ABSTRACT
Background
Though chronic pain is widespread, affecting about one-fifth of the world’s population, its impacts are disproportionately felt across the population according to socioeconomic determinants such as education and income. These factors also influence patients’ access to treatment, including pharmacological pain management.
Aim
A scoping review was undertaken to better understand the association of socioeconomic factors with physicians’ pain management prescribing patterns for adults living with chronic pain.
Methods
An electronic literature search was conducted using the EMBASE, CINAHL, SCOPUS, and Ovid MEDLINE databases and 31 retrieved articles deemed relevant for analyses were critically appraised.
Results
The available evidence indicates that patients’ lower socioeconomic status is associated with a greater likelihood of being prescribed opioids to manage their chronic pain and a decreased likelihood of receiving prescription medications to manage migraines, rheumatoid arthritis, and osteoarthritis.
Conclusions
These results suggest that individuals with lower socioeconomic status do not receive equal prescription medicine opportunities to manage their chronic pain conditions. This is influenced by a variety of intersecting variables, including access to care, the potential unaffordability of certain therapies, patients’ health literacy, and prescribing biases. Future research is needed to identify interventions to improve equity of access to therapies for patients with chronic pain living in lower socioeconomic situations as well as to explain the mechanism through which socioeconomic status affects chronic pain treatment choices by health care providers.
Abbreviation
SES: socioeconomic status; RA: rheumatoid arthritis; IV: intravenous; SC: subcutaneous; bDMARDs: biological disease-modifying antirheumatic drugs; DMARDS; disease-modifying antirheumatic drugs; TNFi: tumour necrosis factor inhibitors; NSAIDs: non-steroidal anti-inflammatory drugs
RÉSUMÉ
Contexte: Bien que la douleur chronique soit répandue, touchant environ un cinquième de la population mondiale, ses effets sont ressentis de manière disproportionnée au sein de la population en fonction de déterminants tels que l’éducation et le revenu. Ces facteurs influencent également l’accès des patients aux traitements, y compris la prise en charge pharmacologique de la douleur.
Objectif: Un examen de la portée a été entrepris pour mieux comprendre l’association entre les facteurs socioéconomiques et les tendances manifestées par les médecins en matière d’ordonnances pour la prise en charge de la douleur chez les adultes vivant avec une douleur chronique.
Méthodes: Une recherche documentaire électronique a été effectuée à l’aide des bases de données EMBASE, CINAHL, SCOPUS et Ovid MEDLINE. Par la suite, 31 articles récupérés qui avaient été jugés pertinents pour les analyses ont été soumis à une évaluation critique.
Résultats: Les données disponibles indiquent que le statut socioéconomique inférieur des patients est associé à une plus grande probabilité de se voir prescrire des opioïdes pour la prise en charge de leur douleur chronique et à une diminution de la probabilité de recevoir des médicaments sur ordonnance pour prendre en charge les migraines, la polyarthrite rhumatoïde et l’ostéoarthrite.
Conclusions: Ces résultats indiquent que les personnes ayant un statut socioéconomique inférieur ne bénéficient pas des mêmes possibilités de médicaments sur ordonnance pour prendre en charge leur douleur chronique. Cela est influencé par une variété de variables croisées, y compris l’accès aux soins, le fait que certains traitements soient potentiellement inabordables, la littératie de santé des patients et les préjugés en matière d’ordonnances. D’autres études sont nécessaires pour recenser les interventions visant à améliorer l’équité d’accès aux traitements pour les patients souffrant de douleur chronique vivant dans des situations socioéconomiques plus faibles, ainsi que pour expliquer le mécanisme par lequel le statut socioéconomique affecte les choix de traitement de la douleur chronique par les prestataires de soins de santé.
Introduction
Recognized to be a disorder unto itself in the 11th revision of the International Classification of Diseases,Citation1,Citation2 chronic pain is “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage … that persists or recurs for longer than 3 months.”Citation3 Chronic pain affects approximately one-fifth of the population and is a leading cause of disability and disease burden.Citation4,Citation5 Given that chronic pain is a complex and multidimensional experience whose assessment, study, and management are influenced by a variety of biopsychosocial contexts,Citation6 it is not surprising that a variety of studies have found that patients’ socioeconomic status (SES) can affect their experiences, treatments, and health outcomes.Citation7–9 Patients’ chronic pain experiences have been shown to be affected by factors such as their gender, ethnicity, social class, income, education, and the neighborhood in which they reside.Citation7,Citation9–11 The Canadian Pain Task ForceCitation12 agrees that such factors play a role in explaining the inconsistent and inequitable experience of pain and access to pain care in Canada. Therefore, among the six recommendations that the Canadian Pain Task Force has suggested to the Canadian federal government to improve the prevention and management of chronic pain are those specifically related to improving both equitable access to pain care and ensuring “equitable approaches for populations disproportionately impacted by pain.”Citation12(p1)
A better understanding of the role of societal inequities in the development and severity of chronic pain is necessary in order to actualize such recommendations. Lower SES has been correlated with increased chronic pain incidence, severity, and pain-associated and psychosocial disability in a variety of studies performed using methodologies ranging from random population sampling to health questionnaires and surveys to prospective cohort studies following patients from birth to adulthood.Citation8,Citation13–17 In addition to being a risk factor for the development for disabling chronic pain,Citation18,Citation19 lower SES has been associated with a higher incidence of other medical comorbidities that can amplify chronic pain.Citation8,Citation18
Associations and interactions between lower SES and chronic pain are due in part to income-related factors. Income has been considered in different ways in chronic pain–related studies, including as measured household income, household wealth that incorporates the financial resources accumulated over a lifetime, employment type, indexes that include a combination of housing tenure, car ownership, and employment status, as well as self-reported ratings of economic hardship and daily financial worry.Citation8,Citation14,Citation17,Citation20–22 Studies report that living or growing up in a lower income household is associated with an increased risk of developing or reporting chronic pain and an increased prevalence and severity of chronic pain.Citation23–28 As wealth increases, the degree to which chronic pain interferes with the performance of activities of daily living decreases.Citation20,Citation29 Self-perceived economic hardship has also been shown to exacerbate chronic pain.Citation22 Income can affect health outcomes in terms of access to resources within health care, as well as resources outside of health care related to healthy living, such as access to healthy food and opportunities for physical activity.Citation20 Lower-paying jobs may be associated with greater occupational hazards or be more physically demanding and so predispose to the development of chronic pain.Citation16,Citation20,Citation30 For patients with lower income and chronic pain, this has meant decreased access to pain therapies or resources to modify their living or work environments to decrease their pain and/or improve their function.Citation20
Closely linked to considerations of income and SES are those related to patients’ educational achievements. Education is associated with the acquisition of beliefs and knowledge that enables individuals to integrate health behaviors into their lifestyles and provides a sense of control over one’s own health.Citation31 In health research, education is often measured by asking participants to identify the highest level of education that they have completed.Citation31,Citation32 Lower educational achievement has been associated with a higher prevalence of pain, more severe pain, and greater occupational and overall disability.Citation6,Citation32–34 Higher educational achievement was found to be a protective factor for developing chronic pain when controlling for occupational class and working conditions.Citation6,Citation32 A variety of hypotheses have been proposed to explain these relationships between lower levels of education and chronic pain. Higher levels of education can facilitate patients’ improved communication with physicians.Citation6,Citation34 Patients are therefore better able to access and understand treatment information, express themselves and their concerns, appropriately use care resources, and navigate health care systems more effectively.Citation6,Citation34–37 Physicians are more likely to spend more time with patients with higher SES, provide them with information, and justify treatment suggestions compared to patients with lower SES.Citation34,Citation38–40 Other studies suggest that lower levels of education are associated with fewer vocational options and occupations that require more physical work and thus workers are more prone to injuries and have less autonomy.Citation33,Citation41–46 Additionally, lower levels of education have been suggested to be a proxy for maladaptive pain beliefs and ineffective and passive coping strategies that exacerbate chronic pain.Citation6,Citation32,Citation47–50 Roth and colleaguesCitation6 found that the belief that chronic pain is synonymous with “harm” and catastrophizing accounted for the association between lower educational achievement and severe disability secondary to chronic pain. They speculated that lower levels of education preclude patients’ abilities to develop accurate models of health and illness such that they develop ineffective ways to manage their pain, such as catastrophizing, praying, and hoping.Citation6,Citation32,Citation47
There is evidence available to suggest that these aforementioned components of patients’ SES can have an association with physician medication prescription patterns. The writing of prescriptions is the most frequent medical intervention performed by physicians and is an integral component of the health care system.Citation51 Therefore, understanding the factors that influence prescribing patterns is important for assessing the quality of care provided to patients. However, though previous studies have evaluated the influence of guidelines on the pharmacological management of chronic pain, the association of patients’ SES on such patterns is not well understood.Citation52–55 The aim of this study was to better identify this knowledge gap by surveying the available literature, examining how research on this topic has been conducted, and providing an overview of its focus and the available evidence. The aim was not to produce a “critically appraised and synthesized result to answer a particular question,” which would be in keeping with a systematic review.Citation56(p3) Thus, a scoping review was deemed to be the most appropriate method of accomplishing these aims.Citation56 Examination of this topic has been suggested to be important given the impetus for chronic pain to be considered a pressing public health problem and increased recognition of and calls to address the issues that contribute to the inequitable burden of chronic pain in society.Citation12,Citation24,Citation57 The demonstration of how the COVID-19 pandemic has disproportionately affected patients with chronic pain living with low SES makes this review topical because it seeks to contribute to greater understanding of how access to chronic pain care can be improved for these patients.Citation58 The information obtained from this review is relevant for clinicians, hospital administrators, and politicians with respect to becoming better informed of the barriers to accessing care for chronic pain. The results of this review may also be beneficial for establishing guidelines to deliver equitable pain management services to patients from differing socioeconomic backgrounds.
Methodology
A scoping review was completed to assess the association of SES on physician prescribing patterns for adult patients experiencing chronic pain. The search strategy extracted articles that assessed the association of SES on physician prescribing practices for chronic pain management in adults. This review focused on studies addressing income and education given the importance of these determinants as previously described. Chronic pain was considered as “pain which has persisted beyond normal tissue healing time,” which, in the absence of other factors, is generally taken to be 3 months.Citation4 We focused on studies that included chronic pain unrelated to cancer and studies that addressed common chronic pain conditions. We included studies related to arthritis, back/neck pain, headache disorders, fibromyalgia, and myofascial pain syndrome.
An electronic search strategy was established in collaboration with an information specialist librarian. The searches completed for this review were restricted to those in the English language and published between the years 2000 and 2021. The electronic databases used for the searches included EMBASE, CINAHL, SCOPUS, and Ovid MEDLINE. The key words related to SES used in the search included “social class,” “social status,” “education,” and “income.” The search terms related to prescribing practices included “physician prescribing practices,” “practice patterns,” and “inappropriate prescribing.” The search terms related to chronic pain that were used for this literature search were as follows: “chronic pain,” “persistent pain,” “arthritis,” “back pain,” “neck pain,” “headache disorders,” “fibromyalgia,” and “myofascial pain syndromes.” The search strategy was conducted to a final date of February 1, 2021, and was continually updated throughout the completion of this review. The search strategy identified 3492 articles to be included in the title and abstract screening for the scoping review. Any duplicate records were removed prior to screening.
The overarching goal of the scoping review selection was to identify and review any available evidence within the eligibility boundaries previously outlined. Specific screening questions were developed for all levels of the assessment and two independent reviewers performed the screening process. Two levels of screening were completed to identify the relevant studies for this scoping review. The first level of screening included a title and abstract review for the articles retrieved from the electronic search in order to remove any studies that did not relate to the review topic. The reports obtained from this screening process were uploaded to Covidence (www.covidence.org) to assist with the overall management of the articles for the review. The articles identified by either one or both reviewers as possibly relevant were retrieved for a full-text review. The second level of screening involved a full-text review based on the eligibility criteria previously outlined. A full paper analysis was completed to ensure that the study provided sufficient information relevant to the objectives of this scoping review. Following the second level of screening, the reference lists of the relevant articles were also manually searched to identify additional references. Disagreements arising between the two independent reviewers during the screening process were resolved by consensus. Thematic analysis of the selected studies was performed independently by each author and then reviewed together to develop consensus. This was an iterative process.
Results
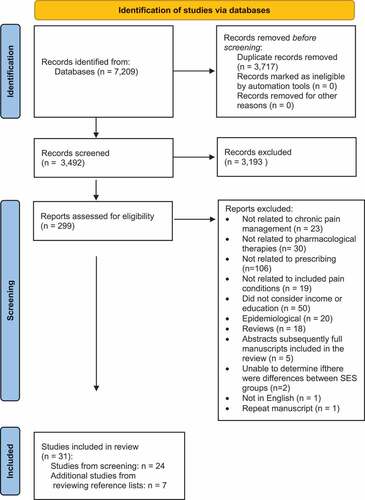
During the primary review, 299 articles were selected to be reviewed further based upon their title and abstract. The full texts of these selected articles were reviewed and 24 papers met the inclusion criteria for this review,Citation59–82 along with an additional 7 studies found after reviewing the reference lists from those studies ().Citation83–89 Reasons for exclusion included that the studies (1) did not cover pain management,Citation90–112 (2) did not deal with pharmacological therapies,Citation29,Citation113–141 (3) were not related to prescribing,Citation142–247 (4) were not related to the pain conditions outlined in the inclusion criteria,Citation248–266 (5) did not consider either income or education,Citation7,Citation11,Citation27,Citation267–313 (6) were epidemiological,Citation314–333 (7) were reviews,Citation23,Citation334–350 (8) were abstracts that were subsequently published as full-length manuscripts that appeared in the search,Citation351–355 (9) did not provide enough information to determine whether there were differences between SES groups,Citation356,Citation357 (10) were repeated,Citation11 or (11) were not in English.Citation358 A PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow diagram summarizes the article management process used for this review ().Citation359
Table 1. List of reviewed studies.
The results of the reviewed studies are summarized in . Overall, these studies demonstrated that SES and, more specifically, income and education typically had an association with physician prescribing patterns for chronic pain management. These associations can be categorized according to the association of SES on the provision of (1) opioid prescriptions, (2) medications to manage arthritis, or (3) triptan medications for headaches.
Association of SES with Opioid Prescribing
Opioid prescriptions were the most common pharmacologic treatment studied among those reviewed. Most studies identified an association between chronic pain, lower SES, and a greater likelihood of receiving an opioid prescription.Citation59,Citation63,Citation65,Citation67,Citation68,Citation72,Citation74–78,Citation85 Looking at SES from an income perspective, studies found that individuals with lower income were more likely to be prescribed opioids for chronic pain management. For example, Grol-ProkopczykCitation67 identified that older American adults in the top income quartile had less than half the odds of using opioids to manage their pain compared to those in the bottom quartile. Frenk and colleaguesCitation85 looked at patients’ income from the perspective of where it related to the American federal poverty line threshold; those with family incomes less than 200% of the threshold were significantly more likely to use an opioid compared to those with incomes greater than 200% of the threshold. Others looked at the receipt of low-income subsidies, being paid hourly, or unemployment status as surrogate markers for low-income status and found that these were similarly associated with higher opioid prescription and use rates.Citation63,Citation65,Citation68,Citation74,Citation77,Citation78 In terms of doses of opioids prescribed, Nielsen and colleagues’Citation72 cohort study of 1514 patients across Australia found that higher doses were provided to patients who had increased barriers, financial and access-wise, to nonopioid treatment. Instead of looking at data obtained from cohorts, cross-sectional surveys, or databases, Anastas and colleaguesCitation59 studied how physicians made pain management decisions using simulated patients with chronic back pain of varying SES backgrounds and also found that they were more likely to recommend opioid use for patients who had lower SES.
Looking at SES from an education perspective, patients with lower levels of education were found to have higher rates of opioid use.Citation76–78 In looking at the management of 4898 American adults with chronic back or arthritis-related pain between 1999 and 2014, Stokes and colleaguesCitation78 observed that prescription opioid use was significantly greater for those without high school matriculation compared to those who had completed high school (22.7% versus 5.7%). These latter findings are consistent with those described by Shmagel and colleagues,Citation76 who found that 94% of patients with chronic back pain to whom opioid prescriptions were provided had less than a college education.
The use of opioid prescriptions for those living with chronic pain in lower SES situations was found to further disadvantage them. Kuo and colleaguesCitation68 identified that individuals with a lower SES were more likely to utilize opioids to manage their chronic pain for a longer time, which resulted in an increased risk of opioid overdose–related emergency room visits and hospitalizations. In addition, Chuang and colleaguesCitation63 found that patients who used opioid analgesics to manage their chronic pain were more likely to be unable to work due to disability and were more likely to receive social security income. When controlling for potential confounders in multivariable analyses, patients who used opioid analgesics had three times the odds of being unable to work due to disability compared with nonusers.Citation63
A limited number of the studies included in this review did not identify an association between SES and the likelihood of receiving an opioid prescription for chronic pain. For example, Nampiaparampil and colleaguesCitation71 did not find that opioid prescriptions were more likely to be prescribed to African American patients of low SES. Additionally, Tamayo-Sarver and colleaguesCitation79 identified that “desirable” patient characteristics increased the likelihood of receiving a prescription of opioids for chronic pain, by which they meant patients with higher SES, who were employed, and who had an established relationship with a primary care physician.(p1239) Other studies found that lower education was found to be a weaker predictor of opioid use than income statusCitation67 or not predictive at all.Citation65
Association of SES with Prescriptions for Medications to Manage Arthritis
Several studies included in this scoping review identified a link between SES and the likelihood of receiving prescriptions of a disease-modifying antirheumatic drug (DMARD) for rheumatoid arthritis. Patients with greater financial resources were found to have a higher probability of being prescribed a biological DMARD.Citation64,Citation70,Citation73,Citation80,Citation82,Citation86,Citation87,Citation89 Patients with low personal incomes were more likely to be prescribed glucocorticoid monotherapy rather than a DMARD.Citation89 Living in communities associated with lower SES was also found to be negatively associated with receipt of a DMARD prescription,Citation86,Citation87 and analyses demonstrated that this effect was evident even beyond the effect of individuals’ low personal income.Citation87 Such a relationship was seen at even larger scales at the county and even national levels, even with the existence of prescribing guidelines.Citation64,Citation73 The time until initiation of a biological DMARD was also significantly longer for patients living in lower SES areas.Citation80
Lower income was also associated with lower odds of being treated with newer or other medications for arthritis. Wang and colleaguesCitation81 gauged patients’ household income from income tax files and found that despite the presence of a universal national health care insurance system in Taiwan, patients were less likely to receive newly enlisted nonsteroidal anti-inflammatory drugs if they had lower incomes. The use of tumor necrosis factor inhibitors was also less likely for patients with lower SES.Citation66
Association of SES with Prescriptions to Treat Migraines
Studies analyzing the variability in triptan prescriptions for patients with migraines living in different socioeconomic situations mostly showed that lower SES was associated with a decreased propensity to receive them.Citation69 Patients were more likely to use triptans if they had insurance coverageCitation62,Citation84,Citation88 or higher incomes, which meant annual household incomes exceeding $60,000 compared to less than $22,500,Citation62 exceeding $40,000 compared to less than $22,500,Citation84 or high income levels compared to middle, lower, or poor income levels when income was referenced with respect to a federal poverty line threshold.Citation88 Achievement of at least college-level education was also seen to be associated with an increased likelihood of using a triptan medication.Citation84,Citation88 It was only an American national stratified sample survey of households using a mailed questionnaire that found that income levels were associated with insignificant differences in rates of prescription medication use for migraine headaches.Citation83
Discussion
This scoping review looked at the association of SES on physician prescribing patterns for adult patients living with chronic pain. Chronic pain is recognized to be affected by a variety of biological, psychological, and social factors and therefore patients’ socioeconomic circumstances affect their pain and pain management experiences.Citation6,Citation14,Citation23,Citation48,Citation360,Citation361 Indeed, as Karran and colleaguesCitation339 wrote, “Health outcomes are explained less by the quality and availability of medical care than they are by the context and circumstances of people’s lives.(p2476) Further, Brady and colleaguesCitation7 noted that “as pain represents higher-order processing, a product of individual interpretation and meaning, it is important that research into pain seeks to understand the ethnocultural and socioenvironmental contexts that shape the way people live with and manage pain.”(p435)
A scoping review was selected because the aim was to synthesize the evidence available regarding the associations of SES with specific chronic pain conditions and identify any knowledge gaps. It is recognized that SES comprises more than just one construct and so factors such as income and education were considered because these are thought to be valid and stable constructs.Citation6,Citation362,Citation363 An approach was utilized to maximize the identification of all relevant studies associated with arthritis, back/neck pain, headache disorders, fibromyalgia, and myofascial pain syndrome, income and education, and prescribing patterns. Most of the studies included in this review utilized data collected from a variety of clinical settings. Many of the included studies had large cohorts of patients across different geographic locations, such as the United States,Citation59–63,Citation65,Citation67–69,Citation71,Citation74,Citation76–79,Citation82–89 Canada,Citation70,Citation80,Citation86 Australia,Citation72 Norway,Citation75 Romania,Citation64 Sweden,Citation66 Taiwan,Citation81 and a multinational trial.Citation73 This increases the generalizability of the findings but also means that the results need to be interpreted in the context of the heterogeneity of the health care systems in which the patients were studied. For example, some studies were performed in countries with universal health care coverage, whereas others were performed in systems in which patients bear more financial responsibility for their health care and prescription medications. Even countries with national universal health care systems may not have universal prescription medication coverage, as is the case in Canada, which means that patients with lower income may face challenges affording medications for pain management.
Medications Prescribed
A variety of hypotheses have been suggested to explain the observed relationships between SES and opioid prescribing and use rates. It was thought that lower SES was associated with decreased resources and therefore abilities to access nonopioid therapies.Citation71 This was due to the costs associated with other types of treatment as well as challenges with taking time to access other services.Citation77,Citation78,Citation364 Nielsen and colleaguesCitation72 found that having private insurance facilitated access to nonopioid therapies as well as access to specialists. They additionally found that the use of higher doses of opioids was associated with greater challenges in getting to pharmacies and physicians, including specialists. As has been pointed out, the lack of coverage for a variety of nonopioid pain therapies therefore means that patients with lower SES may be unable to access more “widely recommended treatment options.”Citation76(p1110),Citation365 Prescribers’ recognition of this may thus lead them to overrely on opioids for pain management for patients with lower SES. At the same time, Grol-ProkopczykCitation67 suggested that caution is required as efforts are made to address the opioid crisis and the “crisis of undertreated pain” by limiting access to opioids: “To shut the door to one form of pain treatment without ensuring that others are open will likely exacerbate the latter crisis, and perhaps the former too, if those unable to access prescribed drugs switch to illicit ones.”(p1017)
From an education perspective, it has been suggested that patients with lower levels of education may have been found to be more likely to use or be prescribed opioids because they were not as well-informed about their lower efficacy in pain management and their associated side effects.Citation76 There is evidence to support this assertion because taking into account health literacy when educating patients about opioid use for pain was an effective way of changing patients’ perceptions about their pain.Citation76,Citation366–368 The contrary finding by a few studies that higher SES was associated with greater rates of opioid prescribing was suggested to be potentially explained by physicians’ concerns that patients with lower SES would be less likely to follow their prescribing and treatment recommendations and more likely to misuse opioids.Citation59,Citation71,Citation345,Citation369,Citation370
The association of lower access to DMARDs among those with lower SES was thought to be due in part to the cost of those medications. Lower incomes were thought to preclude the ability for patients to pay for them.Citation82,Citation87,Citation89 This was thought to be in the context of either lacking or having limits to their health care insurance.Citation82 Nevertheless, this relationship has been observed even when patients have access to universal health care insurance.Citation80,Citation86 Putrik and colleaguesCitation75 found that within such a health care system context, it was also patients’ decreased educational background that contributed to the lower likelihood of receiving a DMARD prescription. Having higher education was hypothesized to enable patients to gain better information about DMARDs and afford them better abilities to negotiate with their physicians about receiving DMARDs.Citation75
The finding that even living in an area associated with lower SES was associated with reduced access to DMARDS suggests that less household or neighborhood income may have served as a proxy for access to specialist care, which was thought to improve patients’ ability to access DMARDs.Citation79,Citation89 Thus, it has been found that patients with lower SES were less likely to see rheumatologists.Citation80,Citation82,Citation89 In the study by Yelin and colleagues,Citation82 patients with lower SES were suggested to have access to federally run health centers that may have had fewer medical resources available to them. Moreover, physicians in those areas may have had less ability to access specialty care, like that provided by rheumatologists.Citation82
The cost of triptan medications was thought to explain the observed association of their reduced prescription and use among patients with lower SES and/or lack of insurance coverage. Wu and colleaguesCitation88 noted that these medications can be costly, with a single triptan pill costing up to $46 in the United States, for example. Bigal and colleaguesCitation62 wondered whether patients’ access to universal health coverage would mitigate the observed decrease in triptan use for migraines because patients’ income would potentially be a less important factor affecting its use. Socioeconomic factors like income and education have been suggested to be surrogate markers for patients’ access to care, and this may explain the observed relationship between lower SES and reduced triptan use.Citation83,Citation84 It was suggested that strategies to improve migraine management for patients without insurance coverage for medication or with lower SES include increasing their use of prophylactic medications rather than triptans and enhancing those patients’ pain self-management skills.Citation88
SES Measures Studied
Income was measured in a variety of ways in the reviewed studies. In some studies, patients’ income was measured and then grouped into categories.Citation62,Citation81 Other studies looked instead at household income; Stokes and colleaguesCitation77,Citation78 additionally looked at whether households received other types of income support, such as housing support, food stamps, child support, and unemployment insurance. Wang and colleaguesCitation81 adjusted for household structure because they argued that such structure influences what resources are available to individual members of the household. A marginalization index was used in one study in order to gather information related to domains such as residential instability, material deprivation, dependency, and ethnicity.Citation80 Alternatively, income was expressed in terms of how it related to federal poverty limits.Citation88,Citation89 Rather than using patient-generated data, some studies relied upon the use of census data in order to determine neighborhood income quintiles.Citation76 It has been argued that a drawback to this is that such estimations may not reflect the true income for individuals in the study.Citation81
Access to health care insurance was another surrogate measure of income that was employed by some studies because it was thought that this would have implications for patients’ abilities to access prescription medications. Indeed, studies confirmed this association. For example, Nielsen and colleaguesCitation72 found that the greatest predictor of patients’ use of nonopioid pain management modalities was having private health insurance. They argued that subsidization of such treatments is therefore important because patients may use medications such as opioids out of necessity rather than choice.Citation67,Citation72 Increased wealth that affords the ability of patients to pay for a greater variety of pain management options also is argued to facilitate other advantages, such as the ability to work fewer hours, or may be associated with occupations that support workplace accommodations or protect against the worsening or progression of chronic pain.Citation14 Patients with lower SES may also experience greater barriers to accessing insurance coverage that extend beyond payment alone, such as navigating bureaucracies.Citation24 Even with universal health care coverage systems or government-sponsored insurance programs, patients with lower SES were found to have decreased access to certain medication therapies; in some cases this was thought to be due to such systems as still not providing adequate coverage for therapies.Citation72,Citation79,Citation82 As BooherCitation23 pointed out, patients with lower SES “become caught in the vicious cycle of chronic pain leading to disability and poverty, and poverty worsening chronic pain.”Citation371(p386)
There was heterogeneity as well in the way in which level of education was measured in the reviewed studies. In some studies, it was viewed in a dichotomous manner looking at whether patients had less than or more than a college education or stratified in terms of the highest level of education obtained.Citation7–78,Citation88 Others looked at census data to obtain levels of education for ZIP code areas and used that information rather than details for individual patients.Citation68 Putrik and colleaguesCitation75 suggested that a better way of looking at education would be to look at patients’ health literacy rather than looking at the level of education they had obtained because this might be more meaningful in terms of determining how or why level of education is associated with particular prescribing patterns.
Prescribing Biases
The social context in which chronic pain clinical encounters take place as well as both implicit and explicit biases held by physicians can influence prescribing patterns and lead to disparities in pain management.Citation15,Citation59,Citation75,Citation372,Citation373 Green and colleaguesCitation15 noted that decisions about pain management are affected by characteristics of patients, health care providers, the clinical practice environment, and the overall health care system in which pain care is provided. Physicians may “unconsciously consider judgements beyond clinical factors in their decisional processes.”Citation75(p1222) This is especially true for conditions like chronic pain, argued Anastas and colleagues,Citation59 because of pain’s “subjective nature” and “clinical uncertainty.”(p772) As they put it, “Providers’ attitudes may ‘fill in the gaps’ of insufficient or ambiguous information, resulting in systematic differences in chronic pain care across race and SES groups.”59(p772)
There is extensive literature demonstrating bias in how health care providers treat patients from specific ethnocultural backgrounds.Citation15,Citation280,Citation374–377 For example, compared to the experience of White patients, Black patients’ pain has been shown to not be as aggressively assessed or treated, they are less likely to be referred to pain specialists,Citation15,Citation280,Citation374,Citation375,Citation377 and they are more likely to be required to provide urine drug screens.Citation376
Tamayo-Sarver and colleaguesCitation79 suggested that inequities in the delivery of pain care may not be due to ethnocultural status alone and that SES is implicated as well. Less common are such studies looking at bias in how patients with lower SES have been treated,Citation59,Citation79 but the studies that have been done that specifically look at the way in which variability in health care provider and patient characteristics affects chronic pain management decision making have confirmed that lower SES can lead to inequities in pain care.Citation71,Citation378,Citation379 Patients with lower SES and chronic pain have been viewed by health care providers to be less competent and compliant in the use of medications and other multidisciplinary therapies for pain.Citation163,Citation369 They have been thought by physicians to have less self-control over medication use with a greater propensity to misuse opioids and so studies have found that physicians disproportionately require them to complete opioid contracts compared to patients with higher SES.Citation59,Citation273,Citation369,Citation380 Patients with lower SES have been considered by health care providers to be more “demanding,”Citation163(p2094) and their pain has been referred to in “dehumanizing” ways.Citation378(p152) Tamayo-Sarver and colleaguesCitation79 wrote that SES was associated with “physicians’ perceptions of patients’ abilities, personalities, behaviors, and role demands” and that physicians “may treat patients preferentially if there is less social distance between them.”(p1245) Green and colleaguesCitation15 suggested that more research is needed to better understand the factors that “systematically influence” physicians’ decisions regarding pain management.(p286)
Characteristics of the communities in which patients reside also affect their ability to access prescription medications for chronic pain. Residing in communities with lower SES has been found to cause increased exposure to violence, psychosocial distress, and social isolation and, thus, to increased risk factors for the development and furtherance of chronic pain.Citation23,Citation166,Citation363,Citation381,Citation382 Poorer communities are less likely to be situated in proximity to pain clinics, making it more challenging for their residents to access them.Citation302,Citation383,Citation384 Moreover, access to medications in communities associated with lower SES has been found to be an issue because pharmacies in those areas may not have sufficient supplies of medications compared to pharmacies located in communities with higher SES.Citation294,Citation382 Morrison and colleaguesCitation385 found that disparities in supply were seen when looking from an ethnocultural perspective as well. Even after adjusting for rates of crime, pharmacies in New York City in Hispanic and African American neighborhoods were less likely to supply opioids compared to those located in White American ones.Citation385
Physician prescribing practices for chronic pain management are influenced by practice guidelines. In their study including in this review, Stokes and colleaguesCitation77 identified that there was a decline in the number of individuals receiving opioids during the years 2013 and 2016 and suggested that this may reflect changes in physician prescribing practices as awareness of the opioid epidemic grew and as Centers for Disease Control and Prevention guidelines about reducing opioid use were developed. Nevertheless, they still found that there was an association between SES and the likelihood of being prescribed opioids for chronic pain management.Citation77
Socioeconomic Status and Intersectionality
In this scoping review, SES was looked at from the perspective of income and education but there are other components of SES that could be investigated. Similar relationships between SES factors such as level of education and income and chronic pain have been found for ethnicity. As Fuentes and colleaguesCitation363 noted, “Race and socioeconomics are entangled constructs sometimes interacting and sometimes acting individually”(p1160) and thus they argued that SES and ethnicity cannot be used interchangeably. Patients considered to be ethnic minorities in North America have been found to have a disproportionate burden of chronic pain and lack of access to pain management.Citation382 For example, African Americans have been found to have increased chronic pain prevalence, intensity, and pain-related distress, disability, and interference of function compared to other ethnic groups.Citation20,Citation280,Citation371,Citation379,Citation382,Citation386–388 Moreover, they have been found to have increased exposures to risk factors for the development of chronic pain and to be offered less comprehensive pain management options.Citation345,Citation382 The etiology of this is multifaceted, including mistrust of the health care system at the individual levelCitation363; decreased access to health care resources such as therapies, primary care physicians, and pain specialists at the health system levelCitation345,Citation382; and negative stereotypes and discrimination at the societal level.Citation273,Citation377,Citation380,Citation382,Citation389 It has therefore been suggested that research related to the effects of SES on access to pain care should also consider patients’ ethnocultural status and recognize that socioeconomic advantages and disadvantages are not uniform across ethnocultural groups.Citation28,Citation59,Citation361 As RiskowskiCitation28 pointed out, patients’ income, education, social status, and ethnicity may each contribute in some way to “the development of pain or in the management of pain, or lack thereof … these factors together can create access to vital, influential, and extensive resources and opportunities to impact health and health behaviors.”(p1517)
Brady and colleaguesCitation7 pointed to Crenshaw’sCitation390 theory of intersectionality as providing a useful way of depicting the manner in which the variety of factors that contribute to patients’ social circumstances and identities interact to affect their experiences with chronic pain. They described that patients’ life experiences and identities “interact to position an individual along a specific trajectory that may confer a position of advantage or disadvantage for health” and “can factor into the creation and maintenance of chronic pain disparities.”Citation7(p435) Bonathan and colleaguesCitation14 outlined in particular how lower SES and chronic pain are interconnected:
The consequences of feeling socially inferior, living in a less socially cohesive neighborhood with a more imminent sense of threat, combined with poorer education and, therefore, poorer job opportunities are likely to interact with the psychological factors known to increase the risk of chronic pain … financial restrictions also constrain coping strategies and since some coping is consistently associated with greater disability, this is a self-maintaining process. Educational level may also influence choice of strategies.(pCitation161)
Therefore, to better understand why chronic pain is not addressed in the same manner for all patients, an understanding of the “conditions in which people are born, grow, live, work, and age, and the inequities in power, money, and resources that are responsible for disparities in health outcomes” is necessary.Citation339(p2476)
Limitations
The results of this review must consider the limitations associated with the reviewed studies. First, there was heterogeneity among the studies from several perspectives. As noted earlier, studies used different ways of measuring SES, in terms of both income and education. Studies used various methodologies; some used data from administrative databases, which can be associated with concerns about the accuracy of diagnoses used and thus the manner in which cases were selected.Citation86 Others used cross-sectional surveys that often use patient self-report in order to acquire date. Such studies are therefore associated with potential recall bias and reporting inaccuracies.Citation62,Citation76 Information may not have been verified by review of charts or provider information.Citation84 Different studies adjusted for different variables when looking at pain-related outcomes. For example, Chuang and colleaguesCitation63 controlled for potential confounders including depression, pain severity, pain interference, global physical and mental functioning, and demographic characteristics, whereas Grol ProkopczykCitation67 controlled for age, sex, race/ethnicity, and marital status. Second, the retrospective nature of most of the reviewed studies meant that they could only show associations and could not determine causality.Citation63 Third, studies that looked at prescribing patterns may be inaccurate, because reports based upon self-report may not reflect what physicians recommended. Additionally, the provision of a prescription does not necessarily mean that it was filledCitation67 or that medications were actually taken by patients.Citation68 There may have been reasons why patients were not provided with certain treatment options, and this was often not reported in studies (contraindications, used in the past and failed, or had adverse events to a particular pharmacological agent).Citation67 Fourth, various elements of SES were at times grouped together. For example, Nampiaparampil and colleaguesCitation71 grouped lower SES related to income and education together with ethnicity and so this would make distinguishing the effects of ethnicity itself from SES challenging.
There are limitations associated with this scoping review as well. BooherCitation23 used key words such as “socioeconomic status” and “chronic pain” but noted that this may have led to missing some studies; this limitation applies to this scoping review as well. A variety of databases that include publications related to medicine, nursing, public health, sociology, and pain were used in order to ensure that as many relevant studies were found as possible. However, limiting the search to particular databases may have led to missing studies. Additionally, the factors that lead to a patient being prescribed a specific medication for chronic pain management are known to be complex and involve a variety of steps.Citation84 For example, patients must be able to access a health care provider, receive a diagnosis, receive a prescription, fill that prescription, and then take that medication.Citation84 There are factors associated with SES that can influence each of these steps. This scoping review’s focus on patients being prescribed or taking a medication therefore misses some of these other factors. Not all types of chronic pain conditions were considered in this scoping review, and so its findings cannot be generalized to chronic pain conditions beyond those described in the inclusion criteria. A final limitation is that this review only includes articles that were published in English. Despite these limitations, we identified a relatively consistent pattern between SES (specifically, income and education) and its association with chronic pain management using prescription medications.
Conclusions
This scoping review provides insight into the complex interactions between SES, specifically income and education, and access to pharmacological treatments for specific chronic pain conditions. The studies examining this topic are heterogenous in terms of how they considered income and education, the methods they used, and the conditions and medications they studied. Nevertheless, an association was found such that patients with lower SES typically do not receive equal prescription medicine opportunities for their chronic pain conditions.
Further research is required to better understand hpw lower SES is associated with disparities in the provision of pharmacological pain management with the aim of reducing the magnitude of such disparities. Green and colleagues’Citation15 recommendations about how to address the gap in knowledge related to ethnicity and pain care can be applied in considerations of how to address this gap related to SES. At the level of the patient, more knowledge is needed from the perspective of patients with chronic pain and lower SES regarding their needs, the challenges they experience obtaining pain management related to their income and education status, and their goals and expectations for treatment. Although this review focused on income and education as they pertain to SES, other areas that require attention are the disparities in access and care that patients receive based upon other factors, such as gender, ethnicity, and language. At the provider level, more research is needed to understand how patients’ income and education affect physicians’ decisions about chronic pain management and about stereotyping and biases that may be involved. At a broader level, more research has been recommended to understand how patients’ income and education intersect with various other social determinants of health to create, influence, or exacerbate chronic pain management disparities.
Addressing disparities in chronic pain care will similarly require a multitiered approach, with particular strategies at the level of the health care provider. With regard to addressing issues related to patients’ education, this could involve improving provider education concerning patients’ health literacy.Citation391 Health care providers have been found to overestimate patients’ understanding of clinical information.Citation391,Citation392 It has been suggested that providers use literacy-dependent teaching methods to provide information using a variety of modalities.Citation393–396 This would help promote shared clinical decision making.Citation393 With regard to addressing issues related to patients’ income, this could involve health care providers having open discussions with patients about the costs of pharmacological therapies.Citation397 Indeed, there is evidence to suggest that many providers consider it their responsibility to have such conversations with patients.Citation398,Citation399 Among the suggestions that Kolhatkar and colleaguesCitation397 have for prescribers to improve medication affordability are their “staying up to date on drug costs, prescribing the most cost-effective alternative, frequently reviewing medication prescription regimens for opportunities to deprescribe and prescribing generic drugs.”(pE549) It is important that health care providers be vigilant in considering the various factors that influence their prescribing practices. Physicians should be encouraged to examine their own implicit assumptions and behaviors toward chronic pain.Citation59 Moreover, it has been suggested that clinicians consider as well the financial conflicts of interest that may influence their prescribing of more expensive medications.Citation400 In accordance with the recommendations of the Canadian Pain Task Force,Citation12 other prescriber-level strategies include improving health care provider education of chronic pain as well as ensuring that its assessment occurs within a biopsychosocial framework that considers patients’ social determinants of health. Using a biopsychosocial approach to chronic pain conditions may assist in addressing the inequitable prescribing patterns among physicians based on a patient’s socioeconomic status because it would involve understanding the association of the patients’ pain with different aspects of their lives and vice versa. Understanding of patients’ socioeconomic situations can help better identify care options that are appropriate for them.
Higher-level strategies could focus on health care system reforms to improve patients’ access to evidence-based pharmacological, physical, psychological, and spiritual treatments for chronic pain.Citation14 First, expansion of insurance coverage for pain therapies is required. Programs in the United States such as Medicaid and those provided by the Veterans Health Administration have been shown to significantly improve patients’ access to pharmacological therapies and increase medication treatment adherence.Citation401–404 It has been suggested that further reducing or eliminating any co-payments that patients need to provide could further improve access to care for patients with lower SES.Citation405,Citation406 In Canada, the Canadian Pain Task ForceCitation12 recommends the expanding of patients’ access to pain treatments under provincial health care coverage and working with private insurers to incentivize expanded coverage of pain management options. Some provincial governments have endorsed improving patients access to currently non-insured nonpharmacological therapies for chronic pain.Citation407 Previous studies have demonstrated that universal access to health insurance is not adequate to achieve total equity in access to appropriate pain management treatments.Citation72,Citation75,Citation80,Citation81,Citation86 Therefore, it has been suggested that programs of universal coverage of prescription medications focused on equity should be developed to ensure that medications can be provided to patients based upon their needs.Citation408,Citation409 Second, the creation of national clinical guidelines to facilitate “evidence-informed, trauma-informed, equity-oriented, and biopsychosocial management of chronic pain across health care settings”Citation12(pCitation10) has been recommended. This sort of advocacy has been done by clinical organizations with the assistance of experts in health policy, pharmacoeconomics, behavioral sciences, clinical care, and the pharmaceutical industry in order to support the development of national policies to increase the affordability of medications.Citation410 The importance of such health system reforms is made clear, as Brady and colleaguesCitation7 noted that “recognizing chronic pain not only as a physical, socioeconomic or psychological problem but as an interaction between social class, migration class, gender, ethnoculture, and the health system encounter would facilitate framing of health disparities as a function of the system, rather than a responsibility of … specific communit[ies].”(p442)
Acknowledgments
The authors thank Robin Parker, an evidence synthesis librarian at Dalhousie University, for her assistance with the electronic literature search.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Nicholas M, Vlaeyen JWS, Rief W, Barke A, Aziz Q, Benoliel R, Cohen M, Evers S, Giamberardino MA, Goebel A, et al. The IASP classification of chronic pain for ICD-11: chronic primary pain. Pain. 2019;160(1):28–37. doi:10.1097/j.pain.0000000000001390.
- World Health Organization. International Classification of Diseases 11th Revision. https://icd.who.int/en [accessed 2021 Dec 4].
- International Association for the Study of Pain. IASP announces revised definition of pain. 2020 [accessed 2021 Dec 4]. https://www.iasp-pain.org/publications/iasp-news/iasp-announces-revised-definition-of-pain.
- Mills SE, Nicolson KP, Smith BH. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br J Anaesth. 2019;123(2):e273–e83. doi:10.1016/j.bja.2019.03.023.
- Sá KN, Moreira L, Baptista AF, Yeng LT, Teixeira MJ, Galhardoni R, de Andrade DC. Prevalence of chronic pain in developing countries: systematic review and meta-analysis. PAIN Rep. 2019;4(6):e779. doi:10.1097/PR9.0000000000000779.
- Roth RS, Punch MR, Bachman JE. Educational achievement and pain disability among women with chronic pelvic pain. J Psychosom Res. 2001;51(4):563–69. doi:10.1016/S0022-3999(01)00242-2.
- Brady B, Veljanova I, Chipchase L. The intersections of chronic noncancer pain: culturally diverse perspectives on disease burden. Pain Med. 2019;20(3):434–45. doi:10.1093/pm/pny088.
- Eachus J, Chan P, Pearson N, Propper C, Smith GD. An additional dimension to health inequalities: disease severity and socioeconomic position. J Epidemiol Community Health. 1999;53:603–11. doi:10.1136/jech.53.10.603.
- Hirsh AT, Miller MM, Hollingshead NA, Anastas T, Carnell ST, Lok BC, Chu C, Zhang Y, Robinson ME, Kroenke K, et al. A randomized controlled trial testing a virtual perspective-taking intervention to reduce race and socioeconomic status disparities in pain care. Pain. 2019;160(10):2229–40. doi:10.1097/j.pain.0000000000001634.
- Jay MA, Howard RF. Inequalities in access to a tertiary children’s chronic pain service: a cross-sectional study. Arch Dis Child. 2016;101(7):657–61. doi:10.1136/archdischild-2015-310280.
- Kapoor S, Thorn BE. Healthcare use and prescription of opioids in rural residents with pain. Rural Remote Health. 2014;14:2879.
- Canadian Pain Task Force. An action plan for pain in Canada. 2021. [accessed 2021 Jan 4]. https://www.canada.ca/en/health-canada/corporate/about-health-canada/public-engagement/external-advisory-bodies/canadian-pain-task-force/report-2021.html.
- Andersson I, Ejlertsson G, Leden I, Rosenberg C. Chronic pain in a geographically defined general population: studies of differences in age, gender, social class, and pain localization. Clin J Pain. 1993;9:174–82. doi:10.1097/00002508-199309000-00004.
- Bonathan C, Hearn L. de Williams CA. Socioeconomic status and the course and consequences of chronic pain. Pain Manag. 2013;3:159–62.
- Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Kaloukalani DA, Lasch KE, Myers C, Tait RC, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4(3):277–94. doi:10.1046/j.1526-4637.2003.03034.x.
- Poleshuck EL, Green CR. Socioeconomic disadvantage and pain. Pain. 2008;136(3):235. doi:10.1016/j.pain.2008.04.003.
- Saastamoinen P, Leino-Arjas P, Laaksonen M, Lahelma E. Socio-economic differences in the prevalence of acute, chronic and disabling chronic pain among ageing employees. Pain. 2005;114(3):364–71. doi:10.1016/j.pain.2004.12.033.
- Macfarlane GJ, Norrie G, Atherton K, Power C, Jones GT. The influence of socioeconomic status on the reporting of regional and widespread musculoskeletal pain: results from the 1958 British Birth Cohort Study. Ann Rheum Dis. 2009;68(10):1591–95. doi:10.1136/ard.2008.093088.
- Morgan CL, Conway P, Currie CJ. The relationship between self-reported severe pain and measures of socio-economic disadvantage. Eur J Pain. 2011;15(10):1107–1011. doi:10.1016/j.ejpain.2011.04.010.
- Janevic MR, McLaughlin SJ, Heapy AA, Thacker C, Piette JD. Racial and socioeconomic disparities in disabling chronic pain: findings from the health and retirement study. J Pain. 2017;18(12):1459–67. doi:10.1016/j.jpain.2017.07.005.
- Pollack CE, Chideya S, Cubbin C, Williams B, Dekker M, Braveman P. Should health studies measure wealth? A systematic review. Am J Prev Med. 2007;33(3):250–64. doi:10.1016/j.amepre.2007.04.033.
- Rios R, Zautra AJ. Socioeconomic disparities in pain: the role of economic hardship and daily financial worry. Health Psychol. 2011;30(1):58–66. doi:10.1037/a0022025.
- Booher L. The impact of low socioeconomic status in adults with chronic pain. Orthopaed Nurs. 2019;38:381–89. doi:10.1097/NOR.0000000000000620.
- Grol-Prokopczyk H. Sociodemographic disparities in chronic pain, based on 12-year longitudinal data. Pain. 2017;158(2):313–22. doi:10.1097/j.pain.0000000000000762.
- Hardt J, Jacobsen C, Goldberg J, Nickel R, Buchwald D. Prevalence of chronic pain in a representative sample in the United States. Pain Med. 2008;9(7):803–12. doi:10.1111/j.1526-4637.2008.00425.x.
- Johannes CB, Le TK, Zhou X, Johnston JA, Dworkin RH. The prevalence of chronic pain in United States adults: results of an internet-based survey. J Pain. 2010;11(11):1230–39. doi:10.1016/j.jpain.2010.07.002.
- Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. J Pain. 2004;5(6):317–28. doi:10.1016/j.jpain.2004.05.005.
- Riskowski JL. Associations of socioeconomic position and pain prevalence in the United States: findings from the National Health and Nutrition Examination Survey. Pain Med. 2014;15(9):1508–21. doi:10.1111/pme.12528.
- Jöud A, Petersson IF, Jordan KP, Lofvendahl S, Grahn B, Englund M. Socioeconomic status and the risk for being diagnosed with spondyloarthritis and chronic pain: a nested case–control study. Rheumatol Int. 2014;34(9):1291–98. doi:10.1007/s00296-014-3039-6.
- Kapteyn A, Smith JP, van Soest A. Dynamics of work disability and pain. J Health Econ. 2008;27(2):496–509. doi:10.1016/j.jhealeco.2007.05.002.
- Yoon YS, Oh SW, Park HS. Socioeconomic status in relation to obesity and abdominal obesity in Korean adults: a focus on sex differences. Obesity. 2006;14(5):909–19. doi:10.1038/oby.2006.105.
- Roth RS, Geisser ME. Educational achievement and chronic pain disability: mediating role of pain-related cognitions. Clin J Pain. 2002;18(5):286–96. doi:10.1097/00002508-200209000-00003.
- Lacey RJ, Belcher J, Croft PR. Does life course socio-economic position influence chronic disabling pain in older adults? A general population study. Eur J Pub Health. 2013;23(4):534–40. doi:10.1093/eurpub/cks056.
- Larson AG, Marcer D. The who and why of pain: analysis by social class. BMJ. 1984;288(6421):883–86. doi:10.1136/bmj.288.6421.883.
- Boulton M, Tuckett D, Olson C, Williams A. Social class and the general practice consultation. Sociol Health Illness. 1986;8(4):325–50. doi:10.1111/1467-9566.ep11340453.
- Waitzkin HB. Doctor-patient communication: clinical implications of social scientific research. JAMA. 1984;252(17):2441–46. doi:10.1001/jama.1984.03350170043017.
- Williams MV, Parker RM, Baker DW. Inadequate health literacy among patients at two public hospitals. JAMA. 1995;274:1677–82. doi:10.1001/jama.1995.03530210031026.
- Pendleton DA, Bochner S. The communication of medical information in general practice consultations as a function of patients’ social class. Soc Sci Med. 1980;14A:669–73.
- Stewart M. Patient characteristics which are related to the doctor-patient interaction. Fam Pract. 1984;1(1):30–35. doi:10.1093/fampra/1.1.30.
- Street RL; Street RL Jr. Information-giving in medical consultations: the influence of patients‘ communicative styles and personal characteristics. Soc Sci Med. 1991;32(5):541–48. doi:10.1016/0277-9536(91)90288-N.
- Bergenudd H, Nilson B. Back pain in middle age: occupational workload and psychological factors: an epidemiological survey. Spine. 1988;13:58–60. doi:10.1097/00007632-198801000-00014.
- Bigos SJ, Battie MC, Spengler DM, Fisher LD, Fordyce WE, Hansson T, Nachemson AL, Zeh J. A Longitudinal, Prospective Study of Industrial Back Injury Reporting. Clin Orthop. 1992;279:21–34. doi:10.1097/00003086-199206000-00004.
- Cats-Baril WL, Frymoyer JW. Demographic factors associated with the prevalence of disability in the general population: analysis of the NHANES I database. Spine. 1991;16(6):671–74. doi:10.1097/00007632-199106000-00019.
- Deyo RA, Diehl AK. Psychosocial predictors of disability in patients with low back pain. J Rheumatol. 1988;15:1557–64.
- Matthews KA. Are sociodemographic variables markers for psychological determinants of health? Health Psychol. 1989;8(6):641–48. doi:10.1037/0278-6133.8.6.641.
- Mnm VP, Koes BW, Deville W, Smid T, Bouter LM. Risk factors for back pain incidence in industry: a prospective study. Pain. 1998;77:81–86. doi:10.1016/S0304-3959(98)00085-2.
- Geisser ME, Robinson ME, Henson CD. Coping Strategies Questionnaire and chronic pain adjustment: a conceptual and empirical analysis. Clin J Pain. 1994;10:98–106. doi:10.1097/00002508-199406000-00003.
- Pincus T. Formal educational level--a marker for the importance of behavioral variables in the pathogenesis, morbidity, and mortality of most diseases? J Rheumatol. 1988;15:1457–60.
- Sullivan MJL, Stanish W, Waite H, Sullivan M, Tripp DA. Catastrophizing, pain, and disability in patients with soft-tissue injuries. Pain. 1998;77(3):253–60. doi:10.1016/S0304-3959(98)00097-9.
- Turner JA, Clancy S. Strategies for coping with chronic low back pain: relationship to pain and disability. Pain. 1986;24(3):355–64. doi:10.1016/0304-3959(86)90121-1.
- Odubanjo E, Bennett K, Feely J. Influence of socioeconomic status on the quality of prescribing in the elderly–a population based study. Br J Clin Pharm. 2004;58:496–502. doi:10.1111/j.1365-2125.2004.02179.x.
- Asamoah-Boaheng M, Badejo OA, Bell LV, Buckley N, Busse JW, Campbell TS, Corace K, Cooper L, Flusk D, Garcia DA, et al. Interventions to influence opioid prescribing practices for chronic noncancer pain: a systematic review and meta-analysis. Am J Prev Med. 2021;60(1):e15–e26. doi:10.1016/j.amepre.2020.07.012.
- Kay C, Wozniak E, Koller S, Ye A, Bernstein J. Adherence to chronic opioid therapy prescribing guidelines in a primary care clinic. J Opioid Manag. 2016;12:333–45. doi:10.5055/jom.2016.0350.
- McCalmont JC, Jones KD, Bennett RM, Friend R. Does familiarity with CDC guidelines, continuing education, and provider characteristics influence adherence to chronic pain management practices and opioid prescribing? J Opioid Manag. 2018;14:103–16. doi:10.5055/jom.2018.0437.
- Victor TW, Alvarez NA, Gould E. Opioid prescribing practices in chronic pain management: guidelines do not sufficiently influence practice. J Pain. 2009;10:1051–57. doi:10.1016/j.jpain.2009.03.019.
- Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. doi:10.1186/s12874-018-0611-x.
- Campbell LC, Robinson K, Meghani SH, Vallerand A, Schatman M, Sonty N. Challenges and opportunities in pain management disparities research: implications for clinical practice, advocacy, and policy. J Pain. 2012;13(7):611–19. doi:10.1016/j.jpain.2012.02.004.
- Webster F, Connoy L, Sud A, Pinto AD, Katz J. Grappling with chronic pain and poverty during the COVID-19 pandemic. Can J Pain. 2020;4:125–28. doi:10.1080/24740527.2020.1766855.
- Anastas TM, Miller MM, Hollingshead NA, Stewart JC, Rand KL, Hirsh AT. The unique and interactive effects of patient race, patient socioeconomic status, and provider attitudes on chronic pain care decisions. Ann Behav Med. 2020;54(10):771–82. doi:10.1093/abm/kaaa016.
- Bankole A. The relationship between socioeconomic factors and DMARDs use in rheumatoid arthritis. Arthritis Rheumatol. 2016;68:150–51.
- Baser O, Wang L, Li L, Xie L. Characteristics associated with initiation of intravenous vs subcutaneous injection tumor necrosis factor inhibitors for rheumatoid arthritis patients. Pharmacoepidemiol Drug Saf. 2013;22:498.
- Bigal ME, Buse DC, Chen Y-T, Golden W, Serrano D, Chu MK, Lipton RB. Rates and predictors of starting a triptan: results from the American migraine prevalence and prevention study. Headache. 2010;50(9):1440–48. doi:10.1111/j.1526-4610.2010.01703.x.
- Chuang E, Gil EN, Gao Q, Kligler B, McKee MD. Relationship between opioid analgesic prescription and unemployment in patients seeking acupuncture for chronic pain in urban primary care. Pain Med. 2019;20(8):1528–33. doi:10.1093/pm/pny169.
- Codreanu C, Popescu CC, Mogoşan, C. Area of residence and socioeconomic factors reduce access to biologics for rheumatoid arthritis patients in Romania. BioMed Res Int. 2018:7458361.
- Fanciullo GJ, Ball PA, Girault G, Rose RJ, Hanscom B, Weinstein JN. An observational study on the prevalence and pattern of opioid use in 25,479 patients with spine and radicular pain. Spine. 2002;27(2):201–05. doi:10.1097/00007632-200201150-00016.
- Frisell T, Baecklund E, Bengtsson K, Di Giuseppe D, Forsblad-d’Elia H, Askling J. Patient characteristics influence the choice of biological drug in RA, and will make non-TNFi biologics appear more harmful than TNFi biologics. Ann Rheum Dis. 2018;77(5):650–57. doi:10.1136/annrheumdis-2017-212395.
- Grol-Prokopczyk H. Use and opinions of prescription opioids among older American adults: sociodemographic predictors. J Gerontol B Psychol Soc Sci. 2019;74:1009–19. doi:10.1093/geronb/gby093.
- Kuo Y-F, Raji MA, Chen N-W, Hasan H, Goodwin JS. Trends in opioid prescriptions among Part D Medicare recipients from 2007 to 2012. Am J Med. 2016;129(2):221.e21–221.e30. doi:10.1016/j.amjmed.2015.10.002.
- Lipton RB, Serrano D, Holland S, Fanning KM, Reed ML, Buse DC. Barriers to the diagnosis and treatment of migraine: effects of sex, income, and headache features. Headache. 2013;53:81–92. doi:10.1111/j.1526-4610.2012.02265.x.
- McKeown T, Jacob B, Li X, Couto S, Bensen W, Ahluwalia V, Karasik A, Bombardier C. Comparison of medication use in rheumatoid arthritis patients between university and private settings – results from ontario best practice research initiative. J Rheumatol. 2015;42:1324.
- Nampiaparampil DE, Nampiaparampil JX, Harden RN. Pain and prejudice. Pain Med. 2009;10(4):716–21. doi:10.1111/j.1526-4637.2009.00612.x.
- Nielsen S, Campbell G, Peacock A, Smith K, Bruno R, Hall W, Cohen M, Degenhardt L. Health service utilisation by people living with chronic non-cancer pain: findings from the Pain and Opioids IN Treatment (POINT) study. Aust Health Rev. 2016;40(5):490–99. doi:10.1071/AH15047.
- Nikiphorou E, van der Heijde D, Norton S, Rbm L, Molto A, Dougados M, Van den Bosch FE, Ramiro S. Inequity in biological DMARD prescription for spondyloarthritis across the globe: results from the ASAS-COMOSPA study. Ann Rheum Dis. 2018;77:405–11. doi:10.1136/annrheumdis-2017-212457.
- Pensa MA, Galusha DH, Cantley LF. Patterns of opioid prescribing and predictors of chronic opioid use in an industrial cohort, 2003 to 2013. J Occup Environ Med. 2018;60(5):457–61. doi:10.1097/JOM.0000000000001231.
- Putrik P, Ramiro S, Lie E, Keszei AP, Kvien TK, van der Heijde D, Landewé R, Uhlig T, Boonen A. Less educated and older patients have reduced access to biologic DMARDs even in a country with highly developed social welfare (Norway): results from Norwegian cohort study NOR-DMARD. Rheumatology. 2016;55(7):1217–24. doi:10.1093/rheumatology/kew048.
- Shmagel A, Ngo L, Ensrud K, Foley R. Prescription medication use among community-based U.S. adults with chronic low back pain: a cross-sectional population based study. J Pain. 2018;19(10):1104–12. doi:10.1016/j.jpain.2018.04.004.
- Stokes A, Berry KM, Hempstead K, Lundberg DJ, Neogi T. Trends in prescription analgesic use among adults with musculoskeletal conditions in the United States, 1999-2016. JAMA Network Open. 2019a;2(12):e1917228. doi:10.1001/jamanetworkopen.2019.17228.
- Stokes A, Berry KM, Hempstead K, Neogi T. Trends in prescription pain management among US adults with arthritis or back pain, 1999-2014. Osteoarthritis and Cartilage. 2019b;27:S279–280. doi:10.1016/j.joca.2019.02.662.
- Tamayo-Sarver JH, Dawson NV, Hinze SW, Cydulka RK, Wigton RS, Albert JM, Ibrahim SA, Baker DW. The effect of race/ethnicity and desirable social characteristics on physicians’ decisions to prescribe opioid analgesics. Acad Emerg Med. 2003;10:1239–48. doi:10.1197/S1069-6563(03)00494-9.
- Tatangelo M, Tomlinson G, Paterson JM, Ahluwalia V, Kopp A, Gomes T, Bansback N, Bombardier C. Association of patient, prescriber, and region with the initiation of first prescription of biologic disease-modifying antirheumatic drug among older patients with rheumatoid arthritis and identical health insurance coverage. JAMA Network Open. 2019;2(12):e1917053. doi:10.1001/jamanetworkopen.2019.17053.
- Wang P-J, Chou Y-J, Lee C-H C-H, Pu C. Diffusion of new medication across different income groups under a universal health insurance program: an example involving newly enlisted nonsteroidal anti-inflammatory drugs for elderly osteoarthritis patients. Int J Public Health. 2010;55(5):497–506. doi:10.1007/s00038-010-0132-9.
- Yelin E, Tonner C, Kim SC, Katz JN, Ayanian JZ, Brookhart MA, Solomon DH. Sociodemographic, disease, health system, and contextual factors affecting the initiation of biologic agents in rheumatoid arthritis: a longitudinal study. Arthritis Care Res. 2014;66(7):980–89. doi:10.1002/acr.22244.
- Celentano DD, Stewart WF, Lipton RB, Reed ML. Medication use and disability among migraineurs: a national probability sample survey. Headache. 1992;32(5):223–28. doi:10.1111/j.1526-4610.1992.hed3205223.x.
- Chu MK, Buse DC, Bigal ME, Serrano D, Lipton RB. Factors associated with triptan use in episodic migraine: results from the American migraine prevalence and prevention study. Headache. 2012;52(2):213–23. doi:10.1111/j.1526-4610.2011.02032.x.
- Frenk SM, Porter KS, Paulozzi LJ. Prescription opioid analgesic use among adults: united States, 1999-2012. NCHS Data Brief. 2015;189:1–8.
- Lacaille D, Anis AH, Guh DP, Esdaile JM. Gaps in care for rheumatoid arthritis: a population study. Arthritis Rheum. 2005;53(2):241–48. doi:10.1002/art.21077.
- Schmajuk G, Trivedi A, Solomon D, Yelin E, Trupin L, Chakravarty E, Yazdany J. Receipt of disease-modifying antirheumatic drugs among patients with rheumatoid arthritis in Medicare managed care plans. JAMA. 2011;305(5):480–86. doi:10.1001/jama.2011.67.
- Wu J, Noxon V, Lu ZK. Patterns of use and health expenses associated with triptans among adults with migraines. Clin J Pain. 2015;31(8):673–79. doi:10.1097/AJP.0000000000000152.
- Yazdany J, Tonner C, Schmajuk G, Lin GA, Trivedi AN. Receipt of glucocorticoid monotherapy among Medicare beneficiaries with rheumatoid arthritis. Arthritis Care Res. 2014;66(10):1447–55. doi:10.1002/acr.22312.
- Abbott S, Hobby L. Welfare benefits advice in primary care: evidence of improvements in health. Public Health. 2000;114(5):324–27. doi:10.1038/sj.ph.1900680.
- Ackerman SJ, Steinberg EP, Bryan RN, BenDebba M, Long DM. Patient characteristics associated with diagnostic imaging evaluation of persistent low back problems. Spine. 1997;22(14):1634–40. doi:10.1097/00007632-199707150-00021.
- Adler NE, Stead WW. Patients in context—EHR capture of social and behavioral determinants of health. N Engl J Med. 2015;70:388–90.
- Alford DP. Opioid Prescribing for Chronic Pain — achieving the Right Balance through Education. N Engl J Med. 2016;374(4):301–03. doi:10.1056/NEJMp1512932.
- Bansback N, Young A, Brennan A, Dixey J. A prognostic model for functional outcome in early rheumatoid arthritis. J Rheumatol. 2006;33:1503–10.
- Bourne PA. Socio-demographic determinants of health care-seeking behaviour, self-reported illness and self-evaluated health status in Jamaica. Int J Collab Res Int Med Public Health. 2009;1:101–30.
- Cerdá M, Gaidus A, Keyes KM, Ponicki W, Martins S, Galea S, Gruenewald P. Prescription opioid poisoning across urban and rural areas: identifying vulnerable groups and geographic areas. Addiction. 2017;112(1):103–12. doi:10.1111/add.13543.
- Chang YP. Factors associated with prescription opioid misuse in adults aged 50 or older. Nurs Outlook. 2018;66:112–20. doi:10.1016/j.outlook.2017.10.007.
- Cooper F, Marx BL, Lee TL, Espesete D. Super-users at an acupuncture and oriental medicine teaching clinic: demographics and unique clinical characteristics. J Altern Complement Med. 2017;23(3):222–26. doi:10.1089/acm.2016.0419.
- Dora-Laskey A, Goldstick J, Bohnert A, Lisa B, Cunningham R, Carter PM. Predictors of misuse among adult emergency department patients using prescription opioids. Acad Emerg Med. 2018;25:S185.
- Feingold D, Goor-Aryeh I, Bril S, Delayahu Y, Lev-Ran S. Problematic use of prescription opioids and medicinal cannabis among patients suffering from chronic pain. Pain Med. 2017;18(2):294–306. doi:10.1093/pm/pnw134.
- Haetzman M, Elliott AM, Smith BH, Hannaford P, Chambers WA. Chronic pain and the use of conventional and alternative therapy. Fam Pract. 2003;20(2):147–54. doi:10.1093/fampra/20.2.147.
- Havens JR, Stoops WW, Leukefeld CG, Garrity TF, Carlson RG, Falck R, Wang J, Booth BM. Prescription opiate misuse among rural stimulant users in a multistate community-based study. The American Journal of Drug and Alcohol Abuse. 2009;35(1):18–23. doi:10.1080/00952990802326298.
- Hurstak EE, Kushel M, Chang J, Ceasar R, Zamora K, Miaskowski C, Knight K. The risks of opioid treatment: perspectives of primary care practitioners and patients from safety-net clinics. Substance Abuse. 2017;38(2):213–21. doi:10.1080/08897077.2017.1296524.
- Khodneva Y, Muntner P, Kertesz S, Howard G, Safford M. Prescription opioid use is associated with increased mortality in the reasons for geographic and racial differences in stroke study. Drug and Alcohol Dependence. 2015;146:e157. doi:10.1016/j.drugalcdep.2014.09.345.
- Kikano GE, Schiaffino MA, Zyzanski SJ. Medical decision making and perceived socioeconomic class. Arch Fam Med. 1996;5:267. doi:10.1001/archfami.5.5.267.
- Licciardone JC, Pandya V. Use of complementary health approaches for chronic low-back pain: a pain research registry-based study. J Altern Complement Med. 2020;26(5):369–75. doi:10.1089/acm.2019.0448.
- Owen-Smith A, Stewart C, Sesay MM, Strasser SM, Yarborough BJ, Ahmedani B, Miller-Matero LR, Waring SC, Haller IV, Waitzfelder BE, Sterling SA. Chronic pain diagnoses and opioid dispensings among insured individuals with serious mental illness. BMC Psychiatry. 2020;20:40. doi:10.1186/s12888-020-2456-1.
- Riley III JL, King C. Self-report of alcohol use for pain in a multi-ethnic community sample. The Journal of Pain. 2009;10(9):944–52. doi:10.1016/j.jpain.2009.03.005.
- Walker MJ, Webster LR. Risk factors for drug diversion in a pain clinic patient population. J Opioid Manag. 2012;8:351–62. doi:10.5055/jom.2012.0135.
- Wallace LS, Keenum AJ, Roskos SE, McDaniel KS. Development and validation of a low-literacy opioid contract. J Pain. 2007;8(10):759–66. doi:10.1016/j.jpain.2007.05.004.
- Ward L, Patel NM, Hanlon A, Eldakar-Hein S, Sherlinski K, Ward SH. Prescription medication borrowing among adult patients at an urban medical center. J Urban Health. 2011;88(6):997–1014. doi:10.1007/s11524-011-9589-y.
- Wilkie R, Jordan JL, Muller S, Nicholls E, Healey EL. Van Der Windt DA. Measures of social function and participation in musculoskeletal populations: impact on participation and autonomy (IPA), Keele Assessment of Participation (KAP), Participation Measure for Post-Acute Care (PM-PAC), Participation Objective, Participation S. Arthritis Care Res. 2011;63:S325–S336.
- Adams J, Peng W, Cramer H, Sundberg T, Moore C, Amorin-Woods L, Sibbritt D, Lauche R. The prevalence, patterns, and predictors of chiropractic use among US adults. Spine. 2017;42(23):1810–16. doi:10.1097/BRS.0000000000002218.
- Armstrong AR, Thiébaut SP, Brown LJ, Nepal B. Australian adults use complementary and alternative medicine in the treatment of chronic illness: a national study. Aust N Z J Public Health. 2011;35:384–90. doi:10.1111/j.1753-6405.2011.00745.x.
- Beer K, Henderson JCR, Papaleontiou M, Olkhovskaya Y, Wigglesworth J, Reid MC. Physical Therapists‘ Use of Cognitive-Behavioral Therapy for Older Adults With Chronic Pain: a Nationwide Survey. Phys Ther. 2009;89(5):456–69. doi:10.2522/ptj.20080163.
- Bruns EB, Befus D, Wismer B, Knight K, Adler SR, Leonoudakis-Watts K, Thompson-Lastad A, Chao MT. Vulnerable Patients‘ Psychosocial Experiences in a Group-Based, Integrative Pain Management Program. J Altern Complement Med. 2019;25(7):719–26. doi:10.1089/acm.2019.0074.
- Bujold E, Huff J, Staton EW, Pace WD. Improving use of narcotics for nonmalignant chronic pain: a lesson from Community Care of North Carolina. J Opioid Manag. 2012;8:363–67. doi:10.5055/jom.2012.0136.
- Cheng T, D’Amico S, Luo M, Lestoquoy AS, Yinusa-Nyahkoon L, Laird LD, Gardiner PM. Health disparities in access to nonpharmacologic therapies in an urban community. J Altern Complement Med. 2019;25(1):48–60. doi:10.1089/acm.2018.0217.
- Cherniack EP, Senzel RS, Pan CX. Correlates of use of alternative medicine by the elderly in an urban population. J Altern Complement Med. 2001;7(3):277–80. doi:10.1089/107555301300328160.
- Forestier R, André-Vert J, Guillez P, Coudeyre E, M-m L-C, Combe B, Mayoux-Benhamou MA. Non-pharmacological non-surgical treatment of rheumatoid arthritis: medicosocial and organizational aspects. Kinestherapie. 2012;12:30–40.
- Hurstak EE, Wismer B, Leonoudakis K, Pace J, Chao MT. Integrative pain management clinic serving high risk primary care patients with chronic pain. J Gen Intern Med. 2017;32:S773.
- Jinks C, Vohora K, Young J, Handy J, Porcheret M, Jordan KP. Inequalities in primary care management of knee pain and disability in older adults: an observational cohort study. Rheumatology. 2011;50(10):1869–78. doi:10.1093/rheumatology/ker179.
- Joines JD, Hertz-Picciotto I, Carey TS, Gesler W, Suchindran C. A spatial analysis of county-level variation in hospitalization rates for low back problems in North Carolina. Soc Sci Med. 2003;56(12):2541–53. doi:10.1016/S0277-9536(02)00295-2.
- Jong M, Kraishi M. A comparative study on the utility of telehealth in the provision of rheumatology services to rural and northern communities. Int J Circumpolar Health. 2004;63(4):415–21. doi:10.3402/ijch.v63i4.17758.
- Laliberté M, Mazer B, Orozco T, Chilingaryan G, Williams-Jones B, Hunt M, Feldman DE. Implicit biases influencing service provision in physical therapy for low back pain. Arthritis Rheumatol. 2016;68:1599.
- Lillefjell M, Haugan T, Martinussen P, Halvorsen T. Treatment Outcomes Among Individuals in a Musculoskeletal Pain Rehabilitation Program Related to the Prevalence and Trends in the Dispensing of Prescribed Medications. J Musculoskelet Pain. 2013;21(4):311–19. doi:10.3109/10582452.2013.848968.
- Loeser JD. Comprehensive pain programs versus other treatments for chronic pain. J Pain. 2006;7(11):800–01. doi:10.1016/j.jpain.2006.09.008.
- Ludwick A, Corey K, Meghani S. Racial and socioeconomic factors associated with the use of complementary and alternative modalities for pain in cancer outpatients: an integrative review. Pain Manag Nurs. 2020;21:142–50. doi:10.1016/j.pmn.2019.08.005.
- Newman AK, Kapoor S, Thorn BE. Health care utilization for chronic pain in low-income settings. Pain Med. 2018;19(12):2387–97. doi:10.1093/pm/pny119.
- Nyiendo J, Haas M, Goldberg B, Sexton G. Patient characteristics and physicians’ practice activities for patients with chronic low back pain: a practice-based study of primary care and chiropractic physicians. J Manipul Physiol Therap. 2001;24:92–100. doi:10.1067/mmt.2001.112565.
- Proctor TJ, Mayer TG, Theodore B, Gatchel RJ. Failure to complete a functional restoration program for chronic musculoskeletal disorders: a prospective 1-year outcome study. Arch Phys Med Rehabil. 2005;86(8):1509–15. doi:10.1016/j.apmr.2005.02.010.
- Roy P, Jackson AH, Baxter J, Brett B, Winter M, Hardesty I, Alford DP. Utilizing a faculty development program to promote safer opioid prescribing for chronic pain in internal medicine resident practices. Pain Med. 2019;20(4):707–16. doi:10.1093/pm/pny292.
- Sundberg T, Cramer H, Sibbritt D, Adams J, Lauche R. Prevalence, patterns, and predictors of massage practitioner utilization: results of a US nationally representative survey. Musculoskelet Sci Pract. 2017;32:31–37. doi:10.1016/j.msksp.2017.07.003.
- Tuakli-Wosornu YA, Selzer F, Losina E, Katz JN. Predictors of exercise adherence in patients with meniscal tear and osteoarthritis. Arch Phys Med Rehabil. 2016;97(11):1945–52. doi:10.1016/j.apmr.2016.05.011.
- Turner BJ, Rodriguez N, Liang Y, Valerio M, Winkler P, Potter J, Avant K, Hill J. Use of exercise versus pain medications for chronic pain: analysis of a population-based sample of Hispanics from five states. J Gen Intern Med. 2016;31:S453–S454.
- Turner B, Rodriguez N, Valerio M, Liang Y, Winkler P, Jackson L. Challenges for U S National Pain Strategy in low income population. J Pain. 2017;18:S62.
- Turner BJ, Rodriguez N, Valerio MA, Liang Y, Winkler P, Jackson L. Less exercise and more drugs: how a low-income population manages chronic pain. Arch Phys Med Rehabil. 2017;98(11):2111–17. doi:10.1016/j.apmr.2017.02.016.
- Ünsal A, Gözüm S. Use of complementary and alternative medicine by patients with arthritis. J Clin Nurs. 2010;19(7–8):1129–38. doi:10.1111/j.1365-2702.2009.03111.x.
- Van Der Vaart R, Van Driel D, Pronk K, Paulussen S. te Boekhorst S, Rosmalen JGM, Evers AWM. The Casing should be initial caps: role of age, education, and digital health literacy in the usability of internet-based cognitive behavioral therapy for chronic pain mixed Methods Study J Med Internet Res. 2019;21:e12883.
- Vina ER, Ran D, Ashbeck EL, Kaur M, Kwoh CK. Relationship between knee pain and patient preferences for joint replacement: health care access matters. Arthritis Care Res. 2017;69(1):95–103. doi:10.1002/acr.23084.
- Vina ER, Ran D, Hannon MJ, Kwoh CK. Understanding ethnic differences in the utilisation of exercise for osteoarthritis. Ann Rheum Dis. 2018;77:519.
- Ahluwalia V, Movahedi M, Rampakakis E, Cesta A, Li X, Couto S, Sampalis J, Bombardier C. Predictors of patient reported decision to discontinue anti-rheumatic medication in rheumatoid arthritis patients: data from a rheumatoid arthritis cohort. J Rheumatol. 2017;44:939–40.
- Ahluwalia V, Movahedi M, Rampakakis E, Cesta A, Li X, Sampalis JS, Bombardier C. Predictors of non-adherence with anti-rheumatic medication in rheumatoid arthritis patients: data from a rheumatoid arthritis cohort. Arthritis Rheumatol. 2016;68:4127–30.
- Alhefny -AE-AM, Mobasher SA, Abd El-Rahman MA, Shedid NH, Sakr HM, Hassan RM. Evaluation of adherence to drug treatment in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76:1145.
- Ali MW, Musami UB, Sa’ad FK, Omoaghe C, Danimoh MA, Ayoola YA, Kanu AO. Profile of migraine patients in a developing country: a multicentre study. SN Comprehensive Clinical Medicine. 2020;2(8):1153–57. doi:10.1007/s42399-020-00394-x.
- Alexandria V. Study indicates low arthritis medication adherence rate in diverse population. PT in Motion. 2013;5:11.
- Alkabbani W, Marrie RA, Bugden S, Alessi‐Severini S, Bolton JM, Daeninck P, Leong C. Persistence of use of prescribed cannabinoid medicines in Manitoba, Canada: a population-based cohort study. Addiction. 2019;114(10):1791–99. doi:10.1111/add.14719.
- Allen C, Murphy A, Kiselbach S, VandenBerg S, Wiebe E. Exploring the experience of chronic pain among female Survival Sex Workers: a qualitative study. BMC Fam Pract. 2015;16(1):182. doi:10.1186/s12875-015-0395-6.
- Allen KD, Bosworth HB, Chatterjee R, Coffman CJ, Corsino L, Jeffreys AS, Oddone EZ, Stanwyck C, Yancy WS, Dolor RJ. Clinic variation in recruitment metrics, patient characteristics and treatment use in a randomized clinical trial of osteoarthritis management. BMC Musculoskelet Dis. 2014;15(1):413. doi:10.1186/1471-2474-15-413.
- Alvarez Nemegyei J, López Salas SJ, Aguilar Erosa JA, Marín Ordóñez J, Angulo Ramírez AV. Effect of clinical and sociodemographic factors on drug treatment adherence in rheumatoid arthritis. J Rheumatol. 2011;38:1199.
- Antoniou T, Ala-Leppilampi K, Shearer D, Parsons JA, Tadrous M, Gomes T. “Like being put on an ice floe and shoved away”: a qualitative study of the impacts of opioid-related policy changes on people who take opioids. Int J Drug Policy. 2019;66:15–22. doi:10.1016/j.drugpo.2019.01.015.
- Argoff C, Stanos S, Irving G, Puenpatom R, Zhao S, Gould E. Demographic characteristics of patients prescribed chronic opioid therapy for chronic noncancer pain: data from the opioid utilization study (OPUS). J Pain. 2010;11(4):S46. doi:10.1016/j.jpain.2010.01.192.
- Bansal K, Rajput SK, Sharma V, Mathur VS. Comparative analysis of socio-economic status in RHD patients: a tertiary care interpretation and exploring reasons to improve adherence. Int J Pharm Sci Res. 2019;10:790–94.
- Barbosa HD, Nogueira AA, Silva JC, Poli Neto OB, Reis FJ. The influence of education and depression on autonomy of women with chronic pelvic pain: a cross-sectional study. Revista Brasileira de Ginecologia e Obstetrícia. 2016;38:47–52. doi:10.1055/s-0035-1570107.
- Barbuto JP, White JGL, Porucznik CA, Holmes EB. Chronic pain: second, do no harm. Am J Phys Med Rehabil. 2008;87(1):78–83. doi:10.1097/PHM.0b013e31815e87b1.
- Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, Dieppe P. The causes and effects of socio-demographic exclusions from clinical trials. Health Technol Assess (Rockv). 2005;9(38):iii–152. doi:10.3310/hta9380.
- Bartuschka A, Dorner TE, Zeinlinger R, Fenzl KH, Mustak M, Erlacher L. Adherence with medication in patients with rheumatoid arthritis and association of socioeconomic variables with adherence. J fur Mineralstoffwechsel. 2014;21:129–30.
- Bazargan M, Cobb S, Wisseh C, Assari S. Psychotropic and opioid-based medication use among economically disadvantaged African-American older adults. Pharmacy. 2020;8(2):74. doi:10.3390/pharmacy8020074.
- Beatty PW, Hagglund KJ, Neri MT, Dhont KR, Clark MJ, Hilton SA. Access to health care services among people with chronic or disabling conditions: patterns and predictors. Arch Phys Med Rehabil. 2003;84:1417–25. doi:10.1016/S0003-9993(03)00268-5.
- Befus DR, Irby MB, Coeytaux RR, Penzien DB. A critical exploration of migraine as a health disparity: the imperative of an equity-oriented, intersectional approach. Curr Pain Headache Rep. 2018;22(12):79. doi:10.1007/s11916-018-0731-3.
- Bekanich SJ, Wanner N, Junkins S, Mahoney K, Kahn KA, Berry CA, Stowell SA, Gardner AJ. A multifaceted initiative to improve clinical awareness of pain management disparities. Am J Med Quality. 2014;29:388–96. doi:10.1177/1062860613503897.
- Benson C, Kim M, Chow W, Wang L. Demographic and characteristics of patients from the Oxycodone Users Registry (OUR): a prospective, multicenter registry of patients with non-malignant pain. J Pain. 2010;11:S47.
- Brandão T, Campos L, De Ruddere L, Goubert L, Bernardes SF. Classism in pain care: the role of patient socioeconomic status on nurses’ pain assessment and management practices. Pain Med. 2019;20(11):2094–105. doi:10.1093/pm/pnz148.
- Breuer B, Pappagallo M, Tai JY, Portenoy PK. U S Board-certified pain physician practices: uniformity and census data of their locations. J Pain. 2007;8:244–50.
- Broekmans S, Dobbels F, Milisen K, Morlion B, Vanderschueren S. Determinants of medication underuse and medication overuse in patients with chronic non-malignant pain: a multicenter study. Int J Nurs Stud. 2010;47(11):1408–17. doi:10.1016/j.ijnurstu.2010.03.014.
- Brown EM, Garneau KL, Tsao H, Solomon DH. DMARD non-use in low-income, elderly rheumatoid arthritis patients: results of 86 structured interviews. Arthritis Res Ther. 2014;16(1):1–7. doi:10.1186/ar4459.
- Buse DC, Nicholson RA, Araujo AB, Reed ML, Shapiro RE, Ashina S, Jaffe DH, Cambron-Mellott M, Li VW, Zagar A, et al. Migraine Care Across the Healthcare Landscape in the United States among those with≥= 4 Migraine Headache Days Per Month: results of the OVERCOME Study. Headache. 2019;59:16–17.
- Cano A, Mayo A, Ventimiglia M. Coping, pain severity, interference, and disability: the potential mediating and moderating roles of race and education. J Pain. 2006;7(7):459–68. doi:10.1016/j.jpain.2006.01.445.
- Cantor M, Leon I, Pulgarin CP. Facts and facets: using a citywide dataset to analyze the impact of community-level social determinants on clinical outcomes. J Gen Intern Med. 2018;33:201.
- Carter C, Goldfarb NI, Hartmann CW, Roumm AR, Vallow SM, Durkin M. Chronic pain management in managed care organizations: a national survey of medical directors. P & T. 2003;28:179–84.
- Chaudhary N, Longworth S, Sell PJ. Management of mechanical low back pain: a survey of beliefs and attitudes in GPs from Leicester and Nottingham. European J Gen Pract. 2004;10:71–72. doi:10.3109/13814780409094238.
- Choi J, Michael A, Michael D, Rothrock JF. A regional comparison of headache clinic populations: demography, diagnoses and frequencies/types of symptomatic medicaton overuse. Headache. 2016;56:45–46.
- Choi NG, Snow AL, Kunik ME. Pain severity, interference, and prescription analgesic use among depressed, low-income homebound older adults. Aging Ment Health. 2016;20:804–13. doi:10.1080/13607863.2015.1037244.
- Cisternas MG, Yelin E, Katz JN, Solomon DH, Wright EA, Losina E. Ambulatory visit utilization in a national, population-based sample of adults with osteoarthritis. Arthritis Care Res. 2009;61(12):1694–703. doi:10.1002/art.24897.
- Curtis JR, Xie F, Smith C, Saag KG, Chen L, Beukelman T, Mannion M, Yun H, Kertesz S. Changing Trends in Opioid Use Among Patients With Rheumatoid Arthritis in the United States. Arthritis Rheumatol. 2017;69(9):1733–40. doi:10.1002/art.40152.
- Curtis J, Xie F, Saag K, Chen L, Beukelman T, Mannion M, Yun H. Changing patterns over time in opiate use in U.S. rheumatoid arthritis patients. Arthritis Rheum. 2016;68:4124–25.
- Curtis JP, Sharma P, Arora T, Bharat A, Barnes I, Morrisey MA, Kilgore M, Saag KG, Wright NC, Yun HG, et al. Physicians’ explanations for apparent gaps in the quality of rheumatology care: results from the US Medicare Physician Quality Reporting System. Arthritis Care Res. 2013;65:235–43. doi:10.1002/acr.21713.
- Dale O, Borchgrevink PC, Fredheim OM, Mahic M, Romundstad P, Skurtveit S. Prevalence of use of non-prescription analgesics in the Norwegian HUNT3 population: impact of gender, age, exercise and prescription of opioids. BMC Public Health. 2015;15(1):461. doi:10.1186/s12889-015-1774-6.
- Davis MM, Patel MS, Halasyamani LK. Variation in estimated Medicare prescription drug plan costs and affordability for beneficiaries living in different states. J Gen Intern Med. 2007;22(2):257–63. doi:10.1007/s11606-006-0018-y.
- DeWitt EM, Lin L, Glick HA, Anstrom KJ, Schulman KA, Reed SD. Pattern and predictors of the initiation of biologic agents for the treatment of rheumatoid arthritis in the United States: an analysis using a large observational data bank. Clin Ther. 2009;31(8):1871–80. doi:10.1016/j.clinthera.2009.08.020.
- Doshi JA, Brandt N, Stuart B. The Impact Of Drug Coverage On COX-2 Inhibitor Use In Medicare: elders with the most generous drug coverage in 2000, not those who met certain clinical criteria, were most likely to use COX-2 inhibitors. Health Aff. 2004;23(Suppl1:W4–94.
- Dubin RE, Kaplan A, Graves L, Ng VK. Acknowledging stigma: its presence in patient care and medical education. Can Fam Phys. 2017;63:906–08.
- Edlund SM, Wurm M, Holländare F, Linton SJ, Fruzzetti AE, Tillfors M. Pain patients’ experiences of validation and invalidation from physicians before and after multimodal pain rehabilitation: associations with pain, negative affectivity, and treatment outcome. Scand J Pain. 2017;17(1):77–86. doi:10.1016/j.sjpain.2017.07.007.
- Elder R, Acheson RM. New Haven survey of joint diseases. XIV Social Class and Behavior in Response to Symptoms of Osteoarthritis Milbank Memorial Fund Quarterly. 1970;48:449–502.
- Elliott RA. Poor Adherence to Medication in Adults with Rheumatoid Arthritis. Dis Manag Health Out. 2008;16(1):13–29. doi:10.2165/00115677-200816010-00003.
- Fletcher A, Lassere M, Black R, Barrett C, Caroll G, Lester S, Richards B, March L, Buchbinder R, Hill C. Socioeconomic differences in opioid use by people with inflammatory arthritis. Arthritis Rheumatol. 2018;70.
- Fletcher A, Lassere M, March L, Buchbinder R, Lester S, Barrett C, Carroll G, Black R, Richards B, Hill C. Socioeconomic differences in opioid use by people with inflammatory arthritis: data from the Australian Rheumatology Association database. Int J Rheum Dis. 2019;22:75.
- Garcia-Gonzalez A, Richardson M, Garcia Popa-Lisseanu M, Cox V, Kallen MA, Janssen N, Ng B, Marcus DM, Reveille JD, Suarez-Almazor ME. Treatment adherence in patients with rheumatoid arthritis and systemic lupus erythematosus. Clin Rheum. 2008;27:883–89. doi:10.1007/s10067-007-0816-6.
- Gibbs SN, Shah S, Deshpande CG, Bensink ME, Broder MS, Dumas PK, Buse DC, Vo P, Schwedt TJ. United States Patients’ Perspective of Living With Migraine: country-Specific Results From the Global “My Migraine Voice” Survey. Headache. 2020;60(7):1351–64. doi:10.1111/head.13829.
- Gilman BH, Gage B, Haber S, Hoover S, Aggarwal J. Impact of drug coverage on medical expenditures among the elderly. Health Care Finan Rev. 2007;29:103.
- Green MA, Little E, Cooper R, Relton C, Strong M. Investigation of social, demographic and health variations in the usage of prescribed and over-the-counter medicines within a large cohort (South Yorkshire, UK). BMJ Open. 2016;6(9):e012038. doi:10.1136/bmjopen-2016-012038.
- Harrold LR, Briesacher BA, Peterson DA, Beard A, Madden J, Zhang F, Gurwitz JH, Soumerai SB. Cost-related medication nonadherence in older patients with rheumatoid arthritis. J Rheumatology. 2013;40:137–43. doi:10.3899/jrheum.120441.
- Heidari P, Cross W, Weller C, Team V, Nazarinia M, Crawford K. Rheumatologists‘ insight into medication adherence in patients with rheumatoid arthritis: a qualitative study. Int J Rheum Dis. 2019;22(9):1695–705. doi:10.1111/1756-185X.13660.
- Ison M, Duggan E, Mehdi A, Thomas R, Benham H. Treatment delays for patients with new-onset rheumatoid arthritis presenting to an Australian early arthritis clinic. Intern Med J. 2018;48(12):1498–504. doi:10.1111/imj.13972.
- Ives TJ, Chelminski PR, Hammett-Stabler CA, Malone RM, Perhac JS, Potisek NM, Shilliday BB, DeWalt DA, Pignone MP. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv Res. 2006;6(1):46. doi:10.1186/1472-6963-6-46.
- Jin XM, Lee J, Choi NK, Seong JM, Shin JY, Kim YJ, Kim MS, Yang BR, Park BJ. Utilization patterns of disease-modifying antirheumatic drugs in elderly rheumatoid arthritis patients. J Korean Med Sci. 2014;29:210–16. doi:10.3346/jkms.2014.29.2.210.
- Kennedy J, Morgan S. A cross-national study of prescription nonadherence due to cost: data from the joint Canada-United States survey of health. Clin Ther. 2006;28(8):1217–24. doi:10.1016/j.clinthera.2006.07.009.
- Kennedy J, Tuleu I, Mackay K. Unfilled prescriptions of medicare beneficiaries: prevalence, reasons, and types of medicines prescribed. J Manag Care Pharm. 2008;14(6):553–60. doi:10.18553/jmcp.2008.14.6.553.
- Kirsch M, Montgomery JR, Hu HM, Englesbe M, Hallstrom B, Brummett CM, Fendrick AM, Waljee JF. Association between insurance cost-sharing subsidy and postoperative opioid prescription refills among Medicare patients. Surgery. 2020;168(2):244–52. doi:10.1016/j.surg.2020.04.013.
- Kuipers JG, Koller M, Zeman F, Müller K, Rüffer JU. Adherence and health literacy as related to outcome of patients treated for rheumatoid arthritis: analyses of a large-scale observational study. Z Rheumatol. 2019;78:74–81. doi:10.1007/s00393-018-0449-y.
- Kurlander JE, Kerr EA, Krein S, Heisler M, Piette JD. Cost-related nonadherence to medications among patients with diabetes and chronic pain: factors beyond finances. Diabetes Care. 2009;32(12):2143–48. doi:10.2337/dc09-1059.
- Li X, Anis AH. Cost sharing of prescription drugs and demand for health-care utilization among seniors with rheumatoid arthritis. Appl Econ Lett. 2013;20(1):23–27. doi:10.1080/13504851.2012.669456.
- Lofchie A, Butler N, Robbins M, Seng E. Predictors of non-adherence with acute migraine medications. Neurology. 2019;92:410–002.
- Löfvendahl S, Jöud A, Petersson IF, Theander E, Å S, Carlsson KS. Income disparities in healthcare use remain after controlling for healthcare need: evidence from Swedish register data on psoriasis and psoriatic arthritis. Eur J Health Econ. 2018;19:447–62. doi:10.1007/s10198-017-0895-5.
- Menon V, Kelley S. A thousand patient voices: knowledge, impact, and experiences of living with osteoarthritis of the knee. Osteoarthritis Cartilage. 2020;28:S491–2. doi:10.1016/j.joca.2020.02.768.
- Mukherjee K, Kamal KM. Socio-demographic factors and out-of-pocket expenditure for prescription drugs in rheumatoid arthritis. Value in Health. 2016;19(3):A232. doi:10.1016/j.jval.2016.03.1141.
- Mukherjee K, Kamal KM. Sociodemographic determinants of out-of-pocket expenditures for patients using prescription drugs for rheumatoid arthritis. Am Health Drug Benefits. 2017;10:7–15.
- Murphy LB, Yelin E, Theis KA. Compromised access to prescriptions and medical care because of cost among US adults with arthritis. Best Pract Res Clin Rheumatol. 2012;26(5):677–94. doi:10.1016/j.berh.2012.10.007.
- Musich S, Wang SS, Slindee L, Kraemer S, Yeh CS. Characteristics associated with transition from opioid initiation to chronic opioid use among opioid-naive older adults. Geriatr Nurs. 2019;40:190–96. doi:10.1016/j.gerinurse.2018.10.003.
- Nair KV, Valuck RJ, Allen RR, Lewis SJ. Impact of increased copayments on the discontinuation/switching rates of nonformulary medications. J Pharm Tech. 2005;21(3):137–43. doi:10.1177/875512250502100304.
- Nascimben J, Cubbison C, Lape EC, Katz JN. Strategies for managing the costs of chronic illness in the context of limited financial resources: a qualitative study in Dominican persons with arthritis. Arthritis Care & Research. 2019;71(10):1379–86. doi:10.1002/acr.23742.
- Nierengarten B. Use of disease-modifying antirheumatic drugs low among unsubsidized beneficiaries with rheumatoid arthritis. Ann Long-Term Care. 2013;21:16–17.
- Oh G, Abner EL, Fardo DW, Freeman PR, Moga DC. Patterns and predictors of chronic opioid use in older adults: a retrospective cohort study. PloS One. 2019a; 14:e0210341.
- Oh TK, Jeon YT, Choi JW. Trends in chronic opioid use and association with five-year survival in South Korea: a population-based cohort study. Br J Anaesth. 2019b;123:655–63. doi:10.1016/j.bja.2019.08.012.
- Orhurhu V, Olusunmade M, Urits I, Viswanath O, Peck J, Orhurhu MS, Adekoya P, Hirji S, Sampson J, Simopoulos T, et al. Trends of opioid use disorder among hospitalized patients with chronic pain. Pain Pract. 2019;19(6):656–63. doi:10.1111/papr.12789.
- Parsons B, Schaefer C, Mann R, Sadosky A, Daniel S, Nalamachu S, Stacey BR, Nieshoff EC, Tuchman M, Anschel A. Economic and humanistic burden of post-trauma and post-surgical neuropathic pain among adults in the United States. J Pain Res. 2013;6:459–69. doi:10.2147/JPR.S44939.
- Parsons LB, Adams J. SAT0762-HPR the Accessibility and Usability of an Australian Web-based Self-management Programme (MYJOINTPAIN) for People with Lower Health Literacy and Joint Pain in the Uk Annals of the Rheumatic Diseases. 2017;76:1525–1526).
- Patel T, Chang F, Mohammed HT, Raman-Wilms L, Jurcic J, Khan A, Sproule B, Lafrenie RM. Knowledge, perceptions and attitudes toward chronic pain and its management: a cross-sectional survey of frontline pharmacists in Ontario, Canada. PLoS One. 2016;11(6):e0157151. doi:10.1371/journal.pone.0157151.
- Peacock A, Nielsen S, Bruno R, Campbell G, Larance B, Degenhardt L. Geographic variation in health service use and perceived access barriers for Australian adults with chronic non-cancer pain receiving opioid therapy. Pain Med. 2016;17(11):2003–16. doi:10.1093/pm/pnw109.
- Power JD, Perruccio AV, Gandhi R, Veillette C, Davey JR, Lewis SJ, Syed K, Mahomed NN, Rampersaud YR. Factors associated with opioid use in presurgical knee, Hip, and spine osteoarthritis patients. Arthritis Care Res. 2019;71(9):1178–85. doi:10.1002/acr.23831.
- Price-Haywood EG, Burton J, Burstain T, Harden-Barrios J, Lefante J, Shi L, Jamison RN, Bazzano A, Bazzano L. Clinical effectiveness of decision support for prescribing opioids for chronic noncancer pain: a prospective cohort study. Value in Health. 2020;23(2):157–63. doi:10.1016/j.jval.2019.09.2748.
- Robinson JP, Dansie EJ, Wilson HD, Rapp S, Turk DC. Attitudes and beliefs of working and work-disabled people with chronic pain prescribed long-term opioids. Pain Med. 2015;16(7):1311–24. doi:10.1111/pme.12770.
- Rosemann T, Joos S, Szecsenyi J, Laux G, Wensing M. Health service utilization patterns of primary care patients with osteoarthritis. BMC Health Serv Res. 2007;7(1):169. doi:10.1186/1472-6963-7-169.
- Rutledge DN, Rakovski C, Zettel-Watson L. Healthcare underutilization in overweight Mexican Americans with chronic pain. Ethnicity and Inequalities in Health and Social Care. 2012;5(4):123–32. doi:10.1108/EIHSC-10-2012-0010.
- Salazar DH, Dy CJ, Choate WS, Place HM Disparities in Access to Musculoskeletal Care: narrowing the Gap: AOA Critical Issues Symposium. J Bone Joint Surg Am. 2019;101:e121.
- Salt E, Frazier SK. Predictors of medication adherence in patients with rheumatoid arthritis. Drug Dev Res. 2011;72(8):756–63. doi:10.1002/ddr.20484.
- Sawyer P, Bodner EV, Ritchie CS, Allman RM. Pain and pain medication use in community-dwelling older adults. Am J Geriatr Pharmacother. 2006;4(4):316–24. doi:10.1016/j.amjopharm.2006.12.005.
- Schuman-Olivier Z, Connery H, Griffin ML, Wyatt SA, Wartenberg AA, Borodovsky J, Renner JJA, Weiss RD. Clinician beliefs and attitudes about buprenorphine/naloxone diversion. The American Journal on Addictions. 2013;22(6):574–80. doi:10.1111/j.1521-0391.2013.12024.x.
- Schwill U, Kordbarlag C, Reutermann P, Listing J, Zaenker M. SAT0066 Interrelationship between Rheumatoid Arthritis, Poverty and Education. Ann Rheum Dis. 2014;73(Suppl 2):614. doi:10.1136/annrheumdis-2014-eular.3720.
- Shipton D, Glazier RH, Guan J, Badley EM. Effects of use of specialty services on disease-modifying antirheumatic drug use in the treatment of rheumatoid arthritis in an insured elderly population. Med Care. 2004;42(9):907–13. doi:10.1097/01.mlr.0000135810.39691.f6.
- Steel N, Hardcastle AC, Bachmann MO, Richards SH, Mounce LT, Clark A, Lang I, Melzer D, Campbell J. Economic inequalities in burden of illness, diagnosis and treatment of five long-term conditions in England: panel study. BMJ Open. 2014;4(10):e005530. doi:10.1136/bmjopen-2014-005530.
- Sung YK, Cho SK, Kim D, Won S, Lee J, Choe JY, Choi CB, Hong SJ, Jun JB, Kim TH, et al. Factors Associated with Time to Diagnosis from Symptom Onset in Early Rheumatoid Arthritis Patients. Arthritis & Rheumatology. 2014;66:S889.
- Thorlund JB, Turkiewicz A, Prieto-Alhambra D, Englund M. Inappropriate use of opioids for incident knee and Hip osteoarthritis. Int J Rheum Dis. 2019a;22:71–72.
- Thorlund JB, Turkiewicz A, Prieto-Alhambra D, Englund M. Inappropriate opioid dispensing in patients with knee and Hip osteoarthritis: a population-based cohort study. Osteoarthritis Cartilage. 2020;28(2):146–53. doi:10.1016/j.joca.2019.10.004.
- Todd A, Akhter N, Cairns J-M, Kasim A, Walton N, Ellison A, Chazot P, Eldabe S, Bambra C. The Pain Divide: a cross-sectional analysis of chronic pain prevalence, pain intensity and opioid utilisation in England. BMJ Open. 2018;8(7):e023391. doi:10.1136/bmjopen-2018-023391.
- Trupin L, Barton J, Gina E-Y, Imboden JB, Gross AJ, Schillinger D, Yelin EH. Decisional conflict among vulnerable patient populations with rheumatoid arthritis is associated with limited health literacy and non-English language. Arthritis Rheum. 2012;64:S1027–S1028.
- Tur‐Sinai A, Shuldiner J, Bentur N. Sociodemographic inequality in joint‐pain medication use among community‐dwelling older adults in Israel. Health Soc Care Community. 2019;27:1167–74. doi:10.1111/hsc.12754.
- Tzouma V, Grepstad M, Grimaccia F, Kanavos P. Clinical, ethical, and socioeconomic considerations for prescription drug use during pregnancy in women suffering from chronic diseases. Ther Innov Regul Sci. 2015;49:947–56. doi:10.1177/2168479015589820.
- van der Meer JB, van den Bos J, Mackenbach JP. Socioeconomic differences in the utilization of health services in a Dutch population: the contribution of health status. Health Policy (New York). 1996;37(1):1–18. doi:10.1016/0168-8510(96)87673-1.
- Viticchi G, Silvestrini M, Falsetti L, Lanciotti C, Cerqua R, Luzzi S, Provinciali L, Bartolini M. Time delay from onset to diagnosis of migraine. Headache. 2011;51(2):232–36. doi:10.1111/j.1526-4610.2010.01778.x.
- Wabe N, Lee A, Wechalekar M, McWilliams L, Proudman S, Wiese M. Factors associated with medication adherence in a longitudinal study of rheumatoid arthritis patients. Int J Clin Pract. 2019;73(7):e13375. doi:10.1111/ijcp.13375.
- Waimann CA, Marengo MF, De Achaval S, Cox VL, Garcia-Gonzalez A, Reveille JD, Richardson MN, Suarez-Almazor ME. Electronic monitoring of oral therapies in ethnically diverse and economically disadvantaged patients with rheumatoid arthritis: consequences of low adherence. Arthritis Rheum. 2013;65(6):1421–29. doi:10.1002/art.37917.
- Werber A, Marschall U, L’hoest H, Hauser W, Moradi B, Schiltenwolf M. Opioid therapy in the treatment of chronic pain conditions in Germany. Pain Physician. 2015;18:E323–31. doi:10.36076/ppj/2015.18.E323.
- White KT, Dillingham TR, González-Fernández M, Rothfield L. Opiates for chronic nonmalignant pain syndromes: can appropriate candidates be identified for outpatient clinic management? Am J Phys Med Rehabil. 2009;88(12):995–1001. doi:10.1097/PHM.0b013e3181bc006e.
- Wong PK. Medication adherence in patients with rheumatoid arthritis: why do patients not take what we prescribe? Rheumatol Int. 2016;36(11):1535–42. doi:10.1007/s00296-016-3566-4.
- Yelin E, Murphy L, Cisternas MG, Foreman AJ, Pasta DJ, Helmick CG. Medical care expenditures and earnings losses among persons with arthritis and other rheumatic conditions in 2003, and comparisons with 1997. Arthritis Rheumat. 2007;56:1397–407. doi:10.1002/art.22565.
- Zänker M, Schwill U, Bielecke C, Jacobi A, Sokoll K, Zeidler G, Scheibert A, Reutermann P, Bohl-Bühler M, Engel JM, et al. FRI0584 The vicious circle of educational level, rheumatoid arthritis, and risk of poverty – results of a cross-sectional multicenter study in Germany. Ann Rheum Dis. 2016;75:653. doi:10.1136/annrheumdis-2016-eular.3316.
- Alvarez-Hernandez E, Upegui-Arango LD, Vazquez-Mellado J, Aranda-Arreola E, Burgos-Vargas R, Pelaez-Ballestas I. Characteristics of adherence, persistence and therapeutic alliance in patients with gout. Annals of Rheumatic Diseases. 2017:1362.
- Bedene A, Lijfering WM, Niesters M, van Velzen M, Rosendaal FR, Bouvy ML, Dahan A, van Dorp EL. Opioid prescription patterns and risk factors associated with opioid use in the Netherlands. JAMA Network Open. 2019;2(8):e1910223. doi:10.1001/jamanetworkopen.2019.10223.
- Bowen‐Davies Z, Muller S, Mallen CD, Hayward RA, Gout Severity RE, Status S. Work Absence: a Cross‐Sectional Study in Primary Care. Arthritis Care Res. 2018;70:1822–28. doi:10.1002/acr.23562.
- Brennan SL, Leslie WD, Lix LM. Is lower income associated with an increased likelihood of qualification for treatment for osteoporosis in Canadian women? Osteoporosis Int. 2014;25(1):273–79. doi:10.1007/s00198-013-2467-6.
- Connelly M, Glynn EF, Hoffman MA, Bickel J. Rates and predictors of using opioids in the emergency department to treat migraine in adolescents and young adults. Pediatr Emerg Care. 2021;37(12):e981–e987. doi:10.1097/PEC.0000000000001851.
- Devold HM, Furu K, Skurtveit S, Tverdal A, Falch JA, Sogaard AJ. Does socioeconomic factors influence the persistence in the use of alendronate? a nationwide register study in Norway. Osteoporosis Int. 2010;21:180–81.
- Gebauer S, Salas J, Scherrer JF. Neighborhood socioeconomic status and receipt of opioid medication for new back pain diagnosis. J Am Board Fam Med. 2017;30(6):775–83. doi:10.3122/jabfm.2017.06.170061.
- Gu X, Chen TC, Su T-L, Steinke D, Chen LC. Investigating the prescribing trajectory and geographical drug utilisation patterns of gabapentinoids in primary care in England. An Ecological Study Br J Clin Pharmacol. 2021;87:4001–12. doi:10.1111/bcp.14827.
- Heins JK, Heins A, Grammas M, Costello M, Huang K, Mishra S. Disparities in analgesia and opioid prescribing practices for patients with musculoskeletal pain in the emergency department. J Emerg Nurs. 2006;32(3):219–24. doi:10.1016/j.jen.2006.01.010.
- Heisler M, Langa KM, Eby EL, Fendrick AM, Kabeto MU, Piette JD. The health effects of restricting prescription medication use because of cost. Med Care. 2004;42(7):626–34. doi:10.1097/01.mlr.0000129352.36733.cc.
- Hopman P, Heins MJ, Korevaar JC, Rijken M, Schellevis FG. Health care utilization of patients with multiple chronic diseases in the Netherlands: differences and underlying factors. Eur J Intern Med. 2016;35:44–50. doi:10.1016/j.ejim.2016.08.025.
- Jendi W, Sherbeny F. Description of prescription opioid use among US adults. Value in Health. 2020;23(Suppl 1):S129. doi:10.1016/j.jval.2020.04.295.
- Laroche F, Guerin J, Azoulay D, Coste J, Perrot S. Fibromyalgia in real life: a national French web-based survey in 4516 patients. Arthritis Rheumatol. 2017;69:10.
- Lee IH, Park S, Lee EK. Sociodemographic factors influencing the use of injections in South Korean outpatient care. Pharmacoepidemiol Drug Saf. 2014;23:51–59. doi:10.1002/pds.3376.
- Lovejoy TI, Dobscha SK, Turk DC, Weimer MB, Morasco BJ. Correlates of prescription opioid therapy in Veterans with chronic pain and history of substance use disorder. J Rehabil Res Dev. 2016;53(1):25–36. doi:10.1682/JRRD.2014.10.0230.
- Miaskowski C, Penko JM, Guzman D, Mattson JE, Bangsberg DR, Kushel MB. Occurrence and characteristics of chronic pain in a community-based cohort of indigent adults living with HIV infection. J Pain. 2011;12(9):1004–16. doi:10.1016/j.jpain.2011.04.002.
- Mordecai L, Reynolds C, Donaldson LJ. de C Williams AC Patterns of regional variation of opioid prescribing in primary care in England: a retrospective observational study. Br J Gen Pract. 2018;68:e225–e233.
- Sanger N, Bhatt M, Shams I, Shahid H, Luo C, Tam SL, Samaan MC, de Souza R, Thabane L, Samaan Z. Association between socio-demographic and health functioning variables among patients with opioid use disorder introduced by prescription: a prospective cohort study. Pain Physician. 2018;21:E623–E632.
- Weiner SS, Weiser SR, Carragee EJ, Nordin M. Managing nonspecific low back pain: do nonclinical patient characteristics matter? Spine. 1987-1994;2011(36).
- Anis AH, Guh DP, Lacaille D, Marra CA, Rashidi AA, Li X, Esdaile JM. When patients have to pay a share of drug costs: effects on frequency of physician visits, hospital admissions and filling of prescriptions. CMAJ. 2005;173:1335–40. doi:10.1503/cmaj.045146.
- Balakrishnan S, Bambery P, Gupta N, Pandhi P. An audit of the use of antirheumatic drugs in a North Indian referral hospital. Pharmacoepidemiol Drug Saf. 2001;10(3):237–43. doi:10.1002/pds.591.
- Bateman C. ‘Opi-phobia’ among doctors leads to unnecessary suffering. S Afr Med J. 2007;97:399–403.
- Bolge SC, Meyer R, Annunziata K. Regional variations in rheumatoid arthritis treatment and health outcomes across the United States. Arthritis Rheum. 2013;65:S451–S45.
- Booker S, Herr K, Tripp-Reimer T. Patterns and perceptions of self-management for osteoarthritis pain in African American older adults. Pain Med. 2019;20(8):1489–99. doi:10.1093/pm/pny260.
- Buonora M, Perez HR, Heo M, Cunningham CO, Starrels JL. Race and gender are associated with opioid dose reduction among patients on chronic opioid therapy. Pain Med. 2019;20(8):1519–27. doi:10.1093/pm/pny137.
- Burgess DJ, Crowley-Matoka M, Phelan S, Dovidio JF, Kerns R, Roth C, Saha S, van Ryn M. Patient race and physicians‘ decisions to prescribe opioids for chronic low back pain. Soc Sci Med. 2008;67(11):1852–60. doi:10.1016/j.socscimed.2008.09.009.
- Butcher L. Practice matters: the CGRP inhibitors for migraine are reaching the market. Why Not All Neurologists May Be Able to Prescribe Them Neurology Today. 2018;18:28–31.
- Canada RE, DiRocco D, Day S. A better approach to opioid prescribing in primary care. J Fam Pract. 2014;63:E1–E8.
- Carter CT, Tunceli O, Bolge SC, Denny L, Fang C, Singer J. Differences in clinical and economic profiles of rheumatoid arthritis patients using frequently doses subcutaneous or less frequently dosed intravenous biologic therapy. Arthritis Rheum. 2010;62:1542.
- Charleston L, Burke J. Do racial/ethnic disparities exist in recommended migraine treatments in US ambulatory care? Cephalalgia. 2018;38(5):876–82. doi:10.1177/0333102417716933.
- Delorme J, Chenaf C, Bertin C, Riquelme M, Eschalier A, Ardid D, Authier N. Chronic pain opioid-maintained patients receive less analgesic opioid prescriptions. Front Psych. 2018:9.
- Gavan S, Harrison M, Barton A, Hyrich K, Isaacs J, Morgan A, Wilson AG, Payne K. Identifying factors which influence the selection of anti-TNF treatment in patients with rheumatoid arthritis in England. Value in Health. 2016;19(3):A238. doi:10.1016/j.jval.2016.03.1106.
- Green CR, Hart-Johnson T. The adequacy of chronic pain management prior to presenting at a tertiary care pain center: the role of patient socio-demographic characteristics. J Pain. 2010;11(8):746–54. doi:10.1016/j.jpain.2009.11.003.
- Grotenhermen F, Schnelle M. Survey on the medical use of cannabis and THC in Germany. Journal of Cannabis Therapeutics. 2003;3(2):17–40. doi:10.1300/J175v03n02_03.
- Harle CA, Cook RL, Kinsell HS, Harman JS. Opioid prescribing by physicians with and without electronic health records. J Med Syst. 2014;38(11):138. doi:10.1007/s10916-014-0138-6.
- Heckman BD, Holroyd KA, O’Donnell FJ, Tietjen G, Utley C, Stillman M, Ellis G. Race differences in adherence to headache treatment appointments in persons with headache disorders. J Natl Med Assoc. 2008;100(2):247–55. doi:10.1016/s0027-9684(15)31213-x.
- Jamison RN, Sheehan KA, Scanlan E, Matthews M, Ross EL. Beliefs and attitudes about opioid prescribing and chronic pain management: survey of primary care providers. J Opioid Manag. 2014;10:375–82. doi:10.5055/jom.2014.0234.
- Kalkan A, Hallert E, Carlsson P, Roback K, Sjöwall C. Individual variations in treatment decisions by Swedish rheumatologists regarding biological drugs for rheumatoid arthritis. Scand J Rheumatol. 2015;44(4):265–70. doi:10.3109/03009742.2014.997286.
- Kim G, Barner JC, Rascati K, Richards K. Factors associated with the initiation of biologic disease-modifying antirheumatic drugs in Texas Medicaid patients with rheumatoid arthritis. J Manag Care Spec Pharm. 2015;21(5):401–07. doi:10.18553/jmcp.2015.21.5.401.
- King C, Liu X. Racial and ethnic disparities in opioid use among US adults with back pain. Spine. 2020;45(15):1062–66. doi:10.1097/BRS.0000000000003466.
- King L, Marshall DA, Hawker G. Patient factors associated with gaps in osteoarthritis care. Arthritis Rheumatol. 2018;70:472–73.
- Loder S, Sheikh HU, Loder E. The prevalence, burden, and treatment of severe, frequent, and migraine headaches in US minority populations: statistics from National Survey studies. Headache. 2015;55(2):214–28. doi:10.1111/head.12506.
- Madsen AM, Stark LM, Has P, Emerson JB, Schulkin J, Matteson KA. Opioid knowledge and prescribing practices among obstetrician–gynecologists. Obstet Gynecol. 2018;131(1):150–57. doi:10.1097/AOG.0000000000002407.
- Mailis-Gagnon A, Lakha SF, Ou T, Louffat A, Yegneswaran B, Umana M, Cohodarevic T, Nicholson K, Deshpande A. Chronic noncancer pain: characteristics of patients prescribed opioids by community physicians and referred to a tertiary pain clinic. Can Fam Physician. 2011;57:e97–e105.
- Martin RW, Head AJ, Birmingham JD, Eggebeen AT. Does biased risk perception explain the underuse of disease modifying anti-rheumatic drugs? Arthritis Rheum. 2012;64:S167.
- Marttinen MK, Kautiainen H, Haanpaa M, Pohjankoski H, Vuorimaa H, Hintikka J, Kauppi M. Pain-associated factors and administration of pain medication in the elderly Finnish population. Scandinavian J Pain. 19:797–803. doi:10.1515/sjpain-2019-0039.
- Meghani SH, Cho E. Self-Reported Pain and Utilization of Pain Treatment Between Minorities and Nonminorities in the United States. Public Health Nurs. 2009;26(4):307–16. doi:10.1111/j.1525-1446.2009.00785.x.
- Mikuls TR, Mudano AS, Pulley L, Saag KG. The association of race/ethnicity with the receipt of traditional and alternative arthritis-specific health care. Med Care. 2003;41(11):1233–39. doi:10.1097/01.MLR.0000093422.67436.E5.
- Moskowitz D, Thom DH, Guzman D, Penko J, Miaskowski C, Kushel M. Is primary care providers’ trust in socially marginalized patients affected by race? J Gen Intern Med. 2011;26(8):846–51. doi:10.1007/s11606-011-1672-2.
- Movahedi M, Joshi R, Rampakakis E, Thorne C, Cesta A, Sampalis JS, Bombardier C. Impact of residential area on the management of rheumatoid arthritis patients initiating their first biologic DMARD: results from the Ontario Best Practices Research Initiative (OBRI). Medicine. 2019;98(20):e15517. doi:10.1097/MD.0000000000015517.
- Navarro-Millan I, Rajan M, Lui G, Kern L, Pinheiro L, Safford M, Curtis J. Racial and ethnic differences in medication use among beneficiaries of social security disability insurance with rheumatoid arthritis. 2020;50:988–95. doi:10.1016/j.semarthrit.2020.07.008.
- Nicholson RA, Rooney M, Vo K, O’Laughlin E, Gordon M. Migraine care among different ethnicities: do disparities exist? Headache. 2006;46:754–65. doi:10.1111/j.1526-4610.2006.00453.x.
- Nieves-Plaza M, Eng H, Metes ID, Shewede A, Wisniewski SR, John A, Levesque MC. Physician and patient characteristics associated with the decision to treat rheumatoid arthritis patients with biologic monotherapy in usual care settings. Arthritis Rheumat. 2013;65:S630.
- Perez HR, Starrels JL, Gonzalez S, Vidot DC, Hua S, Strizich GM, Zeng D, Daviglus M, Gellman MD, Kaplan RC. Factors associated with prescription opioid use among hispanics/latinos with arthritis results from the hispanic community health study/study of latinos. J Gen Intern Med. 2018;33:200–01.
- Prunuske JP, St. Hill CA, Hager KD, Lemieux AM, Swanoski MT, Anderson GW, Lutfiyya MN. Opioid prescribing patterns for non-malignant chronic pain for rural versus non-rural US adults: a population-based study using 2010 NAMCS data. BMC Heath Serv Res. 2014;14:563. doi:10.1186/s12913-014-0563-8.
- Putrik P, Ramiro S, Orueta JF, Keszei A, Alonso-Morán E, Nuño-Solinis R, Boonen A. THU0628 Socio-Economically Deprived Patients Have A Higher Likelihood for Having Any Type of Rheumatic and Musculoskeletal Diseases and Have Higher Healthcare Costs-Results from A Population-Based Administrative Database Including 1.9 Million Persons (Basque Country, Spain). Clin Exp Rheumatol. 2018;36:589–94.
- Rahme E, J-P R, J-p L, Nedjar H, Morin S. Concordance with guideline recommendations: previous and more recent nonsteroidal anti-inflammatory drug prescriptions in Quebec, Canada. Pharmacoepidemiol Drug Safety. 2012;21:420–27. doi:10.1002/pds.2339.
- Robinson S. Poor, scared, and hurting: the experiences of Black elders managing chronic pain. J Pain. 2015;16:S36. doi:10.1016/j.jpain.2015.01.156.
- Rogers KD, Kemp A, McLachlan AJ, Blyth F, Landau R. Adverse selection? a multi-dimensional profile of people dispensed opioid analgesics for persistent non-cancer pain. PLoS ONE. 2013;8(12):e80095. doi:10.1371/journal.pone.0080095.
- Salas J, Scherrer JF, Lustman PJ, Schneider FD. Racial differences in the association between nonmedical prescription opioid use, abuse/dependence, and major depression. Subst Abus. 2016;37(1):25–30. doi:10.1080/08897077.2015.1129523.
- Taff J, Loewenstein J, Wang L, Schwartz K, Wei D. Predictors of opiate prescription for low back pain in outpatient emergency care settings. J Gen Intern Med. 2014;29:S178.
- Turk DC, Brody MC, Okifuji AE. Physicians‘ attitudes and practices regarding the long-term prescribing of opioids for non-cancer pain. Pain. 1994;59(2):201–08. doi:10.1016/0304-3959(94)90072-8.
- Vina E, Hannon M, Dagnino J, Kent Kwoh C. Understanding ethnic differences in the utilization of nonsteroidal anti-inflammatory drugs for osteoarthritis. Ann Rheum Dis. 2019;78:605.
- Webster BS, Cifuentes M, Verma S, Pransky G. Geographic variation in opioid prescribing for acute, work-related, low back pain and associated factors: a multilevel analysis. Am J Ind Med. 2009;52(2):162–71. doi:10.1002/ajim.20655.
- Webster F, Rice K, Katz J, Bhattacharyya O, Dale C, Upshur R, Koester S. An ethnography of chronic pain management in primary care: the social organization of physicians’ work in the midst of the opioid crisis. PLoS One. 2019;14(5):e0215148. doi:10.1371/journal.pone.0215148.
- Wilson FA, Mosalpuria K, Stimpson JP. Association Between Opioid Prescriptions and Non–US-Born Status in the US. JAMA Network Open. 2020;3(6):e206745. doi:10.1001/jamanetworkopen.2020.6745.
- Birke H, Kurita GP, Sjøgren P, Højsted J, Simonsen MK, Juel K, Ekholm O. Chronic non-cancer pain and the epidemic prescription of opioids in the Danish population: trends from 2000 to 2013. Acta Anaesthesiol Scand. 2016;60(5):623–33. doi:10.1111/aas.12700.
- Chang SC, Ma CC, Lee CT, Hsieh SW. Pharmacoepidemiology of chronic noncancer pain patients requiring chronic opioid therapy: a nationwide population-based study. Acta Anaesthesiol Taiwan. 2015;53:89–94. doi:10.1016/j.aat.2015.04.002.
- Eriksson JK, Neovius M, Ernestam S, Lindblad S, Simard JF, Askling J. Incidence of Rheumatoid Arthritis in Sweden: a Nationwide Population-Based Assessment of Incidence, Its Determinants, and Treatment Penetration. Arthritis Care Res. 2013;65(6):870–78. doi:10.1002/acr.21900.
- Frakes KA, Brownie S, Davies L, Thomas J, Miller ME, Tyack Z. The sociodemographic and health‐related characteristics of a regional population with chronic disease at an interprofessional student‐assisted clinic in Queensland Capricornia Allied Health Partnership. Aust J Rural Health. 2013;21:97–104. doi:10.1111/ajr.12017.
- Goodwin JS, Nguyen-Oghalai TU, Kuo Y-F, Ottenbacher KJ. Epidemiology of Medicare abuse: the example of power wheelchairs. J Am Geriatr Soc. 2007;55(2):221–26. doi:10.1111/j.1532-5415.2007.01063.x.
- Hawley S, Edwards CJ, Arden NK, Delmestri A, Cooper C, Judge A, Prieto-Alhambra D. Descriptive epidemiology of Hip and knee replacement of rheumatoid arthritis patients in England and Wales: variation by sex, age, geography and socio-economic status. Semin Arthritis Rheum. 2020;50:237–44. doi:10.1016/j.semarthrit.2019.08.008.
- Hussain S, Wang Y, Peeters G, Wluka AE, Mishra GD, Teede H, Urquhart D, Brown WJ, Cicuttini FM. Demographic, psychosocial factors, musculoskeletal pain and prescription opioid use in community-based middle-aged women - a prospective cohort study. Osteoarthritis Cartilage. 2020;28:S140. doi:10.1016/j.joca.2020.02.231.
- Kashefi S, Lee SM, Mallaysamy S, Thunga PG. Demographic, clinical characteristics and drug prescription pattern in patients with rheumatoid arthritis in south Indian tertiary care hospital. International Journal of Pharmacy and Pharmaceutical Sciences. 2016;8:251–57.
- Luo X, Pietrobon R, Hey L. Patterns and trends in opioid use among individuals with back pain in the United States. Spine. 2004;29(8):884–90. doi:10.1097/00007632-200404150-00012.
- Malon J, Shah P, Koh WY, Cattabriga G, Li E, Cao L. Characterizing the demographics of chronic pain patients in the state of Maine using the Maine all payer claims database. BMC Public Health. 2018;18(1):1–2. doi:10.1186/s12889-018-5673-5.
- McBurney CA, Vina ER. Racial and ethnic disparities in rheumatoid arthritis. Current Rheumatology Reports. 2012;14(5):463–71. doi:10.1007/s11926-012-0276-0.
- Miller GE, Moriya AS Any use and frequent use of opioids among non-elderly adults in 2015-2016, by socioeconomic characteristics. In Statistical Brief (Medical Expenditure Panel Survey); Rockville (MD): Agency for Healthcare Research and Quality; 2001: #515.
- Moody B, Lane M, Botts S, Talbert J. Eastern Kentucky opiate use for chronic non-malignant pain. J Pharm Pract. 2010;23:178.
- Moriya AS, Miller GE. 2001. Any use and frequent use of opioids among elderly adults in 2015-2016, by socioeconomic characteristics. Agency for Healthcare Research and Quality.
- Neovius M, Simard JF, Askling J; Neovius M, Simard JF, Askling J, ARTIS Study Group. Nationwide prevalence of rheumatoid arthritis and penetration of disease-modifying drugs in Sweden. Ann Rheum Dis. 2011;70(4):624–29. doi:10.1136/ard.2010.133371.
- Parsells Kelly J, Cook SF, Kaufman DW, Anderson T, Rosenberg L, Mitchell AA. Prevalence and characteristics of opioid use in the US adult population. Pain. 2008;138(3):507–13. doi:10.1016/j.pain.2008.01.027.
- Lee SW, Shen J, Kim SJ, Chun S-Y, Kim P, Riaz J, Yoo JW, Hwang J. US trends of opioid-use disorders and associated factors among hospitalized patients with spinal conditions and treatment from 2005 to 2014. Spine. 2020;45(2):124–33. doi:10.1097/BRS.0000000000003183.
- Solomon DH, Avorn J, Wang PS, Vaillant G, Cabral D, Mogun H, Stürmer T. Prescription opioid use among older adults with arthritis or low back pain. Arthritis Care Res. 2006;55(1):35–41. doi:10.1002/art.21697.
- Wei YJ, Chen C, Fillingim R, Schmidt SO, Winterstein AG, Strathdee S. Trends in prescription opioid use and dose trajectories before opioid use disorder or overdose in US adults from 2006 to 2016: a cross-sectional study. PLoS Med. 2019;16(11):e1002941. doi:10.1371/journal.pmed.1002941.
- Campbell G, Nielsen S, Bruno R, Lintzeris N, Cohen M, Hall W, Larance B, Mattick RP, Degenhardt L. The Pain and Opioids IN Treatment study: characteristics of a cohort using opioids to manage chronic non-cancer pain. Pain. 2015;156(2):231–42. doi:10.1097/01.j.pain.0000460303.63948.8e.
- Campbell NK, Saadeldin K, De Vera MA. The duality of economic issues with medication non-adherence in patients with inflammatory arthritis. Curr Rheumatol Rep. 2017;19(10):66. doi:10.1007/s11926-017-0691-3.
- Charleston IVL, Royce J, Monteith TS, Broner SW, O’Brien HL, Manrriquez SL, Robbins MS. Migraine care challenges and strategies in US uninsured and underinsured adults: a narrative review, part 2. Headache. 2018;58(5):633–47. doi:10.1111/head.13321.
- Duggan A, Hayes C. Variation in dispensing of opioid analgesics in Australia. Intern Med J. 2017;47(4):473–74. doi:10.1111/imj.13394.
- Gulliford M. Opioid use, chronic pain and deprivation. eClinicalMedicine. 2020 Apr;21(21):100341. doi:10.1016/j.eclinm.2020.100341.
- Hadler NM. Socioeconomics of back pain. Nat Clin Pract Rheumatol. 2006;2(5):230–31. doi:10.1038/ncprheum0170.
- Karran EL, Grant AR, Moseley L. Low back pain and the social determinants of health: a systematic review and narrative synthesis. Pain. 2020;161(11):2476–93. doi:10.1097/j.pain.0000000000001944.
- Knight KR, Kushel M, Chang JS, Zamora K, Ceasar R, Hurstak E, Miaskowski C. Opioid pharmacovigilance: a clinical-social history of the changes in opioid prescribing for patients with co-occurring chronic non-cancer pain and substance use. Soc Sci Med. 2017;186:87–95. doi:10.1016/j.socscimed.2017.05.043.
- Lasser KE. Prescription opioid use among US adults: our Brave New World. AnnIntern Med. 2017;167:351–52.
- Lekpa FK, Ndongo S, Pouye A, Zeba D, Ka O, Doualla M-S, Luma HN, Ka MM, Diop TM. Authors‘ reply to the comment by Pazzaglia et al. Eur J Pain. 2013;17(6):946–47. doi:10.1002/j.1532-2149.2013.00304.x.
- Li E, Khan Z, Balu S. A systematic review of the association between patient out-of-pocket costs and the non-initiation of biologics used to treat rheumatoid arthritis. J Manag Care Spec Pharm. 2018;25:S83.
- Losby JL, Hyatt JD, Kanter MH, Baldwin G, Matsuoka D. Safer and more appropriate opioid prescribing: a large healthcare system’s comprehensive approach. J Eval Clin Pract. 2017;23:1173–79. doi:10.1111/jep.12756.
- Meghani SH, Byun E, Gallagher RM. Time to take stock: a meta-analysis and systematic review of analgesic treatment disparities for pain in the United States. Pain Med. 2012;13(2):150–74. doi:10.1111/j.1526-4637.2011.01310.x.
- Mody GM, Cardiel MH. Challenges in the management of rheumatoid arthritis in developing countries. Best Pract Res Clin Rheumatol. 2008;22(4):621–41. doi:10.1016/j.berh.2008.04.003.
- Richards GC, Mahtani KR, Muthee TB, DeVito NJ, Koshiaris C, Aronson JK, Goldacre B, Heneghan CJ. Factors associated with the prescribing of high-dose opioids in primary care: a systematic review and meta-analysis. BMC Med. 2020;18(1):68. doi:10.1186/s12916-020-01528-7.
- Roa JA, Guevara A, Guevara C, Guevara-Aguirre J. Physician’s role in prescribing opioids in developing countries. BMJ Case Reports CP. 2019;12(6):e227072. doi:10.1136/bcr-2018-227072.
- Stewart J, Kempenaar L, Lauchlan D. Rethinking yellow flags. Man Ther. 2011;16(2):196–98. doi:10.1016/j.math.2010.11.005.
- The Back Letter. Why are disadvantaged whites with chronic pain and other problems dying prematurely? 2016;31:90–93.
- Baser O, Wang L, Lewis-Beck C, Wang H, Li L, Xie L. Demographic and socioeconomic characteristics that affect selection of intravenous versus subcutaneous injection tumor necrosis factor inhibitors for rheumatoid arthritis. Value in Health. 2012;15:A454. doi:10.1016/j.jval.2012.08.1433.
- Codreanu C, Mogosan C, Ionescu R, Ancuta I, Parvu M, Rednic S. Area of residence and socioeconomic factors significantly affect access to biological therapy for rheumatoid arthritis patients in Romania. Arthritis Rheum. 2014;66:S507.
- McKeown T, Jacob B, Li X, Couto S, Bensen W, Ahluwalia V, Karasik A, Bombardier C. Comparison of medication use in rheumatoid arthritis patients between university and private settings – results from Ontario Best Practice Research Initiative. Arthritis Rheumatol. 2014;66:S1058.
- Putrik P, Ramiro S, Lie E, Keszei A, Van Der Heijde D, Landewé R, Kvien TK, Uhlig T, Boonen A. Level of education is associated with access to biologic DMARDS even in a country with highly developed social welfare (Norway). Ann Rheum Dis. 2014;73:87. doi:10.1136/annrheumdis-2014-eular.2036.
- Tatangelo M, Tomlinson GA, Paterson M, Bansback N, Gomes T, Kopp A, Ahluwalia V, Bombardier C. The effect of patient, prescriber and region on the initiation of first biologic for rheumatoid arthritis: a longitudinal population study. Arthritis Rheumatol. 2018;70:289–90.
- Cairns J, Hall N, Walton N, Elison A, Chazot P, Todd A, Eldabe S, Bambra C. Geo-pain: a cross-sectional analysis of spatial inequalities in chronic pain, opioid prescribing and usage in England. Br J Pain. 2017;11:30–31.
- Gomes T, Juurlink DN, Dhalla IA, Mailis-Gagnon A, Paterson JM, Mamdani MM. Trends in opioid use and dosing among socio-economically disadvantaged patients. Open Medicine. 2011;5:E13.
- Guzmán-Ruiz M, Mora-Moscoso R, Delgado-Mediano CM, Pérez-Milena A, Rueda-Rojas M, Gea-Rodríguez LA. Managing chronic pain in primary care, prescription profile for strong opioids: indications, cost and adverse effects. Revista de la Sociedad Espanola Del Dolor. 2014;21:197–204. doi:10.4321/S1134-80462014000400003.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffman TC, Mulrow CD, Shamseer L, Tetzlafff JM, Akl EA, Brennan SE, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71.
- Engel GL. The need for a new medical model: a challenge for biomedicine. Science. 1977;196(4286):129–36. doi:10.1126/science.847460.
- Newman AK, Van Dyke BP, Torres CA, Baxter JW, Eyer JC, Kapoor S, Thorn BE. The relationship of sociodemographic and psychological variables with chronic pain variables in a low-income population. Pain. 2017;158(9):1687–96. doi:10.1097/j.pain.0000000000000964.
- Davey Smith G. Learning to live with complexity: ethnicity, socioeconomic position, and health in Britain and the United States. Am J Public Health. 2000;90:1694–98.
- Fuentes M, Hart-Johnson T, Green CR. The association among neighborhood socioeconomic status, race and chronic pain in black and white older adults. J Natl Med Assoc. 2007;99:1160–69.
- Becker WC, Dorflinger L, Edmond SN, Islam L, Heapy AA, Fraenkel L. Barriers and facilitators to use of non-pharmacological treatments in chronic pain. BMC Fam Pract. 2017;18(1):41. doi:10.1186/s12875-017-0608-2.
- Weeks WJ. Influential U.S. Medical Organizations Call for Insurance Coverage of Non-Pharmacologic Approaches to Pain. J Altern Complement Med. 2016;22(12):947–49. doi:10.1089/acm.2016.29016.jjw.
- Donovan HS, Ward SE, Song M-K, Heidrich SM, Gunnarsdottir S, Phillips CM. An update on the representational approach to patient education. J Nurs Scholar. 2007;39:259–65. doi:10.1111/j.1547-5069.2007.00178.x.
- McCauley JL, Back SE, Brady KT. Pilot of a brief, web-based educational intervention targeting safe storage and disposal of prescription opioids. Addictive Behav. 2013;38:2230–35. doi:10.1016/j.addbeh.2013.01.019.
- Rimer BK, Kedziera P, Levy MH. The role of patient education in cancer pain control. Hospice J. 1992;8:171–91. doi:10.1300/J011v08n01_08.
- Hollingshead NA, Matthias MS, Bair M, Hirsh AT. Healthcare providers’ perceptions of socioeconomically disadvantaged patients with chronic pain: a qualitative investigation. J Health Dispar Res Pract. 2016;9:3.
- Joynt M, Train MK, Robbins BW, Halterman JS, Caiola E, Fortuna RJ. The impact of neighborhood socioeconomic status and race on the prescribing of opioids in emergency departments throughout the United States. J Gen Intern Med. 2013;28(12):1604–10. doi:10.1007/s11606-013-2516-z.
- Reyes-Gibby CC, Aday LA, Todd KH, Cleeland CS, Anderson KO. Pain in aging community-dwelling adults in the United States: non-Hispanic whites, non-Hispanic Blacks, and Hispanics. J Pain. 2007;8:75–84.
- Clark J, Potter D, McKinlay J. Bringing social structure back into clinical decision making. Soc Sci Med. 1991;32(8):853–66. doi:10.1016/0277-9536(91)90241-4.
- Eisenberg JM. Sociologic Influences on Decision-Making by Clinicians. Ann Intern Med. 1979;90(6):957–64. doi:10.7326/0003-4819-90-6-957.
- Chibnall JT, Dabney A, Tait R. Internist Judgments of Chronic Low Back Pain. Pain Med. 2000;1(3):231–37. doi:10.1046/j.1526-4637.2000.00029.x.
- Gatchel RJ, Polatin PB, Kinney RK. Predicting outcome of chronic back pain using clinical predictors of psychopathology: a prospective analysis. Health Psychol. 1995;14(5):415–20. doi:10.1037/0278-6133.14.5.415.
- Hausmann LRM, Gao S, Lee ES, Kwoh KC. Racial disparities in the monitoring of patients on chronic opioid therapy. Pain. 2013;154(1):46–52. doi:10.1016/j.pain.2012.07.034.
- Tait RC, Chibnall JT. Work injury management of refractory low back pain: relations with ethnicity, legal representation and diagnosis. Pain. 2001;91(1):47–56. doi:10.1016/S0304-3959(00)00418-8.
- Diniz E, Castro P, Bousfield A, Bernardes SF. Classism and dehumanization in chronic pain: a qualitative study of nurses’ inferences about women of different socio-economic status. Br J Health Psychol. 2019;25:152–70. doi:10.1111/bjhp.12399.
- Green CR, Baker TA, Smith EM, Sato Y. The effect of race in older adults presenting for chronic pain management: a comparative study of Black and White Americans. J Pain. 2003b;4(2):82–90. doi:10.1054/jpai.2003.8.
- Becker WC, Starrels JL, Heo M, Li X, Weiner MG, Turner BJ. Racial differences in primary care opioid risk reduction strategies. Ann Fam Med. 2011;9(3):219–25. doi:10.1370/afm.1242.
- Davies KA, Silman AJ, Macfarlane GJ, Nicholl BI, Dickens C, Morriss R, Ray D, McBeth J. The association between neighbourhood socio-economic status and the onset of chronic widespread pain: results from the EPIFUND study. Eur J Pain. 2009;13(6):635–40. doi:10.1016/j.ejpain.2008.07.003.
- Maly A, Vallerand AH. Neighborhood, socioeconomic, and racial influence on chronic pain. Pain Manag Nurs. 2018;19:14–22. doi:10.1016/j.pmn.2017.11.004.
- Jordan KM, Sawyer S, Coakley P, Smith HE, Cooper C, Arden NK The use of conventional and complementary treatments for knee.
- Ruhe A-K, Wager J, Hirschfeld G, Zernikow B. Household income determines access to specialized pediatric chronic pain treatment in Germany. BMC Health Serv Res. 2016;16(1):140. doi:10.1186/s12913-016-1403-9.
- Morrison RS, Wallenstein S, Natale DK, Senzel RS, Huang LL. “We don’t carry that”: failure of pharmacies in predominantly nonwhite neighborhoods to stock opioid analgesics. N Engl J Med. 2000;342:1023–26. doi:10.1056/NEJM200004063421406.
- Green CR, Baker TA, Sato Y, Washington TL, Smith EM. Race and chronic pain: a comparative study of young Black and White Americans presenting for management. J Pain. 2003c;4(4):176–83. doi:10.1016/S1526-5900(02)65013-8.
- Shavers VL, Bakos A, Sheppard VB. Race, Ethnicity, and Pain among the U.S. Adult Population. J Health Care Poor Underserved. 2010;21(1):177–220. doi:10.1353/hpu.0.0255.
- Vallerand AH, Pieper B, Crawley J, Nordstrom C, DiNardo E. The prevalence of pain and its association with psychosocial factors for indigent adults enrolled in a primary care clinic. Clin J Pain. 2013;29(10):917–23. doi:10.1097/AJP.0b013e31827c7b30.
- Dovidio JF, Fiske ST. Under the radar: how unexamined biases in decision-making processes in clinical interactions can contribute to health care disparities. Am J Public Health. 2012;102(5):945–52. doi:10.2105/AJPH.2011.300601.
- Crenshaw K. Demarginalizing the intersection of race and sex: a black feminist critique of antidiscrimination doctrine, feminist theory and antiracist politics. Univ Chic Leg Forum. 1989;140:139–1676.
- Mackey LM, Blake C, Casey M-B, Power CK, Victory R, Hearty C, Fullen BM. The impact of health literacy on health outcomes in individuals with chronic pain: a cross-sectional study. Physiotherapy. 2019;105(3):346–53. doi:10.1016/j.physio.2018.11.006.
- Mackert M, Ball J, Lopez N. Health literacy awareness training for healthcare workers: improving knowledge and intentions to use clear communication techniques. Patient Educ Couns. 2011;85(3):e225–e228. doi:10.1016/j.pec.2011.02.022.
- Miller TA. Health literacy and adherence to medical treatment in chronic and acute illness: a meta-analysis. Patient Educ Couns. 2016;99(7):1079–86. doi:10.1016/j.pec.2016.01.020.
- Cooper LA, Roter DL, Johnson RL, Ford DE, Steinwachs DM, Powe NR. Patient-Centered Communication, Ratings of Care, and Concordance of Patient and Physician Race. Ann Intern Med. 2003;139(11):907–15. doi:10.7326/0003-4819-139-11-200312020-00009.
- Schillinger D, Bindman A, Wang F, Stewart A, Piette J. Functional health literacy and the quality of physician–patient communication among diabetes patients. Patient Educ Couns. 2004;52(3):315–23. doi:10.1016/S0738-3991(03)00107-1.
- Shaw A, Ibrahim S, Reid F, Ussher M, Rowlands G. Patients’ perspectives of the doctor–patient relationship and information giving across a range of literacy levels. Patient Educ Couns. 2009;75(1):114–20. doi:10.1016/j.pec.2008.09.026.
- Kolhatkar A, Cheng L, Morgan SG, Goldsmith LJ, Dhalla IA, Holbrook AM, Law MR. Patterns of borrowing to finance out-of-pocket prescription drug costs in Canada: a descriptive analysis. CMAJ Open. 2018;6(4):E544–E550. doi:10.9778/cmajo.20180063.
- Alexander GC, Casalino LP, Meltzer DO. Physician strategies to reduce patients’ out-of-pocket prescription costs. Arch Intern Med. 2005;165(6):633–36. doi:10.1001/archinte.165.6.633.
- Alexander GC, Casalino LP, Tseng C-W, McFadden D, Meltzer DO. Barriers to patient-physician communication about out-of-pocket costs. J Gen Intern Med. 2004;19(8):856–60. doi:10.1111/j.1525-1497.2004.30249.x.
- Goldacre B, Reynolds C, Powell-Smith A, Walker AJ, Yates TA, Croker R, Smeeth L. Do doctors in dispensing practices with a financial conflict of interest prescribe more expensive drugs? a cross-sectional analysis of English primary care prescribing data. BMJ Open. 2019;9:e026886.
- Gaffney A, Bor DH, Himmelstein DU, Woolhandler S, McCormick D. The effect of Veterans Health Administration coverage on cost-related medication nonadherence. Health Aff. 2020;1:33–40.
- Ghosh A, Simon K, Sommers BD. The effect of health insurance on prescription drug use among low-income Adults:evidence from recent Medicaid Expansions. J Health Econ. 2019;63:64–80. doi:10.1016/j.jhealeco.2018.11.002.
- Hill SC, Abdus S. The effects of Medicaid on access to care and adherence to recommended preventive services. Health Serv Res. 2021;56(1):84–94. doi:10.1111/1475-6773.13603.
- Kirsch M, Montgomery JR, Hu HM, Englesbe M, Hallstrom B, Brummett CM, Fendrick AM, Waljee JF. Association between insurance cost-sharing subsidy and postoperative opioid prescription refills among Medicare patients. Surgery. 2020;168:244–52.
- Cong M, Chaisson J, Cantrell D, Mohundro BL, Carby M, Ford M, Liu M, Kemp LS, Ouyang J, Zhang Y, et al. Association of co-pay elimination with medication adherence and total cost. Am J Manag Care. 2021;27:249–54.
- Vogler S, Dedet G, Pedersen HB. Financial burden of prescribed medicines included in outpatient benefits package schemes: comparative analysis of co-payments for reimbursable medicines in European countries. Appl Health Econ Health Pol. 2019;17:803–16.
- Department of Health and Wellness, Province of Nova Scotia. Nova Scotia’s Opioid Use and Overdose Framework. https://novascotia.ca/opioid/nova-scotia-opioid-use-and-overdose-framework.pdf [accessed 27 April, 2022].
- Guo EX, Sweetman A, Guindon GE. Socioeconomic differences in prescription drug supplemental coverage in Canada: a repeated cross-sectional study. Health Pol. 2020;124:252–60.
- Rawson NSB. National pharmacare in Canada: equality or equity, accessibility or affordability. Int J Health Policy Manag. 2020;9:524–27.
- Patel MR, Press VG, Gerald LB, Barnes T, Blake K, Brown LK, Costello RW, Crim C, Forshag M, Gershon AS, et al. Imroving the affordability of prescription medications for people with chronic respiratory disease. Am J Respir Crit Care Med. 2018;198:1367–74.