ABSTRACT
Introduction
Graded Motor Imagery (GMI) is a non-invasive and inexpensive therapy used to treat Phantom Limb Pain (PLP) by sequentially activating motor networks in such a way that movement and pain are unpaired. The objective of this systematic review was to critically appraise relevant data on the efficacy of GMI and its components for reducing PLP and disability in amputees.
Methods
We searched 11 electronic databases for controlled trials investigating GMI and its components in amputees with PLP from inception until February 2023. Two reviewers independently screened studies and extracted relevant data. Study-level data were entered using the inverse variance function of the Review Manager 5 and pooled with the random effects model.
Results
Eleven studies with varying risk of bias were eligible. No eligible study considered left/right judgement tasks in isolation. Studies showed no effect for imagined movements, but positive effects were seen for GMI [weighted mean difference: -21.29 (95%CI: -31.55, -11.02), I2= 0%] and mirror therapy [weighted mean difference: -8.55 (95%CI: -14.74, -2.35, I2= 61%]. A comparison of mirror therapy versus sham showed no difference [weighted mean difference: -4.43 (95%CI: -16.03, 7.16), I2= 51%].
Conclusion
Our findings suggest that GMI and mirror therapy may be effective for reducing PLP. However, this conclusion was drawn from a limited body of evidence, and the certainty of the evidence was very low. Therefore, rigorous, high-quality trials are needed to address the gap in the literature and inform practice.
RÉSUMÉ
Contexte: L’imagerie motrice graduelle (IMG) est un traitement non invasif et peu coûteux utilisé pour traiter la douleur du membre fantôme par activation séquentielle des réseaux moteurs de manière à ce que le mouvement et la douleur soient dissociés. L’objectif de cette revue systématique était d’évaluer de manière critique les données sur l’efficacité de l’IMG et de ses composantes pour réduire la douleur du membre fantôme et l’invalidité chez les amputés.
Méthodes: Nous avons effectué des recherches dans 11 bases de données électroniques afin d’y repérer des essais contrôlés portant sur l’utilisation de l’IMG et de ses composantes auprès des amputés atteints de douleur du membre fantôme depuis le début jusqu’en février 2023. Deux évaluateurs indépendants ont examiné les études et extrait les données pertinentes Les données au niveau de l’étude ont été saisies à l’aide de la fonction de variation inverse de Review Manager 5 et regroupées selon un modèle à effets aléatoires.
Résultats: Onze études présentant un risque de biais variable ont été retenues. Aucune étude admissible ne se penchait sur les tâches de jugement gauche/droite de manière isolée. Les études n’ont montré aucun effet pour les mouvements imaginés, mais des effets positifs ont été observés pour l’IMG [différence moyenne pondérée : -21,29 (IC à 95 % : -31,55, -11,02), I2 = 0 %] et la thérapie miroir [différence moyenne pondérée: -8,55 (IC 95% : -14,74, -2,35, I2 = 61%]. La comparaison de la thérapie miroir à une thérapie factice n’a montré aucune différence [différence moyenne pondérée : -4,43 (IC à 95 % : -16,03, 7.16), I2 = 51 %].
Conclusion: Nos résultats indiquent que l’IMG et la thérapie miroir peuvent être efficaces pour réduire la douleur du membre fantôme. Cependant, cette conclusion a été tirée à partir d’un ensemble limité de données probantes, et la certitude de ces dernières était très faible. Par conséquent, des essais rigoureux et de haute qualité sont nécessaires pour combler les lacunes dans la littérature et éclairer la pratique.
Introduction
Phantom limb pain (PLP) is a common postamputation syndrome characterized by painful sensations in the missing part of the amputated limb. A recent systematic review revealed a PLP incidence of 82% within the first year of undergoing an amputation and a lifetime prevalence of 87%.Citation1 PLP is associated with psychological distress,Citation2 disability,Citation3,Citation4 and poorer health-related quality of life (HRQoL).Citation5,Citation6 PLP remains poorly understood and difficult to treat.Citation7
Treatments recommended for PLP are marginally effective at best and no more effective than placebo. There is systematic review evidence that pharmacological treatments are ineffective: memantine (30 mg/day for 4 days), gabapentin (2.4 g/day for 6 weeks), and amitriptyline (10–125 mg/day for 6 weeks) showed no benefit over placebo.Citation8,Citation9 Moreover, a review of the recent literature showed that of the six treatments investigated (targeted muscle reinnervation, repetitive transcranial magnetic stimulation, imaginal phantom limb exercises, mirror therapy, virtual reality and augmented reality therapies, eye movement desensitization and reprocessing therapy), none were more effective than the control.Citation10 The lack of effectiveness of these interventions suggests that they do not effectively target the mechanisms that underlie PLP.
Neuroimaging evidence has linked PLP to cortical reorganization of the sensorimotor cortex, in which the cortical area that previously represented the missing limb comes to represent other body parts.Citation11,Citation12 However, Makin et al. challenged this association between PLP and cortical reorganization by consistently revealing preserved cortical representation and function of the missing limb in amputees with PLP.Citation13–15 More recently, Ortiz-Catalan argued that PLP is purportedly driven by the stochastic entanglement of somatosensory–motor and pain networks resulting from somatosensory and motor deprivation.Citation16 This therefore suggests that phantom motor execution exercises providing motor and somatosensory feedback, such as mirror therapy, may be effective in reducing PLP.Citation17
Mirror therapy was proposed as a treatment for PLP because it purportedly addresses a theorized mismatch between motor command and sensory feedback.Citation18 Mirror therapy involves positioning a mirror in the sagittal plane of the body and moving the intact limb while viewing its reflection in the mirror, such that the reflection appears to be the missing limb. Mirror therapy has also been used as the third component of a three-phase graded motor imagery (GMI) program, which was developed to progressively target cortical motor networks in people with complex regional pain syndrome (CRPS).Citation19 GMI has systematic review evidence supporting its use in patients with CRPS.Citation20,Citation21 The similarities between the cortical changes seen in patients with CRPS and PLP suggest that the GMI program in its entirety could be a viable treatment for PLP.Citation22
Single studies have investigated mirror therapy, the other components of GMI (left/right judgment exercises and imagined movements), and the full GMI program for alleviating PLP, but to our knowledge there has been no recent attempt to systematically synthesize this literature. We therefore aimed to gather and critically appraise all relevant literature regarding the efficacy of the three components of GMI and the entire GMI program for reducing PLP to guide ongoing research and clinical practice.
Methods
This review was developed using the Cochrane methodology for systematic reviewsCitation23 and has been reported following the PRISMA 2020 statement.Citation24 The protocol of this review has been registered on PROSPERO (Ref. No. CRD42016036471) and published elsewhere.Citation25
Identification of Studies
We used a customized search strategy (Supplementary file 1) to search the following electronic databases: PubMed, Cochrane Central Register of Controlled Trials, Medline (via Ebscohost), PsychINFO (via Ebscohost), Physiotherapy Evidence Database, Scopus, Cumulative Index to Nursing and Allied Health Literature (via Ebscohost), Literatura Latino Americana em Ciências da Saúde, Database of Abstracts of Reviews of effects in the Cochrane Library, Africa-Wide Information (via Ebscohost), and Web of Science. In addition, we searched clinicaltrials.gov, Pactr.gov, and the European Union clinical trials register for ongoing research. Electronic databases and clinical registries were searched from their inception until February 2023.
To identify gray literature, we searched OpenGrey and contacted experts to seek published, unpublished, and ongoing trials that may be eligible for inclusion.
Eligibility Criteria
Studies were eligible for inclusion if they were randomized controlled trials, included adults (≥18 years) with chronic (≥3 months) PLP after amputation of an upper or lower limb, and compared GMI or one of its components to a control treatment. GMI was defined as treatment provided in order of left/right judgments, imagined movements, and mirror therapy. This ordered application of the components is thought to sequentially activate cortical premotor and motor networks and has been shown to produce a superior effect compared to the unordered GMI program.Citation19 Studies had to be published in the English language and needed to report at least one outcome of interest. If studies included participants with other pathologies or measured other outcomes, only the data relevant to the question of this review were extracted and used.
Screening and Study Selection
Two reviewers (S.W. and J.D.) independently screened titles and abstracts of studies retrieved from the literature search in duplicate. We retrieved full-length records of those studies deemed eligible and screened these again to confirm inclusion. Disagreements were resolved through discussion or, when necessary, consultation of a third independent reviewer (K.L.). When further information was required to confirm eligibility, we contacted authors up to four times within a 2-week period.Citation26 We used Cohen’s kappa to determine the measure of agreement between reviewers as either minimal (0–0.39), weak (0.40–0.59), substantial (0.60–0.79), or strong (0.80–0.90).Citation27
Outcomes
The primary outcome of interest was a change in PLP severity assessed by a 0 to 100 mm visual analogue scale (VAS) or 11-point numerical rating scale. The secondary outcomes were disability, HRQoL, adverse effects, psychosocial function, and patient global impression of change.
Data Extraction
Two reviewers (K.L. and J.D.) independently extracted data from included studies using a piloted customized sheet. Extracted data included the study characteristics (e.g., design, setting, exclusion/inclusion criteria, number of participants per group), participant characteristics (e.g., age, gender, amputation type, comorbidities), treatment characteristics (e.g., description, duration, frequency), follow-up period (weeks), number of participants lost to follow-up, adverse effects, and outcome measures (baseline, after intervention, and follow-up results on outcome measures). The two reviewers (K.L. and J.D.) compared the results and resolved disagreements concerning data extraction by discussion.
Assessment of Risk of Bias in Included Studies
Two reviewers (K.L. and S.W.) independently assessed the risk of bias of each included study using a customized risk of bias assessment guide (Supplementary file 2) informed by the Cochrane risk of bias tool.Citation28 The tool assessed the risk of bias across the domains of random sequence generation, allocation concealment, blinding of participants and outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias. Studies received an overall summary risk of bias score of “high risk” if the study was scored as high risk for any individual category, “low risk” if it was scored as low risk for every category, and “unclear” if it was scored as unclear for any category and did not score as high risk for any category. All disagreements were resolved by discussion.
Certainty of the Evidence
Two reviewers (K.L. and J.D.) independently assessed the certainty of the evidence for each analysis using the GRADE system.Citation29 We downgraded the certainty of evidence if a serious flaw was present in the domains of risk of bias, inconsistency, imprecision, indirectness, and publication bias. The certainty of evidence was initially classified as high and then as moderate, low, or very low certainty. All disagreements were resolved by discussion.
Data Analysis
Data were analyzed using Review Manager 5.Citation30 We pooled the results in a meta-analysis using the random effects inverse variance model.Citation23 We pooled studies comparing GMI with routine care, mirror therapy with control treatments, and mirror therapy with sham (covered mirror therapy). We calculated the weighted mean difference with a 95% confidence interval (CI) to determine between-group differences in outcomes for each analysis. A weighted mean difference of >10 mm (on a 0–100 mm VAS) with a 95% CI lower limit of ≥10 mm was considered clinically significant.Citation31 We converted the scores of the two studiesCitation6,Citation32 that assessed pain using a 0 to 10 scale to a 0 to 100 scale by multiplying the mean and standard deviation with the range of the new scale.Citation33 Funnel plots were generated to assess for possible publication bias whenever possible. We assessed statistical heterogeneity using the I2 statistic and rated the level of heterogeneity as low (0%–25%), moderate (>25%–50%), or high (>50%).Citation23 An improvement of ≥20% in HRQoL was considered clinically meaningful in accordance with anchor- and distribution-based methods for assessing the minimum clinically important change in HRQoL.Citation34 In a three-arm study, we reused data from the experimental group such that we had two between-group comparisons. Statistical significance was set at P < 0.05 for all analyses.
Results
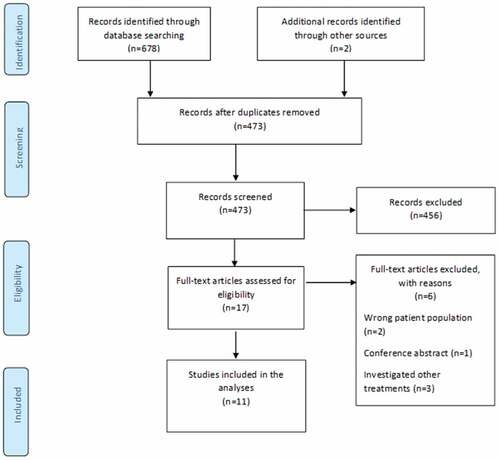
The initial literature search yielded 473 studies after removal of duplicates. Following title and abstract screening, 17 studies proceeded to full-text screening. Eleven studies were eligible and were included in this review (). Two studiesCitation6,Citation35 investigated GMI (versus routine care) and four studiesCitation36–39 investigated mirror therapy (versus sham). Four other studiesCitation32,Citation40–42 investigated mirror therapy (versus imagined movements, phantom movement exercises, tactile training, or transcutaneous electrical nerve stimulation), and two clinically heterogenous studiesCitation37,Citation43 investigated imagined movements (versus direct limb observation and mirror therapy). No study examined left/right judgment exercises as a stand-alone treatment. One three-arm studyCitation37 compared mirror therapy with covered mirror therapy and imagined movements. Therefore, 12 between-group comparisons were included in our analysis. The screening of titles and abstracts and full-text articles reflected strong (kappa = 0.85) and substantial (kappa = 0.76) agreement between reviewers, respectively.
The 11 studies provided data from a total of 373 participants (297 male, 76 female), of whom 37 reported PLP in an upper limb and 336 reported PLP in a lower limb. Further details of participants’ characteristics are provided in . Treatment parameters used in the different studies varied: each treatment session lasted between 10 and 30 min, treatment frequency ranged between one and seven sessions per week, and the total duration of interventions ranged between 1Citation36 and 42 days.Citation35 Three studiesCitation35,Citation40 reported follow-up measures at 6 months after treatment. Treatment details are summarized in Supplementary file 3.
Table 1. Characteristics of patients in included studies.
Nine of the 11 included studies used a 100 mm VAS to assess pain by self-report. One study assessed HRQoL using the VAS (0 = worst imaginable health state to 100 = best imaginable health state) of the EuroQol EQ-5D-5L.6 One studyCitation35 also assessed PLP-related disability as a secondary outcome using a patient-specific task-related numerical rating scale.Citation44 In that study, the participants rated their ability to perform five self-selected activities on a Likert-type scale (0–10 VAS: 0 = completely unable to perform; 10 = able to perform normally). No studies reported data on psychosocial outcomes, adverse effects, and patient global impression of change.
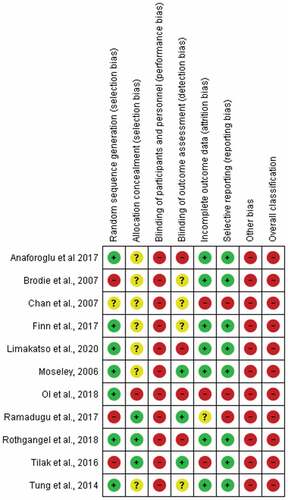
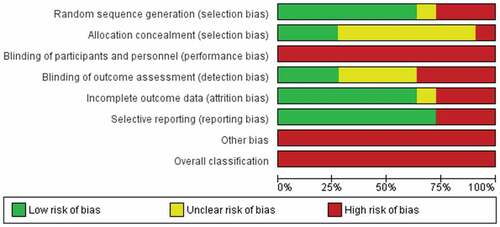
Risk of Bias Assessment
The results of the risk of bias assessment are shown in . All included studies had high risk of bias in the blinding category: no participants or treating clinicians were blinded to group allocation. In addition, only three studiesCitation35,Citation39,Citation42 were scored low risk for blinding outcome assessors to group allocation. All studies scored a high risk of additional bias for using an assessment tool that is not validated for assessing PLP in people with amputations. All of the studies scored a high risk for overall bias.
Effects of the Interventions
Graded Motor Imagery Program
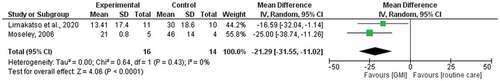
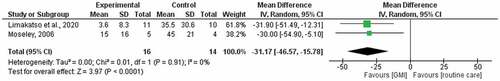
The pooling of twoCitation6,Citation35 studies comparing GMI with routine care at 6 weeks generated a weighted mean difference of −21.29 (95% CI −31.55, −11.02) and low statistical heterogeneity (I2 = 0%; ). The pooling of two studies comparing GMI with routine care at 6 months generated a median difference of −31.17 (95% CI −46.57, −15.78) and low statistical heterogeneity (I2 = 0%; ). The quality of evidence according to the GRADE system is presented in . We found very low-quality evidence that GMI is effective and better than routine care for reducing PLP. The quality of evidence was downgraded by high risk of bias and a small sample size.
Table 2. The certainty of the evidence.
Moseley’sCitation35 comparison of a 6-week course of GMI to a 6-week course of routine physiotherapy also assessed pain-related disability using a patient-specific functional scale.Citation45 Compared with routine physiotherapy, participants who performed GMI had less disability immediately after treatment (−2.40, 95% CI −0.82, −3.98). However, there was no between-group difference in disability scores at 6-month follow-up (0, 95% CI −1.68, 1.68).
Limakatso et al.’sCitation6 comparison of a 6-week course of GMI to a 6-week course of routine physiotherapy also assessed HRQoL using the VAS of the EuroQol EQ-5D-5L.Citation45 Compared with routine physiotherapy, participants who performed GMI had a higher HRQoL immediately after treatment (13.14, 95% CI −4.63, 30.91) and at 6-month follow-up (13.44, 95% CI −3.07, 29.95).
Mirror Therapy
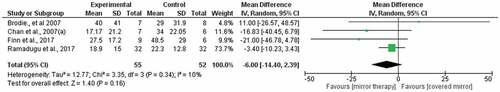
The pooling of eight studies comparing mirror therapy with control treatments generated a weighted mean difference of −8.55 (95% CI −14.74, −2.35) but high statistical heterogeneity (I2 = 61%). Visual inspection of the funnel plot failed to suggest publication bias ( in Supplementary file 4). We found very low-quality evidence that mirror therapy is more effective than control treatments for reducing PLP (). The quality of evidence was downgraded by high risk of bias, a small sample size, and wide 95% CI.
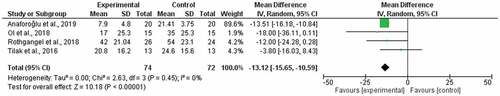
A subgroup analysis of four studiesCitation36–39 comparing mirror therapy with covered mirror therapy generated a weighted mean difference of −4.43 (95% CI −16.03, 7.16) but high statistical heterogeneity (I2 = 51%; ). A subgroup analysis of four studiesCitation32,Citation40–42 comparing mirror therapy to other control treatments generated a weighted mean difference of −13.12 (95% CI −15.65, −10.59; ) and low statistical heterogeneity (I2 = 0%). The certainty of the evidence was very low for both meta-analyses (). The quality of evidence was downgraded by high risk of bias, a small sample size, and a wide 95% CI.
Imagined Movements
Chan et al.’sCitation37 comparison of imagined movements with covered mirror therapy showed a mean difference of −16.40 (95% CI −33.30, 0.50). Tung et al.’sCitation43 comparison of imagined movements with movement observation showed a mean difference of 5.50 (95% CI −5.52, 16.52). Varying treatment protocols and control interventions in these studies meant that pooling them for a meta-analysis was not feasible.
Discussion
The aim of this review was to systematically evaluate the effectiveness of GMI and its components on PLP in people with amputations. Our findings indicate that GMI was probably more effective than routine care for reducing PLP intensity immediately after treatment and at 6-month follow-up. The subgroup analyses based on the type of control treatment showed that mirror therapy was probably more effective than other control treatments but probably no more effective than covered mirror therapy for reducing PLP. The certainty of the evidence for studies evaluating GMI and mirror therapy was ranked as very low, primarily due to a high risk of bias and small sample size. Studies evaluating the efficacy of imagined movements showed no effect.
Efficacy of GMI and Its Components on PLP
Graded Motor Imagery Program
Two studiesCitation6,Citation35 provided evidence that GMI reduced PLP and pain-related disability. These studies corroborate the results of a retrospective case seriesCitation46 that found that GMI had a clinically meaningful and long-lasting effect on PLP. GMI was endorsed in a recent expert Delphi studyCitation47 as a viable treatment for PLP, and the findings of this review further support its clinical utility. However, the generalizability of our findings is limited by a small sample size and a homogenous sample of lower limb amputees in the included studies. Replicating these positive findings in a large randomized, sham-controlled trial is necessary to shed light on the efficacy of GMI for reducing PLP in people with upper and lower limb amputations.
Mirror Therapy
Clinically significant pain reductions suggest that mirror therapy may be a more viable treatment for PLP compared to mental visualization techniques. These positive findings are consistent with those of preliminary studies that were not were not eligible for inclusion in the review.Citation48–53 We found it interesting that one studyCitation40 that provided mirror therapy for a longer term showed superior effects compared to other studies. These findings are in line with expert recommendations that mirror therapy is likely to have a clinically meaningful effect when conducted at least three time a week over a long term.Citation17 Moreover, our findings indicate that the efficacy of mirror therapy can be augmented by combining it with other treatments, as seen in GMI studies.Citation6,Citation35 The studies on mirror therapy included in this review had high risk of bias, and only oneCitation40 followed their participants beyond the immediate posttreatment assessment. This gap in the literature warrants robust clinical trials with a longer follow-up period and mechanisms-based studies to clearly elucidate the mechanisms by which mirror therapy reduces PLP.
Mirror visual feedback is argued to be an active component for mirror therapy.Citation18 However, we found no difference in pain severity between mirror therapy and covered mirror therapy, for which mirror visual feedback is eliminated. Although visual input has been shown to influence phantom limb awareness,Citation54 our results indicate that it is not necessary for PLP reduction. More recently, Ortiz-Catalan hypothesized that PLP is driven by the stochastic entanglement of somatosensory, motor, and pain networks resulting from somatosensory and motor deprivation.Citation16 This concept implies that retraining somatosensory and motor networks, and not visual networks, is sufficient for pain reduction.
Imagined Movements
We found conflicting results for imagined movements in this review. An explanation for the conflicting results after imagined movements emerges from a consideration unique to the use of imagined movements in people with amputations. Raffin et al.Citation55,Citation56 pointed out that clinicians using imagined movements with people with intact limbs verify the absence of movement-generating neural activity by monitoring movement of the intact limb. However, such visual monitoring is not feasible in amputees, because the relevant body part is not present. It is therefore impossible to visually verify the absence of movement-generating neural activity in amputees participating in imagined movements, yet this verification is necessary, because any such activity would place a higher demand on the neural system than is desirable during imagined movements exercises.Citation56
In line with this idea, Raffin et al.Citation55 asked amputees with PLP to perform imagined movements, or movements of the phantom limb, while monitoring cortical activity, residual limb muscle activity (using electromyographic biofeedback), and sensations felt during the task. They found that performance of imagined movements was associated with neural activity in the premotor cortex and posterior lobe of the cerebellum, absent residual limb muscle activity and no triggered or exacerbated pain. In contrast, movements of the phantom limb were associated with neural activity in the primary somatosensory and primary motor cortices and the anterior lobe of the cerebellum, increased residual limb muscle activity, and, in some cases, pain exacerbation. These findings support the suggestion that conflicting findings across studies testing the efficacy of imagined movements for reducing PLP in amputees could be due to discrepancies in accurate performance of the imagined movements tasks; in other words, that participants asked to imagine performing movements are, in fact, performing movements, resulting in more neural activation than is desired.
Left/Right Judgments
We found no studies that evaluated left/right judgment exercises as a stand-alone treatment for PLP. Recent evidence suggests that left/right judgment exercises facilitate inhibitory priming of brain areas that are involved in preparation for movement.Citation57 These data provide a logical rationale for the use of left/right judgment exercises as the first step in the GMI program, yet other studiesCitation35,Citation58 that applied GMI to a group with mixed chronic pain conditions found left/right judgment exercises alone to have no effect on pain. There is a need to investigate the role of left/right judgment exercise in amputees with PLP.
Strengths and Limitations
Our study had several strengths. We conducted this systematic review in accordance with a preregistered and published protocol. In addition, we followed the Cochrane recommendations for conducting a systematic review of controlled trials to ensure the robustness of the review process. A limitation of this review is that we excluded studies written in languages other than English, due to a lack of translation resources. The included studies represented few upper limb amputees, so the results may have limited generalizability to upper limb amputees. Only one study investigating mirror therapy followed up their participants beyond the time of treatment cessation. Therefore, we are unable to draw a firm conclusion on the long-term effects of this treatment on PLP.
In conclusion, this systematic review found weak but promising evidence that GMI is more effective than routine care for producing a clinically meaningful change in pain severity and that mirror therapy is more effective than mental visualization techniques but not covered mirror therapy. Importantly, our conclusion on GMI was derived from the results of few studies with a relatively small sample size. Higher quality studies are needed to generate a robust conclusion regarding the effectiveness of GMI on PLP.
Acknowledgment
The authors thank Mary Shelton (Health Sciences reference librarian, University of Cape Town) for assisting with the development of the search strategy.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
References
- Stankevicius A, Wallwork SB, Summers SJ, Hordacre B, Stanton TR. Prevalence and incidence of phantom limb pain, phantom limb sensations and telescoping in amputees: a systematic rapid review. Eur J Pain. 2021;25(1):23–11. doi:10.1002/ejp.1657.
- Fuchs X, Flor H, Psychological Factors B-BR. Associated with phantom limb pain: a review of recent findings. Pain Res Manage. 2018;2018:5080123. doi:10.1155/2018/5080123.
- Ehde DM, Czerniecki JM, Smith DG, Campbell KM, Edwards WT, Jensen MP, Robinson LR. Chronic phantom sensations, phantom pain, residual limb pain, and other regional pain after lower limb amputation. Arch Phys Med Rehabil. 2000;81(8):1039–44. doi:10.1053/apmr.2000.7583.
- Dietrich C, Nehrdich S, Seifert S, Blume KR, Miltner WHR, Hofmann GO, Weiss T. Leg prosthesis with somatosensory feedback reduces phantom limb pain and increases functionality. Front Neurol. 2018;9:270. doi:10.3389/fneur.2018.00270.
- Polat CS, Konak HE, Altas EU, Akıncı MG, Onat S S. Factors related to phantom limb pain and its effect on quality of life. Somatosens Mot Res. 2021;38(4):322–26. doi:10.1080/08990220.2021.1973405.
- Limakatso K, Madden VJ, Manie S, Parker R. The effectiveness of graded motor imagery for reducing phantom limb pain in amputees: a randomised controlled trial. Physiotherapy. 2020;109:65‐74. doi:10.1016/j.physio.2019.06.009.
- Schone HR, Baker CI, Katz J, Nikolajsen L, Limakatso K, Flor H, Makin TR. Making sense of phantom limb pain. J Neurol Neurosurg Psychiatry. 2022;93(8):833–43. doi:10.1136/jnnp-2021-328428.
- Alviar MJ, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. The Cochrane Database Syst Rev. 2016;10(10):Cd006380. doi:10.1002/14651858.CD006380.pub3.
- Alviar M, Hale T, Dungca M. Pharmacologic interventions for treating phantom limb pain. Cochrane Database Syst Rev. 2011;12.
- Aternali A, Katz J. Recent advances in understanding and managing phantom limb pain. F1000Research. 2019;8.
- Raffin E, Richard N, Giraux P, Reilly KT. Primary motor cortex changes after amputation correlate with phantom limb pain and the ability to move the phantom limb. NeuroImage. 2016;130:134–44. doi:10.1016/j.neuroimage.2016.01.063.
- Kikkert S, Mezue M, Slater DH, Johansen-Berg H, Tracey I, Makin TR. Motor correlates of phantom limb pain. cortex. 2017;95:29–36. doi:10.1016/j.cortex.2017.07.015.
- Makin TR, Scholz J, Filippini N, Henderson Slater D, Tracey I, Johansen-Berg H. Phantom pain is associated with preserved structure and function in the former hand area. Nat Commun. 2013;4(1):1570. doi:10.1038/ncomms2571.
- Makin TR. Phantom limb pain: thinking outside the (mirror) box. Brain. 2021;144(7):1929. doi:10.1093/brain/awab139.
- Muret D, Makin TR. The homeostatic homunculus: rethinking deprivation-triggered reorganisation. Curr Opin Neurobiol. 2021;67:115–22. doi:10.1016/j.conb.2020.08.008.
- Ortiz-Catalan M. The stochastic entanglement and phantom motor execution hypotheses: a theoretical framework for the origin and treatment of phantom limb pain. Front Neurol. 2018;9. doi:10.3389/fneur.2018.00009.
- Wang F, Zhang R, Zhang J, Li D, Wang Y, Yang Y-H WQ. Effects of mirror therapy on phantom limb sensation and phantom limb pain in amputees: a systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2021;35(12):1710–21. doi:10.1177/02692155211027332.
- Ramachandran VS, Rogers-Ramachandran D. Synaesthesia in phantom limbs induced with mirrors. Proc R Soc B: Biol Sci. 1996;263(1369):377–386.
- Moseley GL. Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial. Pain. 2004;108(1‐2):192‐8. doi:10.1016/j.pain.2004.01.006.
- Bowering KJ, O’Connell NE, Tabor A, Catley MJ, Leake HB, Moseley GL, Stanton TR. The effects of graded motor imagery and its components on chronic pain: a systematic review and meta-analysis. The Journal of Pain. 2013;14(1):3–13. doi:10.1016/j.jpain.2012.09.007.
- Méndez-Rebolledo G, Gatica-Rojas V, Torres-Cueco R, Albornoz-Verdugo M, Guzmán-Muñoz E. Update on the effects of graded motor imagery and mirror therapy on complex regional pain syndrome type 1: a systematic review. J Back Musculoskelet Rehabil. 2017;30(3):441–49. doi:10.3233/BMR-150500.
- Acerra NE, Souvlis T, Moseley GL. Stroke, complex regional pain syndrome and phantom limb pain: can commonalities direct future management? J Rehabil Med. 2007;39(2):109–14. doi:10.2340/16501977-0027.
- Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane handbook for systematic reviews of interventions. 2nd ed. Chichester (UK): John Wiley & Sons; 2019.
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, et al. 2020 statement: an updated guideline for reporting systematic reviews. Syst Rev. 2021;10(1):1–11. doi:10.1186/s13643-021-01626-4.
- Limakatso K, Corten L, Parker R. The effects of graded motor imagery and its components on phantom limb pain and disability in upper and lower limb amputees: a systematic review protocol. Syst Rev. 2016;5(1):145. doi:10.1186/s13643-016-0322-5.
- Meursinge Reynders R, Ladu L, Di Girolamo N. Contacting of authors by systematic reviewers: protocol for a cross-sectional study and a survey. Syst Rev. 2017;6(1):1–12. doi:10.1186/s13643-017-0643-z.
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas. 1960;20(1):37–46. doi:10.1177/001316446002000104.
- Higgins J, Altman DG. Assessing risk of bias in included studies. In: Cochrane handbook for systematic reviews of interventions: cochrane book series. Chichester (UK): 2008. p. 187–241.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–26. doi:10.1136/bmj.39489.470347.AD.
- RevMan R. The nordic cochrane centre, the cochrane collaboration. Book [computer program]; 2014.
- Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, Farrar JT, Hertz S, Raja SN, Rappaport BA. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain. 2009;146(3):238–44. doi:10.1016/j.pain.2009.08.019.
- Rothgangel A, Braun S, Winkens B, Beurskens A, Smeets R. Traditional and augmented reality mirror therapy for patients with chronic phantom limb pain (PACT study): results of a three-group, multicentre single-blind randomized controlled trial. Clin Rehabil. 2018;32(12):1591–608. doi:10.1177/0269215518785948.
- Wewege MA, Jones MD, Williams SA, Kamper SJ, McAuley JH. Rescaling pain intensity measures for meta-analyses of analgesic medicines for low back pain appears justified: an empirical examination from randomised trials. BMC Med Res Methodol. 2022;22(1):1–9. doi:10.1186/s12874-022-01763-x.
- Olsen MF, Bjerre E, Hansen MD, Hilden J, Landler NE, Tendal B, Hróbjartsson A. Pain relief that matters to patients: systematic review of empirical studies assessing the minimum clinically important difference in acute pain. BMC Med. 2017;15(1):1–18. doi:10.1186/s12916-016-0775-3.
- Moseley GL. Graded motor imagery for pathologic pain a randomized controlled trial. Neurology. 2006;67:2129–34.
- Brodie EE, Whyte A, Niven CA. Analgesia through the looking‐glass? A randomized controlled trial investigating the effect of viewing a ‘virtual’limb upon phantom limb pain, sensation and movement. Eur J Pain. 2007;11(4):428–36. doi:10.1016/j.ejpain.2006.06.002.
- Chan BL, Witt R, Charrow AP, Magee A, Howard R, Pasquina PF, Heilman KM, Tsao JW. Mirror therapy for phantom limb pain. N Engl J Med. 2007;357(21):2206–07. doi:10.1056/NEJMc071927.
- Finn SB, Perry BN, Clasing JE, Walters LS, Jarzombek SL, Curran S, Rouhanian M, Keszler MS, Hussey-Andersen LK, Weeks SR, et al. A randomized, controlled trial of mirror therapy for upper extremity phantom limb pain in male amputees. Front Neurol. 2017;8(JUL). doi:10.3389/fneur.2017.00267.
- Ramadugu S, Nagabushnam SC, Katuwal N, Chatterjee K. Intervention for phantom limb pain: a randomized single crossover study of mirror therapy. Indian J Psychiatry. 2017;59(4):457–64. doi:10.4103/psychiatry.IndianJPsychiatry_259_16.
- Anaforoğlu Külünkoğlu B, Erbahçeci F, Alkan A. A comparison of the effects of mirror therapy and phantom exercises on phantom limb pain. Turkish J Med Sci. 2019;49(1):101‐9. doi:10.3906/sag-1712-166.
- Ol HS, Van Heng Y, Danielsson L, Husum H. Mirror therapy for phantom limb and stump pain: a randomized controlled clinical trial in landmine amputees in Cambodia. Scand Actuarial J. 2018;18(4):603–10. doi:10.1515/sjpain-2018-0042.
- Tilak M, Isaac SA, Fletcher J, Vasanthan LT, Subbaiah RS, Babu A, Bhide R, Tharion G. Mirror therapy and transcutaneous electrical nerve stimulation for management of phantom limb pain in amputees—a single blinded randomized controlled trial. Physiother Res Int. 2016;21(2):109–15. doi:10.1002/pri.1626.
- Tung ML, Murphy IC, Griffin SC, Alphonso AL, Hussey‐Anderson L, Hughes KE, Weeks SR, Merritt V, Yetto JM, Pasquina PF, et al. Observation of limb movements reduces phantom limb pain in bilateral amputees. Ann Clin Transl Neurol. 2014;1:633–38.
- Nugent SM, Lovejoy TI, Shull S, Dobscha SK, Morasco BJ. Associations of pain numeric rating scale scores collected during usual care with research administered patient reported pain outcomes. Pain Med. 2021;22(10):2235–41. doi:10.1093/pm/pnab110.
- Horn KK, Jennings S, Richardson G, Van Vliet D, Hefford C, Abbott JH. The patient-specific functional scale: psychometrics, clinimetrics, and application as a clinical outcome measure. J Orthop Sports Phys. 2012;42(1):30–D17. doi:10.2519/jospt.2012.3727.
- Hinkel M. Graded motor imagery for the treatment of phantom limb pain. Arch Phys Med Rehabil. 2017;98(10):e72. doi:10.1016/j.apmr.2017.08.225.
- Limakatso K, Parker R. Treatment recommendations for phantom limb pain in people with amputations: an expert consensus Delphi study. Pm R. 2021;13(11):1216–26. doi:10.1002/pmrj.12556.
- Kim SY, Kim YY. Mirror therapy for phantom limb pain. Korean J Pain. 2012;25(4):272–74. doi:10.3344/kjp.2012.25.4.272.
- MacLachlan M, McDonald D, Waloch J. Mirror treatment of lower limb phantom pain: a case study. Disabil Rehabil. 2004;26(14–15):901–04. doi:10.1080/09638280410001708913.
- Ramachandran VS, Altschuler EL. The use of visual feedback, in particular mirror visual feedback, in restoring brain function. Brain. 2009;132(7):1693–710. doi:10.1093/brain/awp135.
- Schmalzl L, Ragno C, Ehrsson HH. An alternative to traditional mirror therapy illusory touch can reduce phantom pain when; 2013.
- Mercier C, Sirigu A. Training with virtual visual feedback to alleviate phantom limb pain. Neurorehabil Neural Repair. 2009;23(6):587–94. doi:10.1177/1545968308328717.
- Mallik AK, Pandey SK, Srivastava A, Kumar S, Kumar A. Comparison of relative benefits of mirror therapy and mental imagery in phantom limb pain in amputee patients at a tertiary care center. Arch Rehabil Res Clin Transl. 2020;2(4):100081. doi:10.1016/j.arrct.2020.100081.
- Hunter JP, Katz J, Davis KD. The effect of tactile and visual sensory inputs on phantom limb awareness. Brain: A J Neurol. 2003;126(3):579–89. doi:10.1093/brain/awg054.
- Raffin E, Giraux P, Reilly KT. The moving phantom: motor execution or motor imagery? Cortex. 2012;48(6):746–57. doi:10.1016/j.cortex.2011.02.003.
- Raffin E, Mattout J, Reilly KT, Giraux P. Disentangling motor execution from motor imagery with the phantom limb. Brain. 2012;135:582–95. doi:10.1093/brain/awr337.
- Beisheim-Ryan EH, Pohlig RT, Medina J, Hicks GE, Sions JM. Body representation among adults with phantom limb pain: results from a foot identification task. Eur J Pain (London, England). 2022;26(1):255–69. doi:10.1002/ejp.1860.
- Moseley G. Graded motor imagery is effective for long-standing complex regional pain syndrome: a randomised controlled trial. Pain. 2004;108:192–98.