
- Liossi C, Howard RF. Pediatric chronic pain: biopsychosocial assessment and formulation. Pediatrics. 2016;138(5):e20160331. doi:10.1542/peds.2016-0331.
- Caes L, Boerner KE, Chambers CT, Campbell-Yeo M, Stinson J, Birnie KA, Parker JA, Huguet A, Jordan A, MacLaren Chorney J. A comprehensive categorical and bibliometric analysis of published research articles on pediatric pain from 1975 to 2010. PAIN. 2016;157(2):302–13. doi:10.1097/j.pain.0000000000000403.
- Chambers CT. From evidence to influence: dissemination and implementation of scientific knowledge for improved pain research and management. PAIN. 2018;159:S56–S64. doi:10.1097/j.pain.0000000000001327.
- Morris ZS, Wooding S, Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104(12):510–20. doi:10.1258/jrsm.2011.110180.
- MacDonald NE, Flegel K, Hebert PC, Stanbrook MB. Better management of chronic pain care for all. Can Med Assoc J. 2011;183(16):1815–1815. doi:10.1503/cmaj.111065.
- Palermo TM, Slack M, Zhou C, Aaron R, Fisher E, Rodriguez S. Waiting for a pediatric chronic pain clinic evaluation: a prospective study characterizing waiting times and symptom trajectories. J Pain. 2019;20(3):339–47. doi:10.1016/j.jpain.2018.09.009.
- Peng P, Stinson JN, Choiniere M, Dion D, Intrater H, LeFort S, Lynch M, Ong M, Rashiq S, Tkachuk G. Dedicated multidisciplinary pain management centres for children in Canada: the current status. Can J Anaesth. 2007;54(12):985–91. doi:10.1007/BF03016632.
- Graham ID, Logan J, Harrison MB, Straus SE, Tetroe J, Caswell W, Robinson N. Lost in knowledge translation: time for a map? J Contin Educ Health Prof. 2006;26(1):13–24. doi:10.1002/chp.47.
- Snilstveit B, Vojtkova M, Bhavsar A, Stevenson J, Gaarder M. Evidence & gap maps: a tool for promoting evidence informed policy and strategic research agendas. J Clin Epidemiol. 2016;79:120–29. doi:10.1016/j.jclinepi.2016.05.015.
- Saran A. Evidence and gap maps. Campbell Syst Rev. 2020 Mar:16. Epub ahead of print. doi:10.1002/cl2.1075.
- Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, Welch V., eds. Cochrane handbook for systematic reviews of interventions version 6.0. Cochrane; updated July 2019., Available from . www.training.cochrane.org/handbook.
- Saran A, White H. Evidence and gap maps: a comparison of different approaches. Version 1.0. Discussion Paper 6. Oslo (Norway): The Campbell Collaboration; 2018 Oct.
- Boulkedid R, Abdou AY, Desselas E, Monégat M, de Leeuw TG, Avez-Couturier J, Dugue S, Mareau C, Charron B, Alberti C. The research gap in chronic paediatric pain: a systematic review of randomised controlled trials. Eur J Pain. 2018;22(2):261–71. doi:10.1002/ejp.1137.
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi:10.1371/journal.pmed.1000100.
- Pollock M, Fernandes RM, Becker LA, Featherstone R, Hartling L. What guidance is available for researchers conducting overviews of reviews of healthcare interventions? A scoping review and qualitative metasummary. Syst Rev. 2016;5(1):190. doi:10.1186/s13643-016-0367-5.
- International Initiative for Impact Evaluation (3ie); Snilstveit B, Bhatia R, Rankin K, Leach B. 3ie evidence gap maps: a starting point for strategic evidence production and use. International Initiative for Impact Evaluation (3ie); 2017 Feb. doi:10.23846/WP0028.
- Littell JH. Conceptual and practical classification of research reviews and other evidence synthesis products. Version 1.0. Discussion Paper 5. Oslo (Norway): The Campbell Collaboration; 2018 July.
- Moher D, Stewart L, Shekelle P. All in the family: systematic reviews, rapid reviews, scoping reviews, realist reviews, and more. Syst Rev. 2015;4(1):183–84. doi:10.1186/s13643-015-0163-7.
- McGrath PJ, Walco GA, Turk DC, Dworkin RH, Brown MT, Davidson K, Eccleston C, Finley GA, Goldschneider K, Haverkos L. Core outcome domains and measures for pediatric acute and chronic/recurrent pain clinical trials: PedIMMPACT recommendations. J Pain. 2008;9(9):771–83. doi:10.1016/j.jpain.2008.04.007.
- James Lind Alliance Priority Setting Partnerships. The James lind alliance guidebook version 8. UK: NHS National Institute for Health Research; 2018 Nov. http://www.jla.nihr.ac.uk.
- Covidence. Australia: Veritas Health Innovation; 2019. www.covidence.org.
- Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson E. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi:10.1136/bmj.j4008.
- Manafò E, Petermann L, Vandall-Walker V, Mason-Lai P. Patient and public engagement in priority setting: a systematic rapid review of the literature. PLoS One. 2018;13(3):e0193579. doi:10.1371/journal.pone.0193579.
- American Academy of Pediatrics and the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition Subcommittee on Chronic Abdominal Pain. Chronic abdominal pain in children. PEDIATRICS. 2005;115(3):812–15. doi:10.1542/peds.2004-2497.
- Di Lorenzo C, Colletti RB, Lehmann HP, Boyle JT, Gerson WT, Hyams JS, Squires RH, Walker LS, Kanda PT. Chronic abdominal pain in children: a technical report of the American academy of pediatrics and the North American society for pediatric gastroenterology, hepatology and nutrition: AAP subcommittee and NASPGHAN committee on chronic abdominal pain. J Pediatr Gastroenterol Nutr. 2005;40(3):249–61. doi:10.1097/01.MPG.0000154661.39488.AC.
- Häuser W, Bernardy K, Wang H, Kopp I. Methodogical fundamentals used in developing the guideline. Der Schmerz. 2012;26(3):232–46. doi:10.1007/s00482-012-1189-6.
- Zernikow B, Gerhold K, Bürk G, Häuser W, Hinze CH, Hospach T, Illhardt A, Mönkemöller K, Richter M, Schnöbel-Müller E. Definition, Diagnostik und Therapie von chronischen Schmerzen in mehreren Körperregionen und des sogenannten Fibromyalgiesyndroms bei Kindern und Jugendlichen: systematische Literaturübersicht und Leitlinie. Der Schmerz. 2012;26(3):318–30. doi:10.1007/s00482-012-1168-y.
- Abbott RA, Martin AE, Newlove-Delgado TV, Bethel A, Whear RS, Thompson Coon J, Logan S. Recurrent abdominal pain in children: summary evidence from 3 systematic reviews of treatment effectiveness. J Pediatr Gastroenterol Nutr. 2018;67(1):23–33. doi:10.1097/MPG.0000000000001922.
- Abbott RA, Martin AE, Newlove-Delgado TV, Bethel A, Thompson-Coon J, Whear R, Logan S. Psychosocial interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev. 2017;(1). doi:10.1002/14651858.CD010971.pub2.
- Martin AE, Newlove-Delgado TV, Abbott RA, Bethel A, Thompson-Coon J, Whear R, Logan S. Pharmacological interventions for recurrent abdominal pain in childhood. Cochrane Database Syst Rev. 2017;(3). doi:10.1002/14651858.CD010973.pub2.
- Abujaradeh H, Safadi R, Sereika SM, Kahle CT, Cohen SM. Mindfulness-based interventions among adolescents with chronic diseases in clinical settings: a systematic review. J Pediatr Health Care. 2018;32(5):455–72. doi:10.1016/j.pedhc.2018.04.001.
- Cooper TE, Wiffen PJ, Heathcote LC, Clinch J, Howard R, Krane E, Lord SM, Sethna N, Schechter N, Wood C. Antiepileptic drugs for chronic non-cancer pain in children and adolescents. Cochrane Database Syst Rev. 2017;(8). doi:10.1002/14651858.CD012536.pub2.
- Eccleston C, Fisher E, Law E, Bartlett J, Palermo TM. Psychological interventions for parents of children and adolescents with chronic illness. Cochrane Database Syst Rev. 2015;(4). doi:10.1002/14651858.CD009660.pub3.
- Eccleston C, Cooper TE, Fisher E, Anderson B, Wilkinson NM. Non-steroidal anti-inflammatory drugs (NSAIDs) for chronic non-cancer pain in children and adolescents. Cochrane Database Syst Rev. 2017;(2). doi:10.1002/14651858.CD012537.pub2.
- Egunsola O, Wylie CE, Chitty KM, Buckley NA. Systematic review of the efficacy and safety of gabapentin and pregabalin for pain in children and adolescents. Anesth Analg. 2019;128(4):811–19. doi:10.1213/ANE.0000000000003936.
- Fisher E, Law E, Dudeney J, Palermo TM, Stewart G, Eccleston C. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2018;(9). doi:10.1002/14651858.CD003968.pub5.
- Fisher E, Law E, Dudeney J, Eccleston C, Palermo TM. Psychological therapies (remotely delivered) for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2019;(4). doi:10.1002/14651858.CD011118.pub3.
- Hechler T, Kanstrup M, Holley AL, Simons LE, Wicksell R, Hirschfeld G, Zernikow B. Systematic review on intensive interdisciplinary pain treatment of children with chronic pain. PEDIATRICS. 2015;136(1):115–27. doi:10.1542/peds.2014-3319.
- Kichline T, Cushing CC. A systematic review and quantitative analysis on the impact of aerobic exercise on pain intensity in children with chronic pain. Children Health Care. 2019;48(2):244–61. doi:10.1080/02739615.2018.1531756.
- Liossi C, Johnstone L, Lilley S, Caes L, Williams G, Schoth DE. Effectiveness of interdisciplinary interventions in paediatric chronic pain management: a systematic review and subset meta-analysis. Br J Anaesth. 2019;123(2):e359–e371. doi:10.1016/j.bja.2019.01.024.
- Lonergan A. The effectiveness of cognitive behavioural therapy for pain in childhood and adolescence: a meta-analytic review. Ir J Psychol Med. 2016;33(4):251–64. doi:10.1017/ipm.2015.59.
- Michel E, Anderson BJ, Zernikow B. Buprenorphine TTS for children - a review of the drug’s clinical pharmacology: buprenorphine in paediatrics. Pediatr Anesth. 2011;21(3):280–90. doi:10.1111/j.1460-9592.2010.03437.x.
- Palermo TM, Eccleston C, Lewandowski AS, de Williams AC, Morley S. Randomized controlled trials of psychological therapies for management of chronic pain in children and adolescents: an updated meta-analytic review. Pain. 2010;148(3):387–97. doi:10.1016/j.pain.2009.10.004.
- Shah RD, Cappiello D, Suresh S. Interventional procedures for chronic pain in children and adolescents: a review of the current evidence. Pain Pract. 2016;16(3):359–69. doi:10.1111/papr.12285.
- Tomé-Pires C, Miró J. Hypnosis for the management of chronic and cancer procedure-related pain in children. Int J Clin Exp Hypn. 2012;60(4):432–57. doi:10.1080/00207144.2012.701092.
- Velleman S, Stallard P, Richardson T. A review and meta-analysis of computerized cognitive behaviour therapy for the treatment of pain in children and adolescents: CCBT for pain in children. Child Care Health Dev. 2010;36(4):465–72. doi:10.1111/j.1365-2214.2010.01088.x.
- Weydert JA, Ball TM, Davis MF. Systematic review of treatments for recurrent abdominal pain. PEDIATRICS. 2003;111(1):e1–e11. doi:10.1542/peds.111.1.e1.
- Wicksell RK, Kanstrup M, Kemani MK, Holmström L, Olsson GL. Acceptance and commitment therapy for children and adolescents with physical health concerns. Curr Opin Psychol. 2015;2:1–5. doi:10.1016/j.copsyc.2014.12.029.
- Brent M, Lobato D, LeLeiko N. Psychological treatments for pediatric functional gastrointestinal disorders. J Pediatr Gastroenterol Nutr. 2009;48(1):13–21. doi:10.1097/MPG.0b013e3181761516.
- Horvath A, Dziechciarz P, Szajewska H. Systematic review of randomized controlled trials: fiber supplements for abdominal pain-related functional gastrointestinal disorders in childhood. Ann Nutr Metab. 2012;61(2):95–101. doi:10.1159/000338965.
- Sprenger L, Gerhards F, Goldbeck L. Effects of psychological treatment on recurrent abdominal pain in children — A meta-analysis. Clin Psychol Rev. 2011;31(7):1192–97. doi:10.1016/j.cpr.2011.07.010.
- Arruda MA, Chevis CF, Bigal ME. Recent advances in the management of chronic migraine in children. Expert Rev Neurother. 2018;18(3):231–39. doi:10.1080/14737175.2018.1438191.
- Bailey B, McManus BC. Treatment of children with migraine in the emergency department: a qualitative systematic review. Pediatr Emerg Care. 2008;24(5):321–30. doi:10.1097/PEC.0b013e31816ed047.
- Barnes NP. Migraine headache in children. Child Health. 2015;6:318.
- Ng QX, Venkatanarayanan N, Kumar L. A systematic review and meta-analysis of the efficacy of cognitive behavioral therapy for the management of pediatric migraine. Headache. 2017;57(3):349–62. doi:10.1111/head.13016.
- Trautmann E, Lackschewitz H, Kröner-Herwig B. Psychological treatment of recurrent headache in children and adolescents - a meta-analysis. Cephalalgia. 2006;26(12):1411–26. doi:10.1111/j.1468-2982.2006.01226.x.
- Cohen EM, Morley-Fletcher A, Mehta DH, Lee YC. A systematic review of psychosocial therapies for children with rheumatic diseases. Pediatr Rheumatol. 2017;15(1):6. doi:10.1186/s12969-016-0133-1.
- Ferro MA, Speechley KN. Complementary and alternative medicine use in juvenile idiopathic arthritis: a systematic review of prevalence and evidence. J Complement Integr Med. 2008;5(1):33. doi:10.2202/1553-3840.1179.
- Nijhof LN, Nap-van der Vlist MM, van de Putte EM, van Royen-kerkhof A, Nijhof SL. Non-pharmacological options for managing chronic musculoskeletal pain in children with pediatric rheumatic disease: a systematic review. Rheumatol Int. 2018;38(11):2015–25. doi:10.1007/s00296-018-4136-8.
- Fellas A, Coda A, Hawke F. Physical and mechanical therapies for lower-limb problems in juvenile idiopathic arthritis: a systematic review with meta-analysis. J Am Podiatr Med Assoc. 2017;107(5):399–412. doi:10.7547/15-213.
- Bredlau AL, Thakur R, Korones DN, Dworkin RH. Ketamine for pain in adults and children with cancer: a systematic review and synthesis of the literature. Pain Med. 2013;14(10):1505–17. doi:10.1111/pme.12182.
- Cooper TE, Heathcote LC, Anderson B, Grégoire M-C, Ljungman G, Eccleston C. Non-steroidal anti-inflammatory drugs (NSAIDs) for cancer-related pain in children and adolescents. Cochrane Database Syst Rev. 2017;(7). doi:10.1002/14651858.CD012563.pub2.
- Wiffen PJ, Cooper TE, Anderson A-K, Gray AL, Grégoire M-C, Ljungman G, Zernikow B. Opioids for cancer-related pain in children and adolescents. Cochrane Database Syst Rev. 2017;(2). doi:10.1002/14651858.CD012564.pub2.
- Anie KA, Green J. Psychological therapies for sickle cell disease and pain. Cochrane Database Syst Rev. 2015;5. doi:10.1002/14651858.CD001916.pub3.
- Badawy SM, Cronin RM, Hankins J, Crosby L, DeBaun M, Thompson AA, Shah N. Patient-centered ehealth interventions for children, adolescents, and adults with sickle cell disease: systematic review. J Med Internet Res. 2018;20(7):e10940. doi:10.2196/10940.
- Cairns G, Owen T, Kluzek S, Thurley N, Holden S, Rathleff MS, Dean BJF. Therapeutic interventions in children and adolescents with patellar tendon related pain: a systematic review. BMJ Open Sport Exerc Med. 2018;4(1):e000383. doi:10.1136/bmjsem-2018-000383.
- Huertas-Ceballos. Pharmacologicalinterventions for recurrent abdominal pain [RAP] and irritable bowel syndrome [IBS] in childhood. Cochrane Database Syst Rev. 2008;(1).
- Huertas-Ceballos. Psychosocial interventions for recurrent abdominal pain [RAP] and irritable bowel syndrome [IBS] in childhood. Cochrane Database Syst Rev. 2008;(1).
- Yeung P, Gupta S, Gieg S. Endometriosis in adolescents: a systematic review. J Endometr Pelvic Pain Disord. 2017;9(1):17–29. doi:10.5301/je.5000264.
- Ostojic K, Paget SP, Morrow AM. Management of pain in children and adolescents with cerebral palsy: a systematic review. Dev Med Child Neurol. 2019;61(3):315–21. doi:10.1111/dmcn.14088.
- Scheper MC, Engelbert RHH, Rameckers EAA, Verbunt J, Remvig L, Juul-Kristensen B. Children with generalised joint hypermobility and musculoskeletal complaints: state of the art on diagnostics, clinical characteristics, and treatment. Biomed Res Int. 2013;2013:1–13. doi:10.1155/2013/121054
- Mental health in the balance: ending the health care disparity in Canada. Canadian Mental Health Association; 2018.
- Miró J, McGrath PJ, Finley GA, Walco GA. Pediatric chronic pain programs: current and ideal practice. PAIN Report. 2017;2(5):e613. doi:10.1097/PR9.0000000000000613.
- Breau LM, Camfield CS, McGrath PJ, Finley GA. The incidence of pain in children with severe cognitive impairments. Arch Pediatr Adolesc Med. 2003;157(12):1219. doi:10.1001/archpedi.157.12.1219.
- King S, Chambers CT, Huguet A, MacNevin RC, McGrath PJ, Parker L, MacDonald AJ. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152(12):2729–38. doi:10.1016/j.pain.2011.07.016.
- Hogan M-E, Taddio A, Katz J, Shah V, Krahn M. Incremental health care costs for chronic pain in Ontario, Canada: a population-based matched cohort study of adolescents and adults using administrative data. PAIN. 2016;157(8):1626–33. doi:10.1097/j.pain.0000000000000561.
- Campbell F, Stinson J, Ouellette C, Ostapets V, Salisbury G. The association between pediatric chronic pain clinic attendance and health care utilization: a retrospective analysis. Can J Pain. 2018;2(1):30–36. doi:10.1080/24740527.2017.1415701.
- Hurtubise K, Brousselle A, Noel M, Camden C. What really matters in pediatric chronic pain rehabilitation? Results of a multi-stakeholder nominal group technique study. Disabil Rehabil. 2019:1–12. doi:10.1080/09638288.2018.1532462.
- Vinall J, Pavlova M, Asmundson G, Rasic N, Noel M. Mental health comorbidities in pediatric chronic pain: a narrative review of epidemiology, models, neurobiological mechanisms and treatment. Children. 2016;3(4):40. doi:10.3390/children3040040.
- Banner D, Bains M, Carroll S, Kandola DK, Rolfe DE, Wong C, Graham ID. Patient and public engagement in integrated knowledge translation research: are we there yet? Res Involv Engagem. 2019;5(1):8. doi:10.1186/s40900-019-0139-1.
- Birnie KA, Dib K, Ouellette C. Co-building a new landscape in pediatric chronic pain research: patient partner and researcher perspectives on meaningful patient engagement. 2018;20:21–27.
- Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–26. doi:10.1097/MLR.0b013e3182408812.
- Wittmeier KDM, Klassen TP, Sibley KM. Implementation science in pediatric health care: advances and opportunities. JAMA Pediatr. 2015;169(4):307. doi:10.1001/jamapediatrics.2015.8.
- King S, Boutilier JA, MacLaren Chorney J. Managing chronic pain in the classroom: development and usability testing of an ehealth educational intervention for educators. Can J Sch Psychol. 2018;33(2):95–109. doi:10.1177/0829573516674308.
- Bhandari RP, Goddard J, Campbell F, Sangster M, Stevens B. Becoming a pediatric pain specialist: training opportunities to advance the science and practice of pediatric pain treatment. 2019;21:10.