?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Background
Because of the high initial cost of intrathecal drug delivery (ITDD) therapy, this study investigated the cost-effectiveness and cost–utility of ITDD therapy in refractory cancer pain management in Thailand over the past 10 years.
Methods
The retrospective study was conducted in patients with cancer pain who underwent ITDD therapy from January 2011 to 2021 at three university hospitals. Clinical outcomes included the numerical rating scale (NRS), Palliative Performance Scale, and the EQ-5D. The direct medical and nonmedical as well as indirect costs were also recorded. Cost-effectiveness and cost–utility analyses were performed comparing ITDD therapy with conventional therapy (extrapolated from costs of the same patient before ITDD therapy) from a societally oriented economic evaluation.
Results
Twenty patients (F:M: 10:10) aged 60 ± 15 years who underwent implantation of an intrathecal percutaneous port (IT port; n = 15) or programmable intrathecal pump (IT pump; n = 5) were included. The median survival time was 78 (interquartile range = 121–54) days after ITDD therapy. At 2-month follow-up, the incremental cost-effectiveness ratio (ICER)/pain reduction of an IT port (US$2065.36 (CA$2829.54)/2-point NRS reduction/lifetime) was lower than for patients with an IT pump (US$5479.26 (CA$7506.58)/2-point NRS reduction/lifetime) compared with continued conventional therapy. The ICER/quality-adjusted life years (QALYs) gained for an IT port compared with conventional treatment was US$93,999.31(CA$128,799.06)/QALY gained, which is above the cost-effectiveness threshold for Thailand.
Conclusion
The cost-effectiveness and cost–utility of IT port therapy for cancer pain was high relative to the cost of living in Thailand, above the cost-effectiveness threshold. Prospective cost analysis studies enrolling more patients with diverse cancers that investigate the benefit of early ITDD therapy with devices over a range of prices are warranted.
RÉSUMÉ
Contexte: En raison du coût initial élevé du traitement par administration intrathécale de médicaments (AIM), cette étude a étudié le rapport coût-efficacité et le rapport coût-utilité du traitement par AIM dans la prise en charge de la douleur cancéreuse réfractaire en Thaïlande au cours des 10 dernières années.
Méthodes: L’étude rétrospective a été menée auprès de patients souffrant de douleur cancéreuse ayant subi un traitement par AIM de janvier 2011 et 2021 dans trois hôpitaux universitaires. Les résultats cliniques comprenaient l’échelle d’évaluation numérique (EEN), l’échelle de performance palliative et l’EQ-5D. Les coûts médicaux et non médicaux directs et indirects ont également été consignés. Les analyses coûts-efficacité et coût-utilité ont été effectuées en comparant le traitement par AIM au traitement conventionnel (extrapolé à partir des coûts pour le même patient avant le traitement par AIM) à partir d’une évaluation économique sociétale.
Résultats: Vingt patients (F : M : 10 : 10) âgés de 60 ± 15 ans ayant subi l’implantation d’un port percutané intrathécal (port IT; n = 15) ou d’une pompe intrathécale programmable (pompe IT; n = 5) ont été inclus. Le temps de survie médian était de 78 jours (intervalle interquartile = 121–54) après le traitement par AIM. À deux mois de suivi, le ratio coût-efficacité incrémental (RCEI/réduction de la douleur d’un port IT (2 065,36 $ US (2 829,54 $ CA) /réduction de 2 points sur l’EEN/durée de vie) était inférieur à celui des patients avec une pompe IT (5479,26 $ US (7506,58 $ CA) /réduction de 2 points sur l’EEN/durée de vie) comparativement au traitement conventionnel en continu. Le RCEI/année de vie pondérée par la qualité (AVPQ) gagnée pour un port IT comparativement au traitement conventionnel était de 93 999,31 $ US (128 799,06 $ CA) /AVPQ gagné, ce qui est au-dessus du seuil de rentabilité pour la Thaïlande.
Conclusion: Le rapport coût-efficacité et le rapport coût-utilité du traitement par port IT pour la douleur cancéreuse étaient élevés par rapport au coût de la vie en Thaïlande, soit au-dessus du seuil de rentabilité. Les études d’analyse de coût prospectives portant sur un plus grand nombre de patients atteints de divers cancers qui étudient les avantages des traitements par AIM précoces à l’aide d’appareils de prix différents sont justifiées.
Introduction
More than half of patients with advanced stage or terminal cancer experience moderate to severe pain.Citation1 Up to 15% of patients with advanced-stage cancer experiencing refractory pain require advanced pain management techniques.Citation2,Citation3 Intrathecal drug delivery (ITDD) therapy is an alternative route of analgesic administration that provides significant pain relief by using small doses of medications administered directly into the intrathecal space. In patients who experience sufficient pain relief with opioids but have intolerable side effects (e.g., sedation, gastrointestinal effects), ITDD may improve the side effect profile, leading to better outcomes.Citation4 Ninety percent of patients who receive ITDD therapy provides significant pain reduction and well-controlled pain within 1 weekCitation5–8 with a low incidence of major complications.Citation9
However, the initial cost of ITDD therapy is high, especially the upfront costs of equipment, as well as facility and implantation fees. Although several cost-effectiveness studies have been performed in industrialized Western countries,Citation10 economic evaluations of continuous intrathecal opioid infusions for refractory cancer pain in developing countries are extremely limited. Moreover, the high fixed costs for devices in developing countries pose a barrier and limit the number of patients that can be studied. In the United States, one retrospective study performed in 36 patients with cancer found the threshold for cost-effectiveness of ITDD to be approximately 7 months in patients with high costs of care, with cost equivalence predicted at 344 months in the entire cohort.Citation7 In Korea, ITDD systems were shown to be cost beneficial at 28 months with 50% government financial coverage in patients with cancer who experienced intolerable side effects or uncontrolled pain despite high-dose and nonopioid therapy.Citation11 In China, ITDD therapy delivered via an intrathecal percutaneous port (IT port) for refractory cancer pain achieved cost equivalence at 2.89 months in patients on extremely high doses of opioids (morphine equivalent daily dose [MEDD] > 599 mg/day),Citation12 9.71 months in those on high-dose opioid therapy (MEDD = 300–599 mg/day), and 28.83 months in patients receiving more typical opioid doses (MEDD < 300 mg/day).Citation6 Because the cost of IT port implantation is significantly less than the cost of an IT pump, this approach might prove to be a cost-effective treatment option in patients with cancer with a very limited life expectancy (e.g., less than 6 months). Yet, there have been no cost-effectiveness studies performed in Southeast Asia evaluating the cost-effectiveness or utility of any form of ITDD therapy. The objectives of this study are to investigate the cost-effectiveness and cost–utility of ITDD therapy in cancer pain management in Thailand and to report pain and quality of life outcomes of patients with cancer treated with ITDD.
Materials and Methods
Participants
After obtaining institutional review board approval from three university hospitals (Ramathibodi, Siriraj, and King Chulalongkorn Memorial hospital; ID No. COA. MURA2021/382, May 12, 2021), the study was registered with the Thai Clinical Trials Registry (TCTR20210607005) on May 28, 2021 (https://www.thaiclinicaltrials.org/#). All patients with cancer pain for more than 3 months who had an ITDD system implanted between January 2011 and January 2021 were enrolled in this study. Indications for intrathecal therapy were intolerable side effects from conventional therapy or poorly pain controlled pain with multimodal pain management including radiation and palliative chemotherapy with opioid therapy for at least 3 months. The systems evaluated were either an IT port or an IT pump. All patients received conventional pain therapy such as oral, intravenous, or transdermal opioids and nonsteroidal anti-inflammatory drugs or adjuvants as indicated at least 1 month before ITDD therapy. The participants or their relatives provided verbal informed consent via the telephone. The exclusion criteria were lack of data records and loss of contact with participants or their relatives.
Data Extraction
The medical records were retrospectively reviewed. Data were extracted and entered into case report forms in deidentified format. Demographic and clinical data including age, sex, type of cancer, categorization of pain, and opioid and nonopioid analgesic consumption. Indication for and type of ITDD therapy, pain scores, and quality of life measurements were recorded. Preimplant data for hospital visits and medication usage were recorded for 30 days, with adjunctive care such as integrative analgesic therapies and pain-alleviating (e.g., neurolytic) injections recorded for up to 3 months before implant. Therapies related to cancer treatment (e.g., radiation and chemotherapy) and general well-being (e.g., yoga) were not included in cost analysis but were recorded pre- and postimplant. Postimplant, data were recorded until death or 12 months to include survival time after ITDD therapy, total opioid consumption, nonanalgesic usage, hospital visits, injections, integrative care, and complications related to implantation. Phone interviews with patients and health care proxies were conducted to record direct nonmedical and indirect costs.
Interventions
An intrathecal or continuous epidural morphine trial was performed in every case (mean duration 5 ± 2 days). The implantation cutoff threshold was ≥50% pain reduction. Implantation of both IT ports (Celsite Spinal, B Braun) and IT pumps (SynchroMed II, Medtronic) was performed using standardized techniques in sterile fashion. Medications used in all IT port cases consisted of morphine mixed with bupivacaine, whereas morphine alone was used in IT pumps. Other interventions were continued as indicated such as physiotherapy, radiation, or chemotherapy.
Clinical Outcomes
The clinical outcomes were recorded at 1 month and 1 day pre-implantation (pre-ITDD) and every month after ITDD therapy (post-ITDD) for 12 months or until the patient died. The primary outcome measures were the cost-effectiveness ratios for ITDD for pain score reduction and the incremental cost-effectiveness ratio (ICER) for ITDD compared to conventional therapy. Secondary clinical outcomes included the following:
Average pain score using a 0–10 numerical rating scale (NRS) in the past 24 h.
Opioid consumption was converted to an MEDD by using the opioid conversion table in the 2022 revised U.S. Centers for Disease Control and Prevention opioid guidelinesCitation13 and a 1:300 ratio for intrathecal to oral morphine conversion.Citation14
Functional status using the Palliative Performance Scale (PPS). This measures the progressive decline of a palliative patient from 0% to 100% on five dimensions including (1) ambulation, (2) activity level and evidence of disease, (3) self-care, (4) oral intake, and (5) level of consciousness. A score of 0% to 30% on the PPS indicates a patient with terminal cancer, 31% to 70% indicates a patient requiring close monitoring and care (i.e., who may require hospice care), and >70% indicates a stable patient.Citation15
Quality of life using utility scores calculated from the EQ-5D, which consists of five dimensions with five levels of severity including (1) mobility, (2) self-care, (3) usual activities, (4) pain and discomfort, and (5) anxiety and depression. The utility score ranges from 0 to 1, with 0 = death and 1 = completely healthy.Citation16
Cost Estimates and Comparisons
We employed a pre–post design comparing pain-related costs before and after implantation. The fixed time period was time from ITDD implantation to death, with the total costs sustained over that period divided by the time in days to come up with a cost per day in U.S. and Canadian dollars (e.g., US$20,000/CA$25,000 total costs for a person who died in 100 days would be US$200/CA$250/per day). For pre-implant costs, we searched back 3 months to estimate costs for ancillary procedures including injections and integrative pain treatments. Pre-implant medication costs were based on the 30-day period (or less if 30 days were not available) before implant and divided by 30 (or the number of days available if less than 30) to come up with a cost per day. Extrapolating medication costs from the 30-day period pre-implant until death is based on the assumption that though costs of pharmacotherapy are difficult to predict, including data from 30 days before implant would best reflect (and possibly underestimate) them because worsening pain and analgesic escalation are usually the basis for ITDD. Assuming that patients will remain on their pre-implant medications until death without escalation or reduction has previous precedent in the cost-effectiveness intrathecal drug delivery literature.Citation6,Citation7
Cost Analyses
The total cost included direct medical and nonmedical and indirect costs based on publicly available economic data from Thailand. All costs were adjusted according to the Thailand Consumer Price Index price in 2021 and are presented in U.S. and Canadian dollars using a 0.80 (US$)/1 (CA$) to 25.56 (Thai Baht) conversion rate, the average exchange rates during 2021.
Costs for ITDD Period
Direct medical costs were based on an average wholesale price from the three participating hospitals.
The fixed costs included ITDD implantation costs (ITDD equipment, infusion set for IT percutaneous port, operating room facility, and professional fees). The ITDD implantation cost was divided by the patient’s survival days to obtain the cost/day (US$ and CA$/day).
Variable costs included
ITDD maintenance costs (cost of the ITDD refill kit and procedural fees)
Medication costs (cost of intrathecal and nonintrathecal analgesics such as nonopioids, intravenous opioids)
Non-ITDD-related analgesic procedure costs (e.g., chemotherapy, radiation therapy, injections). The cost for each refill period was divided by the number of days between refills to calculate the cost per day for that period (US$ and CA$/day), after which refill period costs were added to determine a total cost.
The costs of hospital readmissions were included only if the admission was deemed due to ITDD-related complications.
Direct nonmedical costs (US$ and CA$/OPD (outpatient department) visit) included the costs of meals, transportation, and caregiving.
Indirect costs included patient and caregiver productivity losses including time-adjusted lost income for patients and caregivers.
Costs for Pre-ITDD Period
The data extraction included direct medical and nonmedical cost and indirect costs, similar to the post-ITDD period.
Statistical Analysis
Continuous data including NRS pain scores, PPS scores, and opioid requirement are presented as medians and interquartile ranges (IQRs); 75th to 25th percentile (Q3–Q1). For comparisons of nonnormally distributed data, the Friedman test was used. The Wilcoxon signed rank test was used for comparisons between pretreatment and each follow-up time point. A P value < .05 was considered statistically significant.
Cost analyses were conducted monthly up to 3 months and at 6 and 12 months in those who survived through these time periods. As noted above, the costs for patients when they received conventional therapy were calculated from medical records and phone interviews over the 1-month period pre-implant and divided by 30 to come up with a cost per day. The cost of conventional therapy was calculated based on the costs of hospital visits and analgesic usage, categorized as follows: (1) no change in opioid or any analgesic medication dose or (2) increase in opioid or nonopioid analgesic dose or starting new therapy, with costs calculated accordingly. For postimplant costs, total costs were divided by the survival time to come up with a cost per day. Due to the short evaluation period, no discount rate was used for cost estimation. The study adopted Thai-relevant economic references for cost evaluations to inform decision making among national policymakers and payers. All estimated costs were based on actual individual patient data, not model-based systems.
An ICER was calculated by dividing the difference in cost by the difference in clinical outcomes (NRS and PPS) between the ITDD and conventional therapy groups. Quality-adjusted life days (QALDs) and quality-adjust life years (QALYs) were calculated from the results of EQ-5D questionnaires. The ICER for QALD and QALY were calculated using the following formulas:
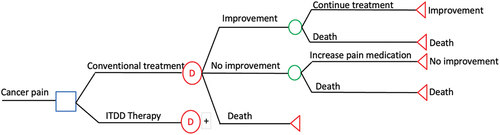
The cost-effectiveness threshold for a QALY was considered to be 1.5 times the 2021 Thailand gross domestic product per capita (about 377,600 baht), or US$10,783 (CA$14,773).Citation17 The threshold of willingness to pay for universal health coverage in Thailand has been estimated at US$4,848.48 (CA$6,400)/QALY gained (160,00 baht/QALY gained) since 2013.Citation18 The decision model depicted in was used for our analytical model using a Thai societal perspective for health economic evaluations.
Post Hoc Power Analysis
A prestudy power analysis was not performed because our study encompassed all patients with cancer with an implant at participating institutions over a 10-year period, and similar to other cost-effectiveness studies lacking power analyses,Citation5–7,Citation11 no reliable assumptions could be made regarding ancillary care requirements and life expectancy. However, a post hoc sample size calculation showed that our study would have a 72% chance of detecting a difference between IT therapy and conventional therapy for the secondary outcome of pain score based on the unweighted mean of two Asian studies conducted in Korea and China that evaluated IT port therapy and one international study (costs evaluated on only the U.S. subset) that evaluated cost-effectiveness and cost–utility.Citation5,Citation6,Citation11
Results
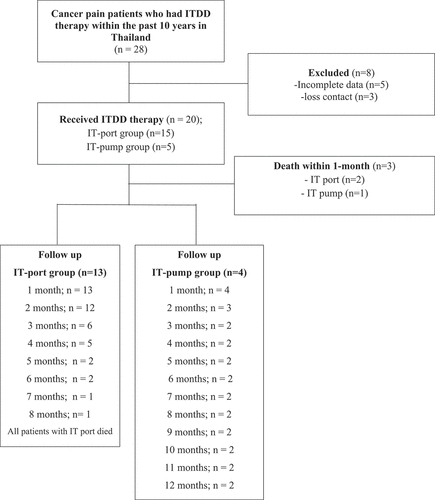
Twenty-eight patients with cancer pain who received an ITDD system between January 2011 and January 2021 at three university hospitals were eligible for the study. Eight patients were excluded owing to incomplete data (n = 5) and loss of contact with the patient or family (n = 3). Twenty patients were enrolled in this study, including 1 at Ramathibodi Hospital, 4 at Siriraj Hospital, and 15 at Chulalongkorn Memorial Hospital ().
Patients’ demographic data are shown in . The most common type of pain was mixed nociceptive–neuropathic pain (n = 12; 60%). Six patients received chemotherapy in the 3 months before implant (30%) and 5 underwent radiation therapy (25%; not included in cost analyses). Postimplant, 1 patient received chemotherapy (6.6%) and 4 received radiation treatment (26.7%). None received integrative analgesic therapies or pain management procedures. Fifteen patients had an IT port implanted (75%) and 5 had an IT pump implanted (25%). Median opioid consumption before ITDD therapy was 140.00 mg MEDD (IQR = 183.40–115.00) in the IT port group and 160.00 mg MEDD (IQR = 168.00–128.00) in the IT pump group. The IT pump group received intrathecal morphine alone, whereas all patients with an IT port received morphine and bupivacaine; no patients received other adjuvants such as clonidine or baclofen. The median survival time after ITDD implantation was 78 days (IQR = 121–54). Only 2 patients survived longer than 12 months after receiving ITDD, with both surviving over 2 years. No patient was readmitted because of an ITDD implant-related complication.
Table 1. Demographic data (n = 20).
Clinical Outcomes
Pain: The average pain intensity was reduced by 50% from 8/10 (IQR = 8.25–7.75) to 4/10 (IQR = 5.00–3.50) at 2-month follow-up. At 2 months (n = 15), 3 months (n = 8), 6 months (n = 4), and 12 months (n = 2), 93.3%, 87.5%, 75.0%, and 50.0% of patients experienced a clinically meaningful reduction in pain, defined as ≥2 points on the NRSCitation19 (). For repeated measures analyses, significant pain reductions were observed up to 4 months of follow-up (P = .02), with significant changes from pretreatment found at 1-month (P < .01), 2-month (P < .01), 3-month (P = .01), and 4-month (P = .02) time points.
Table 2. Clinical outcomes.
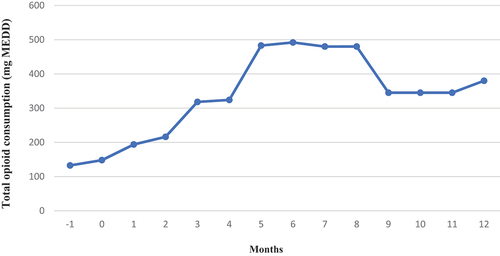
Opioid consumption: The opioids used in conventional therapy included morphine, fentanyl, methadone, oxycodone, and oxycodone/naloxone. In contrast, the opioid for ITDD therapy was exclusively morphine (). After ITDD therapy, the total opioid consumption increased over time, approximately 1.88% per month, despite decreases in non-IT opioid consumption ().
There was no difference in repeated measurement comparisons for total opioid consumption (P = .15) or PPS (P = .16).
Nonopioid analgesic consumption: 5/20 of patients were taking acetaminophen or nonsteroidal anti-inflammatory drugs pre-implant (25%), and 17/20 were receiving adjuvants (85%; membrane stabilizers, antidepressants). Postimplant, 13% (2/15), 12.5% (1/8), 25% (1/4), and 50% (1/2) were taking nonsteroidal anti-inflammatory drugs or acetaminophen at 2, 3, 6, and 12 months, respectively, with statistical significance for analgesic reduction occurring only at 3 months (P = .047). For adjuvants, the proportion or patients receiving therapy at these time points were 73% (11/15), 75% (6/8), 50% (2/4), and 50% (1/2), respectively. Overall, at 2 months, the total number of adjuvant prescriptions decreased from 85% pre-implant to 73%.
PPS: The median PPS slightly increased after ITDD therapy, as shown in .
Costs
The implantation cost of an IT port was lower than the cost of an IT pump, US$2587 (CA$3544.44) vs. US$15,015 (CA$20,570.88), which was similar to the pattern for maintenance costs (IT port, US$86 [CA$118]/day vs. IT pump, US$500 [CA$686]/day; ). The median frequency of IT medication refills was 1.5 times/month for patients with an IT port and once per month for patients with an IT pump. The IT pump group had lower daily medical costs after receiving ITDD therapy (reduction from US$6.50 [CA$8.91] to US$1.97 [CA$2.70]/day), whereas the cost slightly increased in the IT port group to US$7.43 (CA$10.18)/day.
Table 3. Lifetime costs of treatment: Conventional therapy vs. ITDD therapy (n = 15) after 2 months.
There were no significant differences in direct nonmedical costs before and after treatment, US$0.60 (CA$0.82)/day in the conventional therapy period vs. US$0.84 (CA$1.15)/day in the IT port group and US$0.31 (CA$0.42)/day in patients in the an IT pump group. The median frequency of OPD visits after ITDD therapy was 1.5 visits/month in the IT port group and 1 visit/month in the IT pump group.
There were no patient- or caregiver-reported productivity losses during therapy.
Cost Equivalence
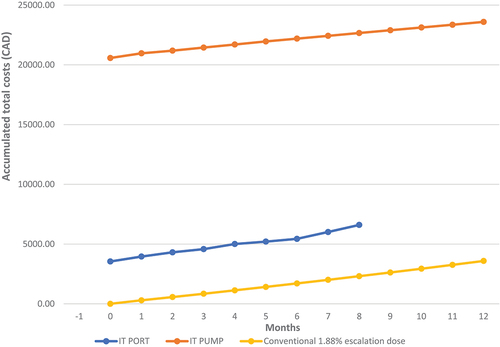
There was no approximated point of cost equivalence between ITDD therapy and conventional therapy within 12 months of follow-up ().
Figure 4. The accumulation of total costs.
Cost-Effectiveness Analysis
Because 60% of patients died after 2-month follow-up (n = 12), cost-effectiveness and cost–utility analyses were conducted using 2 months’ data for 15 patients (75% of the population). The utility score of conventional therapy assumed that the same patient without ITDD therapy would have the same NRS pain and PPS scores until death. The cost-effectiveness ratio (CER) of a 1-point pain score reduction in the IT port group was less than that in the IT pump group, US$1032.68 (CA$1414.77) vs. US$2739.63 (CA$3753.29) per 1-point NRS reduction; ). For a 2-point reduction (minimal clinically important difference), the CERs were US$2065.36 (CA$2829.54) in the IT port group and US$5479.26 (CA$7506.58) in the IT pump group. IT pump therapy was associated with increased PPS scores (i.e., improvement), but worsening PPS scores were observed in those receiving IT port therapy. The ICER of IT port therapy compared with conventional therapy was less than that for IT pump therapy for pain reduction, US$862.73 (CA$1181.94) vs. US$2635.68 (CA$3610.88)/1-point NRS reduction; US$1725.46 (CA$2363.88) vs. US$5271.36 (CA$7221.76)/ 2-point reduction. Because IT port therapy was associated with worsening PPS, an ICER could not be calculated for improvement; the ICER/1-point PPS reduction was US$316.62 (CA$433.77). For IT pump therapy, the ICER/1-point PPS increase was US$592.95 (CA$812.34).
Table 4. Cost-effectiveness and cost–utility analyses at 2 months after ITDD therapy.
Cost-Utility Analysis
Ten patients (nine who received an IT port and one who received an IT pump) had complete EQ-5D data. In the IT port group, the utility score of pre-ITDD therapy was extremely low (0.09) but slightly increased after ITDD therapy (0.23). The ICER was analyzed only in the IT port group. The ICER for IT port therapy compared with conventional therapy was US$259.19 (CA$355.09)/QALD gained and US$93,999.31 (CA$128,779.06)/QALY gained, which was 8.7 times above the cost-effectiveness threshold in Thailand and just below the US$104,000 (CA$142,480) cost-effectiveness threshold in the United States based on 2019 dollars.Citation20 For the single patient with quality of life data who received IT pump therapy, the utility score was higher after starting therapy (increase from 0.04 to 0.54). Compared with conventional therapy, the ICER of an IT pump was US$39.24 (CA$53.76)/QALD gained and US$14,269.95 (CA$19,544.83)/QALY gained, which was nearly three times higher than Thailand’s threshold of willingness to pay.
Complications from ITDD
ITDD implantation resulted in no major adverse events, reoperations, or readmissions among participants during the study.
Discussion
Our study investigated 10 years of experience with ITDD therapy in Thailand, which provided approximately 50% pain reduction with only modest changes in function and quality of life. The lifetime cost of ITDD therapy per patient was much higher than that for conventional therapy, even without device-related complications, which occur at a rate exceeding 30% in some studies,Citation21 13 times higher with IT port therapy and 70 times higher for IT pump therapy. Up to 90% of total costs were from equipment and implantation fees. However, the ICER per QALD and QALY gained from IT port therapy was higher than both the cost-effectiveness threshold and willingness to pay threshold of the Thai government.
Clinical Outcomes
Similar to previous studies,Citation5,Citation6,Citation8,Citation22–25 our study showed that ITDD therapy significantly decreased pain scores in patients with cancer, with 82.35% of patients experiencing clinically meaningful (≥2 points) improvement at 50% or more of their follow-ups until death or up to 12 months (14/17). However, our study also showed that patients receiving ITDD therapy experienced minimal change in PPS scores after initiation of therapy. Patients who received IT port therapy may have reported worse PPS scores because an external infusion system might pose an obstacle to movement, thereby limiting mobility.
Cost Comparisons between Countries
Our study found an enormous difference in total costs between ITDD and conventional therapy, which was predominantly due to the high upfront costs of ITDD implantation, including the device and facility fees. The cost of IT pump implantation was similar to the cost in Korea (US$14,900.00, CA$20,413.00) but lower than that in the United States (US$35,601.00, CA$48,773.37).Citation7,Citation11 In Canada, the budget impact of ITDD therapy in patients with cancer was estimated to be approximately US$100,000 in the first year and US$500,000 by the fifth year, which is more costly than routine conventional pain therapy.Citation10 There is limited data regarding IT port implantation costs in any country, with the only study from China reporting an implant cost of US$6,771 and a median postimplant daily analgesic cost of US$0.67 per day (CA$0.92/day).Citation6 The cost of conventional therapy in Thailand (US$6.48, CA$8.91/day) was lower compared with other countries, including the United States (US$21.26, CA$29.13/day)Citation7 and China (US$18.80, CA$25.76/day).Citation6 Although our study included patients receiving oxycodone and oxycodone/naloxone (eight to ten times more expensive than morphine in Thailand) during conventional therapy, the daily medication cost was still relatively low.
Cost Equivalents and Cost-Effectiveness Analysis
In a previous study, patients with high pre-ITDD opioid consumption (>1000 mg MEDD) reached a cost beneficial cutoff point faster than those with lower opioid consumption.Citation7 Qin et al. showed that an IT port with an external infusion system cost substantially less than an IT pump and was suitable for patients with cancer with very short life expectancies in whom an implanted IT pump would not be cost-effective.Citation6 Possible causes for the lack of cost equivalence demonstrated in this study included (1) a very high upfront IT device cost, (2) the low cost of conventional therapy, and (3) a short survival time. Contributors to the low cost of conventional therapy in Thailand, which may not be relevant in Canada, the United States and other industrialized countries, included (1) low medication costs (e.g., a generic 12 mcg/h fentanyl patch costs US$2.40, CA$3); (2) lower utilization of opioids due to limited availability and greater concerns about side effects for both providers and patients, and (3) less access to expensive medications due to limited government health care coverage. Opioid consumption pre-implantation was low (<200 mg MEDD) in this study, contributing to the low cost of conventional therapy. The survival time of patients in this study was shorter than that in previously published studies (median 78 days vs. 168 days).Citation7 Our study demonstrated a high ICER and cost-effectiveness ratio for ITDD therapy due to the high initial costs of the devices. Ironically, the clinical threshold required for many patients with cancer to be considered for IT therapy is not reached until the end of their lives, which decreases the likelihood that an implanted device will be cost-effective. Moreover, uncontrollable pain during the last stages of terminal cancer may include high affective-motivational and cognitive-evaluative components, which may not be as responsive to opioids as the sensory-discriminative component.Citation26 One potential strategy to optimize cost-effectiveness would be to calculate “the minimal market price” for a device for different contingencies, though predicting analgesic response and life expectancy in end-stage cancer can be challenging. A retrospective study that compared 376 patients with cancer treated with ITDD therapy to 4839 patients who received only conventional medical management demonstrated that ITDD therapy provided cost savings and lower health care utilization at 2 and 12 months, but not 6 months, postimplantation.Citation27 Herein, health care utilization increased after IT port implantation because patients needed to have IT port medications refilled every 1 to 2 weeks owing to the dynamic nature of terminal cancer pain.
Cost-Utility Analysis
The ICER/QALY gained from IT port therapy was 19 times higher than Thailand’s willingness-to-pay threshold, US$93,999.31 (CA$128,779.06) vs. US$4848.48 (CA$6400)/QALY gained]. In the United States, the willingness-to-pay threshold is estimated at US$50,000 to US$100,000/QALY gained.Citation28 To optimize the cost-effectiveness and cost–utility gained from ITDD therapy, early ITDD implantation could be considered before patients’ health status precipitously declines, though patients in earlier stages may not meet established criteria for ITDD (e.g., uncontrollable pain) and be nonterminal. This could leave them with a bulky, expensive implantable device that carries significant risks, including pump malfunction, pocket fills, and medication errors that can lead to overdose and withdrawal.Citation21 In Korea, the government provides 50% financial support for IT pumps in patients with cancer with uncontrollable pain and a life expectancy exceeding 1 year.Citation11 In contrast, in the United States, current guidelines recommend considering ITDD therapy in patients with refractory pain and a life expectancy greater than 6 months.Citation14,Citation29,Citation30 Considering the differences in disposable income and insurance coverage between Thailand and industrialized Western and East Asian countries, the cost of IT devices must be commensurately reduced to improve cost-effectiveness.
Generalizability
With widespread and immediate dissemination of knowledge worldwide, the concept of regional standards of care is becoming obsolete in principle, yet, in practice, cost impediments continue to pose barriers to equal access to care in the developing world. There are extremely few cost-effectiveness and cost–utility studies evaluating expensive devices and therapies (e.g., chemotherapy) in developing countries because the catch-22 of high costs and subsequent lack of availability prohibit performing these studies or enrolling high numbers of patients. Thailand is a regional hub for medical tourism (which exacerbates disparities in care), home to around 4 million foreigners, and its gross domestic product (ranked 26th worldwide) is similar to or better than most surrounding and even distant countries. This, along with the relatively fixed upfront costs for intrathecal drug delivery devices, makes the cost-effectiveness and cost–utility analyses in this study generalizable particularly to the Southeast Asia region. It should also spur industry to provide less expensive, more cost-efficient alternatives to intrathecal drug delivery because this will dramatically expand access to care worldwide.
Limitations
There are several limitations to this study that warrant attention. First, there were missing data that might have affected the results, including a lack of quality of life data in more than half of the patients and data used to calculate cost-effectiveness and cost–utility, especially in patients with an IT pump. To compare similar time frames for conventional therapy to the fixed time frames for ITDD, we also extrapolated existing data for the pre-implant phase (including costs, analgesic consumption, and pain and PPS scores), which may both overestimate (because time frames closer to implantation are likely to be associated be greater pain and disease burden than earlier in the disease course and after ITDD placement) and underestimate (because pain and disease burden tend to worsen with advancing cancer) costs and pain levels. Second, some direct nonmedical and indirect costs were estimated based on recall from patients’ relatives, which can be either underestimated or overestimated. Third, the sample size was small, and there is considerable variability in life expectancy in patients with cancer, both of which could influence cost-effectiveness and cost–utility analyses and render conclusions regarding effectiveness statistically “fragile.” However, these patients represent the entire sample of individuals who received ITDD therapy for cancer pain over a 10-year period at Thailand’s largest research university, with the high overhead costs in a developing country serving as a vicious circle to limit utilization. Fourth, our study was not designed to tease out or measure the different types of cancer pain (e.g., neuropathic vs. nonneuropathic, constant vs. breakthrough pain). The prevalence of breakthrough cancer pain ranges between 40% and 86% and may be subcategorized as incident pain (related to specific events), idiopathic (including affective-motivational pain from psychosocial stressors), and end-of-dose failure (which typically occurs with nonintrathecal delivery systems).Citation31 Whereas the intrathecal pumps implanted in this study have the capability of patient-controlled “flex dosing,” the onset of effect is unlikely to effectively treat breakthrough pain episodes, which typically resolve within 30 min,Citation32 and we did not query patients on their use of flex dosing. In several reviews, expensive transmucosal opioids such as fentanyl have been found to have the most supporting evidence for rapid onset and offset breakthrough pain.Citation33–35 If transmucosal opioid formulations had been readily available in Thailand, this could have further adversely affected cost–utility in patients with an IT pump with high expectations and ample means to secure the medications. Last, our study did not investigate the satisfaction of patients and their relatives regarding ITDD therapy, which is one of the essential goals in end-of-life palliative care.
Conclusions
In the past 10 years, ITDD therapy for cancer pain management in Thailand has expanded, albeit slowly. Although ITDD therapy can provide significant pain reduction (albeit with minimal changes in function and quality of life), the high upfront costs limit its cost-effectiveness and cost–utility, and consequently utilization, in developing countries. To optimize ITDD therapy, earlier implantation in well-selected patients based on evidence-based algorithms and actuarial tables and reducing device acquisition costs are solutions that warrant further investigation.
Authors’ Contributions
AT, NT, PE, PP, BL, and OP contributed to the conception and design of the study. AT, PE, and PP were involved in data acquisition. All authors analyzed and interpreted data, drafted the article, and critically revised the article for important intellectual content. Additionally, all authors approved the final version of the article and agreed to be accountable for all aspects of the work in ensuring that questions related to the integrity of any part of the work were appropriately investigated and resolved.
Acknowledgment
The authors gratefully acknowledge the assistance of Wareeya Vongspanich for statistical analysis.
Disclosure Statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- van den Beuken-van Everdingen MH, Hochstenbach LM, Joosten EA, Tjan-Heijnen VC, Janssen DJ. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(6):1070–1090.e9. doi:10.1016/j.jpainsymman.2015.12.340.
- Portenoy RK. Treatment of cancer pain. Lancet. 2011;377:2236–14. doi:10.1016/S0140-6736(11)60236-5.
- NHS England. Clinical commissioning policy: intrathecal pumps for treatment of severe cancer pain; 2015. https://www.england.nhs.uk/commissioning/wp-content/uploads/sites/12/2015/10/d08pb-intra-pumps-trtmnt.pdf.
- Bruel BM, Burton AW. Intrathecal therapy for cancer-related pain. Pain Med. 2016;17(12):2404–21. doi:10.1093/pm/pnw060.
- Smith TJ, Coyne PJ, Staats PS, Deer T, Stearns LJ, Rauck RL, Boortz-Marx RL, Buchser E, Catala E, Bryce DA, et al. An implantable drug delivery system (IDDS) for refractory cancer pain provides sustained pain control, less drug-related toxicity, and possibly better survival compared with comprehensive medical management (CMM). Ann Oncol. 2005;16(5):825–33. doi:10.1093/annonc/mdi156.
- Qin W, Li Y, Liu B, Liu Y, Zhang Y, Zhang X, Li P, Fan B. Intrathecal morphine infusion therapy via a percutaneous port for refractory cancer pain in China: an efficacy, safety and cost utilization analysis. J Pain Res. 2020;13:231–37. doi:10.2147/JPR.S233905.
- Brogan SE, Winter NB, Abiodun A, Safarpour R. A cost utilization analysis of intrathecal therapy for refractory cancer pain: identifying factors associated with cost benefit. Pain Med. 2013;14(4):478–86. doi:10.1111/pme.12060.
- Dupoiron D. Intrathecal therapy for pain in cancer patients. Curr Opin Support Palliat Care. 2019;13(2):75–80. doi:10.1097/SPC.0000000000000427.
- Kiehelä L, Hamunen K, Heiskanen T. Spinal analgesia for severe cancer pain: a retrospective analysis of 60 patients. Scand J Pain. 2017;16:140–45. doi:10.1016/j.sjpain.2017.04.073.
- Health Quality Ontario. Intrathecal drug delivery systems for cancer pain: a health technology assessment. Ont Health Technol Assess Ser. 2016;16(1):1–51.
- Kim EK, Shin JY, Castaneda AM, Lee SJ, Yoon HY, Kim YC, Moon JY. Retrospective analysis of the financial break-even point for intrathecal morphine pump use in Korea. Korean J Pain. 2017;30(4):272–80. doi:10.3344/kjp.2017.30.4.272.
- Bruera E, Schoeller T, Wenk R, MacEachern T, Marcelino S, Hanson J, Suarez-Almazor M. A prospective multicenter assessment of the Edmonton staging system for cancer pain. J Pain Symptom Manage. 1995;10(5):348–55. doi:10.1016/0885-3924(95)00052-Z.
- Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC clinical practice guideline for prescribing opioids for pain - United States, 2022. MMWR Recomm Rep. 2022 Nov 4;71(3):1–95. doi:10.15585/mmwr.rr7103a1.
- Deer TR, Pope JE, Hayek SM, Bux A, Buchser E, Eldabe S, De Andres JA, Erdek M, Patin D, Grider JS, et al. The Polyanalgesic Consensus Conference (PACC): recommendations on intrathecal drug infusion systems best practices and guidelines. Neuromodulation. 2017;20(2):96–132. doi:10.1111/ner.12538.
- Chewaskulyong B, Sapinun L, Downing GM, Intaratat P, Lesperance M, Leautrakul S, Somwangprasert A, Leerapun T. Reliability and validity of the Thai translation (Thai PPS Adult Suandok) of the Palliative Performance Scale (PPSv2). Palliat Med. 2012;26:1034–41. doi:10.1177/0269216311424633.
- Pattanaphesaj J, Thavorncharoensap M, Ramos-Goñi JM, Tongsiri S, Ingsrisawang L, Teerawattananon Y. The EQ-5D-5L valuation study in Thailand. Expert Rev Pharmacoecon Outcomes Res. 2018;18(5):551–58. doi:10.1080/14737167.2018.1494574.
- Cai D, Shi S, Jiang S, Si L, Wu J, Jiang Y. Estimation of the cost-effective threshold of a quality-adjusted life year in China based on the value of statistical life. Eur J Health Econ. 2022;23(4):607–15. doi:10.1007/s10198-021-01384-z.
- Tanvejsilp P, Taychakhoonavudh S, Chaikledkaew U, Chaiyakunapruk N, Ngorsuraches S. Revisiting roles of health technology assessment on drug policy in universal health coverage in Thailand: where are we? and what is next? Value Health Reg Issues. 2019;18:78–82. doi:10.1016/j.vhri.2018.11.004.
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, Haythornthewaite JA, Jensen MP, Kerns RD, Ader DN. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–21. doi:10.1016/j.jpain.2007.09.005.
- Vanness DJ, Lomas J, Ahn H. A health opportunity cost threshold for cost-fffectiveness analysis in the United States. Ann Intern Med. 2021;174(1):25–32. doi:10.7326/M20-1392.
- Necking E, Levi R, Ertzgaard P. Complications of intrathecal drug delivery therapy (ITDD): a retrospective study of 231 implantations between 1999 and 2014. Clin Neurol Neurosurg. 2021;205:106630. doi:10.1016/j.clineuro.2021.106630.
- Kim EJ, Moon JY, Kim YC, Park KS, Yoo YJ. Intrathecal morphine infusion therapy in management of chronic pain: present and future implementation in Korea. Yonsei Med J. 2016;57(2):475–81. doi:10.3349/ymj.2016.57.2.475.
- Kumar K, Hunter G, Demeria DD. Treatment of chronic pain by using intrathecal drug therapy compared with conventional pain therapies: a cost-effectiveness analysis. J Neurosurg. 2002;97(4):803–10. doi:10.3171/jns.2002.97.4.0803.
- Bhatia G, Lau ME, Koury KM, Gulur P. Intrathecal Drug Delivery (ITDD) systems for cancer pain. F1000Res. 2013;2:96. doi:10.12688/f1000research.2-96.v4.
- Deer TR, Pope JE, Hanes MC, McDowell GC. Intrathecal therapy for chronic pain: a review of morphine and ziconotide as firstline options. Pain Med. 2019;20(4):784–98. doi:10.1093/pm/pny132.
- Sela RA, Bruera E, Conner-spady B, Cumming C, Walker C. Sensory and affective dimensions of advanced cancer pain. Psychooncology. 2002;11(1):23–34. doi:10.1002/pon.551.
- Stearns LJ, Narang S, Albright RE Jr, Hammond K, Xia Y, Richter HB, Paramanandam GK, Haagensen KK, Doth AH. Assessment of health care utilization and cost of targeted drug delivery and conventional medical management vs conventional medical management alone for patients with cancer-related pain [published correction appears in JAMA Netw Open. 2019;2(5):e195248]. JAMA Netw Open. 2019;2(4):e191549. doi:10.1001/jamanetworkopen.2019.1549.
- Menzel PT. How should willingness-to-pay values of quality-adjusted life-years be updated and according to whom? AMA J Ethics. 2021;23(8):E601–E606. doi:10.1001/amajethics.2021.601.
- De Andres J, Rubio-Haro R, Andres-serrano CD, Asensio-Samper JM, Fabregat-Cid G. Intrathecal drug delivery. Methods Mol Biol. 2020;2059:75–108.
- Aman MM, Mahmoud A, Deer T, Sayed D, Hagedorn JM, Brogan SE, Singh V, Gulati A, Strand N, Weisbein J, et al. The American Society of Pain and Neuroscience (ASPN) best practices and guidelines for the interventional management of cancer-associated pain. J Pain Res. 2021;14:2139–64. doi:10.2147/JPR.S315585.
- Mishra S, Bhatnagar S, Chaudhary P, Rana SP. Breakthrough cancer pain: review of prevalence, characteristics and management. Indian J Palliat Care. 2009;15(1):14–18. doi:10.4103/0973-1075.53506.
- McCarberg BH. The treatment of breakthrough pain. Pain Med. 2007;8(Suppl 1):S8–13. doi:10.1111/j.1526-4637.2006.00270.x.
- Brant JM, Rodgers BB, Gallagher E, Sundaramurthi T. Breakthrough cancer pain: a systematic review of pharmacologic management. Clin J Oncol Nurs. 2017;21(3):71–80. doi:10.1188/17.CJON.S3.71-80. PMID: 28524907.
- Cascella M, Monaco F, Nocerino D, Chinè E, Carpenedo R, Picerno P, Migliaccio L, Armignacco A, Franceschini G, Coluccia S, et al. A bibliometric network analysis on rapid-onset opioids for breakthrough cancer pain treatment. J Pain Symptom Manage. 2022;63(6):1041–50. doi:10.1016/j.jpainsymman.2022.01.023. PMID:35151801.
- Mercadante S. Treating breakthrough pain in oncology. Expert Rev Anticancer Ther. 2018;18(5):445–49. doi:10.1080/14737140.2018.1443813. PMID:29478355.