ABSTRACT
Background
Chronic pain is a complex condition that poses challenges in assessment and treatment. Primary care teams, especially in rural areas, may have a role in managing this population, providing interprofessional care to optimize patient outcomes. Tools are needed to aid these clinicians in assessing chronic pain.
Aims
The aim of this article is to present the case application of a clinical reasoning framework proposed by Walton and Elliott, which is used to identify drivers of chronic pain in a 61-year-old male patient with a remote history of spinal injury. Furthermore, it aims to demonstrate that an interprofessional, individualized intervention strategy can improve patient outcomes.
Methods
This case took place in a multidisciplinary primary care team in rural northern Ontario, Canada. An assessment was completed by the author, including collection of the patient’s history, a medication review, and the use of multiple validated patient-reported outcome measures (PROMs), all of which were used in applying the framework.
Results
Three relevant drivers of his pain experience were identified: central nociplastic, cognitive/belief, and emotional/affective. A pharmacist and social worker then used multimodal interventions to address these drivers, which yielded improvements in scores on multiple validated pain measures but also improved the patient’s self-reported quality of life.
Conclusions
A clinical reasoning framework can provide a basis for identifying drivers of chronic pain during assessment and guide primary care clinicians to targeted interventions. Broader applications of this framework by primary care providers could serve to increase capacity for managing chronic pain in Canada.
RÉSUMÉ
Contexte: La douleur chronique est une affection complexe qui pose des problèmes d’évaluation et de traitement. Les équipes de soins primaires, en particulier dans les zones rurales, peuvent jouer un rôle dans la prise en charge de cette population et fournir des soins interprofessionnels pour optimiser les résultats pour les patients. Des outils sont nécessaires pour aider ces cliniciens dans l’évaluation de la douleur chronique.
Objectifs: Le but de cet article est de présenter l’application d’un cadre de raisonnement clinique proposé par Walton et Elliott, utilisé pour déterminer les facteurs de douleur chronique, à un patient masculin âgé de 61 ans ayant des antécédents lointains de lésion de la colonne vertébrale. En outre, il vise à démontrer qu’une stratégie d’intervention interprofessionnelle et individualisée peut améliorer les résultats pour les patients.
Méthodes: Ce cas a eu lieu dans une équipe de soins primaires multidisciplinaire en milieu rural dans le nord de l’Ontario, au Canada. Une évaluation a été réalisée par l’auteur, y compris la collecte des antécédents du patient, une revue des médicaments et l’utilisation de plusieurs mesures validées des résultats rapportés par les patients, qui ont tous été utilisés dans l’application du cadre.
Résultats: Trois facteurs pertinents de son expérience de la douleur ont été répertoriés: nociplastique centralisée, cognitif/croyance, et émotionnel/affectif. Un pharmacien et un travailleur social ont ensuite eu recours à des interventions multimodales pour traiter ces facteurs, ce qui a permis d’améliorer les scores sur plusieurs mesures de douleur validées, tout en améliorant la qualité de vie autodéclarée du patient.
Conclusions: Un cadre de raisonnement clinique peut fournir une base pour déterminer les facteurs de maladies de douleur chronique lors de l’évaluation et guider les cliniciens de soins primaires vers des interventions ciblées. Des applications plus larges de ce cadre par les prestataires de soins primaires pourraient servir à accroître la capacité de prise en charge de la douleur chronique au Canada.
Introduction
The prevalence of chronic pain among adults in Canada is estimated at nearly 20%.Citation1 With significant individual and societal costs, in the form of disability, health care utilization, and health care spending,Citation2 there is a need for effective ways to manage this complex condition. Recently, interprofessional collaboration using a biopsychosocial approach has been endorsed as one of the best strategiesCitation3; however, specialized chronic pain programs remain difficult for many patients to access, especially in rural and remote areas. To address this access issue, primary care teams have been highlighted for their potential role in chronic pain management,Citation4 yet research suggests that primary care providers report a lack of confidence in managing this population.Citation5
Given the complexity of treating chronic pain and the challenges that primary care providers face, tools that could enhance the process of interprofessional chronic pain assessment and management would be useful. Despite over a decade of clinical experience in primary care and involvement in numerous chronic pain cases over the years, the author has found that many of these cases are complex can be overwhelming to manage. This sentiment would resonate with many other primary care clinicians attempting to help these patients in a health care system where access to specialized pain management resources is limited. Walton and ElliottCitation6 proposed a clinical reasoning framework and radar plot to aid clinicians in identifying drivers of a patient’s pain. Their framework was attractive as a tool because the biopsychosocial model of pain formed a basis for its multidomain approach to pain assessment, it was easy to understand, and straightforward to implement.
To identify potential contributors to a patient’s pain experience, the framework suggests categorizing patient data into seven pain domains: nociceptive/physiological, peripheral neuropathic, central nociplastic, emotional/affective, cognitive/belief, socioenvironmental, and sensorimotor dysintegration. The authors of the framework note that these domains are not meant to be comprehensive in their description of a patient’s pain experience. Instead, they represent reasonable domains that include commonly accepted dimensions of the biopsychosocial model of pain, domains that would also be responsive to intervention. Data from multiple sources, which might include a patient’s narrative report of their pain experience, the results from objective pain measures, and information from their medical history, are categorized into one of the seven pain domains and a radar plot is generated by assigning a qualitative level of contribution (very low, low, moderate, high, and very high) to each domain. The level of contribution assigned is subjective according to the user, but Walton and Elliott applied the concept of triangulation in determining the level of contribution; with each additional data point for a given domain there comes a greater certainty of its contribution to the patient’s pain. The radar plot is a graphical representation of this process and can be particularly useful in scenarios where multiple pain domains are identified and prioritization is needed due to time or resource limitations.
The author, a pharmacist practicing in a rural multidisciplinary primary care team, presents this patient case involving the use of Walton and Elliott’s novel clinical reasoning framework to assess the drivers of a patient’s chronic pain.Citation6 Proposed as a means to support pattern recognition when assessing a variety of musculoskeletal pain conditions, this framework provides a method for identifying potential drivers of chronic pain that can subsequently guide interprofessional interventions. Furthermore, this case demonstrates how an individualized and interprofessional care plan, adopting a patient-centered strategy to target the most relevant chronic pain drivers, can be enhanced through the use of validated PROMs to improve the quality of life of a patient with a long-standing history of chronic pain.
Materials and Methods
This is a case study of a single patient seen in a rural primary care team in Elliot Lake, Ontario, Canada. It was compiled as a component of the author’s postprofessional graduate studies at Western University in London, Ontario, Canada; ethics approval was not required as per Western University’s policy on case reports. The patient has read this case report, approved its content, and given informed written consent for its publication.
The 61-year-old male patient was referred to the author for an opioid taper and chronic pain management. He presented with chronic low back pain, described as spasms and shooting, burning pain that radiated down his right leg. His primary concern was, “These pills are controlling my life.” He reported frustration with his dependence on opioids and persistent pain that was not particularly responsive to his current treatment plan, which consisted entirely of drug therapy. He also expressed difficulties performing his daily activities, problems sleeping, and decreased libido due to his persistent pain. The patient’s pain-related medications at the time of presentation were oxycodone extended release 20 mg twice daily, oxycodone/acetaminophen 5/325 mg three times daily, duloxetine 60 mg daily, pregabalin 150 mg three times daily, and baclofen 10 mg four times daily. Noted medication side effects included constipation and what he called “brain fog.”
The patient’s 20-year chronic pain history originated from a workplace accident, resulting in a crush injury to his lumbosacral spine that ultimately required surgery. Postoperatively, he remained in significant pain and was severely deconditioned, requiring months of physiotherapy while using opioids and other pain medications to regain his ability to walk. Due to persistent chronic pain despite a return to somewhat normal function, a spinal cord stimulator was implanted with some benefit, but he remained dependent on medication. Significant additional medical history included depression, obesity, coronary artery disease with myocardial infarction, migraines, and bariatric surgery.
The author’s assessment involved gathering the patient’s history and completing a medication review. The patient’s morphine equivalent dose (MED) was calculated at 82.5 mg of morphine daily, just below the maximum dose of 90 mg daily recommended by the Canadian “Guideline for opioid therapy and chronic noncancer pain.”Citation7 The Opioid Risk Tool was completed and the patient scored a 7, indicating moderate risk of opioid abuse.Citation8 The patient was also asked to complete several PROMs during this assessment,Citation9–15 the results of which were used as data in applying Walton and Elliott’s framework.Citation6 They included the Brief Pain Inventory Short Form (BPI-SF),Citation9 which measures pain severity and impact on function; the Brief Illness Perception Questionnaire (BIPQ),Citation10 which assesses the emotional and cognitive representations of illness; the Pain Catastrophizing Scale (PCS),Citation11 which assesses pain-related catastrophic thinking; and the Depression, Anxiety, and Stress Scale (DASS21),Citation12 which measures levels of depression, anxiety, and stress. Additional PROMs included the Central Sensitization Inventory (CSI),Citation13 which measures symptoms commonly associated with central nervous system hypersensitivity; the Self-Administered Leeds Assessment of Neuropathic Symptoms and Signs (S-LANSS),Citation14 which is used to identify neuropathic pain symptoms; and the Tampa Scale for Kinesiophobia (TSK),Citation15 which measures fear of movement and reinjury. Once they were completed by the patient, they were reviewed and scored by the author.
Results
Baseline scores on the completed PROMs with interpretations are presented in . These data, along with information from the patient’s narrative and medical history, were used by the author to generate the radar plot () by assigning a very low, low, moderate, high, or very high level of contribution for each of the seven pain domains. The central nociplastic, cognitive/belief, and emotional/affective drivers were deemed the most relevant in this patient’s case.
Figure 1. Radar plot generated for the patient using the clinical reasoning framework described by Walton and Elliott.Citation6
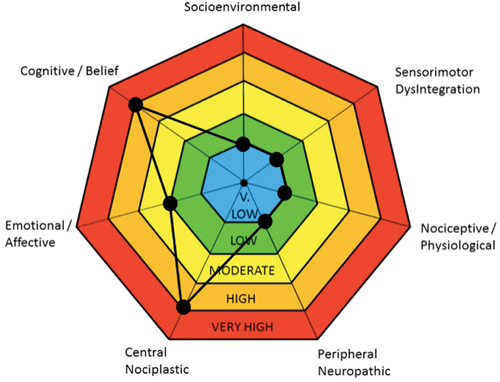
Table 1. Baseline PROM scores with interpretation.
The central nociplastic driver refers to central pain sensitization,Citation6 one of the most challenging components of chronic pain to treat. Items from the patient’s narrative and medical history that suggested central sensitization included his continued pain for over 20 years after his precipitating injury would have been expected to heal, pain that had been persistent and only partially responsive to surgical and pharmacological intervention, and his chronic use of high dose opioids, which has previously been implicated as a contributor to central sensitization and hyperalgesia.Citation16 From the PROMs collected, his severe CSI score of 56 was also highly suggestive of a central nociplastic driver; scores >40 indicate a likelihood of central sensitization. Based on these data, the author assigned a high/very high contribution to the central nociplastic driver.
The cognitive/belief driver refers to false and potentially irrational thoughts or beliefs about pain.Citation6 Previous research has demonstrated that addressing maladaptive cognitions in patients living with chronic pain through modalities such as cognitive behavioral therapy (CBT) can be beneficial.Citation17 During the initial interview, the patient noted an irrational fear that he would reinjure his back, even from engaging in his activities of daily living. This fear was further made evident when reviewing his TSK score of 51, which was high enough to indicate a clinically significant fear of movement (i.e., score >37). The patient’s baseline score on the BIPQ was also high at 60, indicative of an exaggerated perception of threat related to his chronic pain condition. Delving into individual BIPQ items, low scores on both the personal and treatment control items suggested that he had poor self-efficacy and little confidence that any intervention could provide him with pain relief. A high score on the identity item suggested that chronic pain had become a defining characteristic of his self-image. Furthermore, he reported rumination and magnification of his pain at night when the activities of the day were no longer present to distract him. This was also evident from his score of 39 on the PCS at baseline; a score >30 indicates clinically relevant catastrophizing. Based on these data, the author also assigned a high/very high contribution to the cognitive/belief driver.
The emotional/affective driver refers to “diagnosable psychopathology.”Citation6(p18) The patient had a long-standing diagnosis of depression since shortly after his spinal injury. The connection between depression and pain has been well documented, and both conditions can interact with each other in a bidirectional manner.Citation18 Though the patient only scored 16 (moderate) for depression and 8 (mild) for anxiety on the DASS21 at baseline, his stress score was 26 (severe). At the initial interview, this stress was largely driven by fear of pain and reinjuring himself, as well as a lack of perceived control over the management of his chronic pain. Despite his history of depression and obvious state of distress, the patient also exhibited a positive attitude and remained open-minded and motivated about attempting whatever interventions the team felt might benefit him. Because of the presence of these potential protective factors, a low/moderate contribution was assigned to the emotional/affective driver relative to the other identified pain drivers.
The other pain domains proposed by Walton and Elliott were deemed less relevant in this case and assigned a very low/low level of contribution. The patient’s pain presentation was consistent with the pattern he had been experiencing since his injury and he denied any new onset of acute pain symptoms, suggesting that a nociceptive/physiological driver was unlikely, especially given how remote his precipitating injury was. The author acknowledges that there may have been a nociceptive component to his pain due to years of deconditioning and sedentary lifestyle, which may have been beyond the skills of the author to assess. The care plan had initially included consulting a physiotherapist for input on this domain, but due to challenges in accessing physiotherapy services in the area and the fact that the cost would be prohibitive to the patient, this was deferred. The patient also reported having a supportive relationship with his wife, engaging in meaningful time spent volunteering at his church, and a stable financial situation. All of this suggested negligible contribution from a socioenvironmental driver.
Though the patient’s score of 19 on the S-LANSS suggested a neuropathic component to his pain (i.e., score >12), the patient denied any peripheral symptoms such as burning, tingling, or numbness in his extremities. The S-LANSS does not differentiate between central and peripheral neuropathy; any neuropathic symptoms the patient reported appeared to originate from his spinal injury. For this reason, the peripheral neuropathy domain was not deemed significant. Lastly, sensorimotor dysintegration also did not appear to be relevant because the patient denied any symptoms suggestive of deficits in sensory processing.
As a pharmacist, the author felt uniquely positioned to help the patient attempt an opioid taper, which was the patient’s goal, but also in the hope of addressing his central nociplastic driver. Chronic opioid use, particularly at higher doses, has been implicated in nociplastic changes in the central nervous system that result in pain sensitization, a condition called opioid-induced hyperalgesia.Citation16 Reducing the opioid dose or tapering off completely is the primary means of addressing this central pain sensitization. Interventions began after the patient was given educational materials on pain and opioid tapering. The taper proceeded by decreasing his opioid dose by roughly 10% of his initial MED monthly. The option for temporary pauses in the taper was outlined for the patient at the initial assessment; patients’ ability to have input into the progress of their taper has been identified as critically important in previous literature.Citation19 At the time of writing this case report, the patient had successfully tapered off opioids completely and maintained his opioid-free status for a period of 6 months.
Additional pharmacological interventions included titration of his duloxetine dose to 90 mg daily to facilitate his coping during the taper and to improve his mood, sleep, and neuropathic pain. The use of serotonin-norepinephrine reuptake inhibitors has been suggested in the management of comorbid pain and depressionCitation18 and is considered first line in the management of chronic neuropathic pain.Citation20 Though there is some evidence that increasing duloxetine doses beyond 60 mg daily is not beneficial,Citation21 the author has found that sometimes it is worth conducting an “n-of-1” trial because some patients may respond to a higher dose. From a medication management perspective, this approach was preferable to titrating his pregabalin because he had previously been intolerant to a pregabalin dose increase and adding a tricyclic antidepressant would only further contribute to polypharmacy. The patient placed significant value on minimizing his dependence on medication.
To address the identified cognitive/belief and emotional/affective drivers, the author felt it important to enlist the team’s social worker. Collaboration was necessary because addressing the patient’s maladaptive cognitions around pain and his emotional distress was beyond a pharmacist’s skill to manage alone. The social worker and the patient initially met twice monthly to focus on behavior activation and coping strategies. The patient was taught relaxation methods, meditation, stress management techniques, mindfulness, and calm breathing. He also began to increase his socialization by setting goals he strived to achieve. CBT was employed to help the patient address maladaptive pain-related thoughts and anxiety. For example, early on the patient noted that he was not using his cane to ambulate, stating that he viewed it as a “weakness.” The social worker worked on reframing this thought with him, suggesting that he view his cane as an assistive device just like glasses are to someone with poor vision. Restructuring this thought helped the patient utilize his cane more; this, in turn, gave him more confidence to move where otherwise he would have avoided activity out of fear. Throughout his sessions, a proactive approach was stressed, encouraging him to practice the strategies he had learned on a routine basis rather than waiting for his symptoms to escalate. The patient was seen for ten sessions that slowly tapered to monthly visits.
The patient was reassessed on the completed PROMs at roughly 20 weeks (for the BPI-SF, BIPQ, and DASS21) and 30 weeks (for the PCS, CSI, and TSK) after his baseline assessment. The S-LANSS was not repeated because his pain was already identified as being primarily of neuropathic origin and this was not expected to change. The postintervention scores, changes from baseline, and interpretations for the six PROMs are summarized in . It is important to note that though there was an improvement in raw scores across these PROMs, caution must be used in interpreting the clinical significance of these changes. The minimal clinically important difference (MCID), defined as “the smallest improvement considered worthwhile by a patient,”Citation22 is often cited in the literature when attempting to quantify meaningful changes in outcome measures, but the MCID for many measures is unknown. The known MCIDs for the PROMs used in this case are also presented for reference in .
Table 2. Postintervention PROM scores with interpretation.
At week 20, when the patient had decreased his MED by nearly a third, there were significant decreases in his BPI-SF scores; mean pain severity decreased by 38% and mean pain interference decreased by 36%. These decreases exceeded a threshold of 30%, which has been previously reported by Williams and Arnold as the MCID for the BPI-SF.Citation23 The patient indicated a twofold improvement (from 30% to 60%) on the BPI-SF item regarding the effectiveness of pain interventions, and this was despite a reduction in his opioid use. On the DASS21, there were decreases in depression (from 16 to 6) and stress (from 26 to 12) scores, both now normal. It was noted that there was a slight increase (from 8 to 10) in his anxiety score, although it is difficult to say how clinically meaningful this was. His score on the BIPQ also decreased markedly (from 60 to 34), suggesting that he was perceiving his chronic pain condition as much less threatening.
At week 30, the patient had further progressed in his opioid taper, decreasing his MED by 45% overall. His scores on the PCS, CSI, and TSK all improved from baseline. His PCS score dropped from 39 to 8, suggesting a decrease in pain catastrophizing. For patients treated with a spinal cord stimulator, Sabourin et al. have reported an MCID for the PCS ranging from 1.9 to 13.6.Citation24 Even accepting the high end of this range, the patient’s change in PCS score could be considered clinically significant (31-point decrease). His CSI score also dropped from 56 (severe) to 33 (mild), suggesting an overall decrease in central sensitization. Lastly, his TSK score decreased from 51 to 36, below the cutoff for kinesiophobia and suggesting a decline in his fear of movement.
Overall, the patient tolerated the interventions well and he endorsed a congruence between these results and his own perception of changes in his condition. Though he still had pain, he reported it was no longer the dominant theme in his life; he had new motivation to engage socially, better ability to cope with his pain, and saw improvements in his mood, sleep, and personal relationships. Other than some mild and transient withdrawal symptoms (e.g., shakiness, chills, restlessness) for several days after an opioid dose reduction, his taper proceeded without any significant issues. The patient reported increased pain at certain points in the taper, but this was mitigated through his coping strategies, pauses in the taper, and adjustments to his other medication.
Discussion
This patient had a prolonged history of chronic pain secondary to a workplace spinal injury and had explored a wide variety of chronic pain interventions since that time: surgical, pharmacological, and self-management. Despite this, he still reported poor quality of life and persistent pain that was interfering in his daily activities, and he was deeply concerned about physical and psychological dependence on opioids. The subjective nature of pain and the complex interacting factors that contribute to it would be overwhelming for many clinicians seeking to help him. Walton and Elliott’sCitation6 novel approach to pain phenotyping was useful in identifying this patient’s unique combination of pain drivers and guiding an interprofessional, collaborative care plan aimed at addressing them. The use of PROMs not only added useful information to the assessment of his pain drivers but also served to provide an objective measure of improvements in his condition over time. This case also demonstrates that this process is feasible when conducted by interdisciplinary health professionals working in a primary care team.
The author and the social worker share this case report in the hope that other clinicians, particularly those in primary care who may be struggling with managing this population, might find it useful. The author acknowledges that Walton and Elliott’s framework is not a validated assessment tool but suggests that there is value in using it to help describe a patient’s pain experience from a biopsychosocial perspective. Patients with a long history of chronic pain tend to be exceedingly complex, with extensive medical and social histories as well as a variety of potentially interacting factors that contribute to their pain. A degree of clinical inertia can set in as clinicians become overwhelmed trying to manage these patients, particularly in primary care where time, resources, and pain management expertise may be limited. In the author’s experience, this results in care plans that are often one-dimensional (e.g., focused solely on medication management) or that are targeted at the wrong pain driver (e.g., pushing counseling on a patient with no evidence of cognitive or emotional distress). Walton and Elliott suggested that their framework supports pain assessment through pattern recognition, helping clinicians eliminate the “white noise” and home in on the most relevant pain drivers for each individual patient. From a primary care clinician’s perspective, the author found this approach exceedingly helpful in this case and in other subsequent chronic pain cases.
Interprofessional collaboration was a key component of this case, and research suggests that it is a prerequisite for achieving optimal patient outcomes in chronic pain.Citation3 The Canadian Interprofessional Health Collaborative’s interprofessional competency framework describes six competency domains for interprofessional collaboration including interprofessional communication, patient-centered care, role clarification, team functioning, collaborative leadership, and interprofessional conflict resolutionCitation25; many of these competencies were demonstrated in this case.
For example, role clarification was evident from the beginning when the author recognized the patient’s emotional and cognitive distress, acknowledged that he did not possess the expertise to help the patient manage it, and recommended a referral to the social worker. A team-based approach was explained to the patient, highlighting the author’s role in helping him manage his opioid taper and the social worker’s role in helping him manage his mental health. The patient’s role as a decision maker and participant in his own care was also highlighted. Through sharing of their patient notes within the electronic medical record and informal case conferences to discuss the patient’s progress and challenges, the author and the social worker engaged in interprofessional communication. Collaborative leadership was evident when each clinician took the lead on matters involving their expertise (e.g., the pharmacist guiding the care plan when it came to medications and the social worker doing the same in matters of mental health). The patient also served in a leadership role on matters of his own pain experience, advising both clinicians on how he responded to the various interventions and how the process influenced his quality of life. Lastly, patient-centered care was embraced by both clinicians, who acted as guides for the patient rather than directors of his care. For example, when the patient struggled with his opioid taper, the author was open to pausing until the patient stated he was ready to proceed. Similarly, when the patient did not find success with a progressive muscle relaxation technique, the social worker supported him in moving on to try other modalities that he found more helpful.
The author would not have been able to help the patient achieve the reported outcomes without the social worker’s aid. It would be negligent, however, not to highlight the most important collaborator of all: the patient himself. Without a doubt, his progress would not have been as significant without his commitment to the process. Much of his success can be attributed to innate characteristics of the patient himself, but he did highlight the importance of having a trusting, nonjudgmental relationship with both providers. In the author’s and the social worker’s opinion, this is another critical piece in successfully helping patients living with chronic pain.
As with all case reports, there are limitations in terms of the generalizability of these findings to the broader population of patients living with chronic pain. The value of this case report, however, lies not in the specific methods of intervention but in highlighting the utility of a thorough, individualized chronic pain assessment aimed at identifying the highest priority drivers of pain. Once clinicians better understand what is driving a patient’s pain, appropriate interventions can be selected to target them. In this case, only two clinicians were involved; the author hypothesizes that including a more diverse complement of health care providers from different disciplines would yield even more robust assessments of each domain. It would be interesting to see whether future applications of Walton and Elliott’s model, using input from more providers, could produce more accurate or nuanced assessments of these domains and whether that might also translate to even better patient outcomes. Even with this limitation, the patient in this case had improved scores on multiple validated PROMs and reported improved quality of life.
Walton and Elliott noted that their approach may be too “reductionistic,”Citation6(p19) ignoring the myriad potential interactions that may exist between these seven pain domains. How does the presence of central sensitization influence cognitive and emotional factors and vice versa? When targeting interventions at multiple domains simultaneously, it becomes impossible to tease out the contribution of each individual intervention. For example, was this patient successful in his opioid taper because he was concurrently developing coping strategies with the social worker, or did “lifting the fog” from his opioid use allow him to engage more meaningfully in his counseling sessions to address his cognitive/emotional distress? The truth is likely a bit of both. In this case, multiple drivers were targeted simultaneously. Did this influence the outcome? If the interventions had been carried out sequentially, would the patient have fared better, worse, or the same? In clinical practice, particularly in primary care, prioritization will be based on patient and clinician goals, the perceived weight of contribution from each pain driver, and access to intervention resources. In addition to investigating further applications of this framework in primary care and beyond, all of these questions serve as interesting potential topics of future study as we continue to search for effective ways to manage chronic pain.
This case represents the first practical application of Walton and Elliott’sCitation6 clinical reasoning framework in the literature, applied to the case of a patient with long-standing chronic pain. It highlights the potential for improved patient outcomes when an individualized chronic pain management intervention, based on the identified pain drivers, is implemented by collaborative health care professionals in a primary care setting. With specialized pain clinics out of reach for many patients due to geography and long wait times, leveraging the accessibility of primary care clinicians may be one solution to addressing the chronic pain problem in Canada.Citation1,Citation2
The complexity of chronic pain and the subjective nature of each patient’s pain experience suggests that the identification of generalizable interventions that can be applied to the majority of patients living with chronic pain may not be reasonable or achievable. Health care providers do these patients a disservice by attempting to manage their chronic pain with a formulaic, one-size-fits-all approach. This framework provides an opportunity for primary care providers to engage in identifying biopsychosocial contributors to patients’ chronic pain, focusing on interventions aimed at addressing the unique combination of pain drivers for each individual patient.
Informed Consent
The patient has read this case report, is satisfied with the content, and has provided informed written consent for publication.
Acknowledgments
The author thanks C. Hull, MSW, for her contribution to the care of this patient, for providing input on the description of her interventions, and for reviewing the case report for accuracy.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Schopflocher D, Taenzer P, Jovey R. The prevalence of chronic pain in Canada. Pain Res Manage. 2011;16(6):445–9. doi:10.1155/2011/876306.
- Henry JL. The need for knowledge translation in chronic pain. Pain Res Manage. 2008;13(6):465–76. doi:10.1155/2008/321510.
- Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. 2021;397(10289):2082–97. doi:10.1016/S0140-6736(21)00393-7.
- Hassan S, Carlin L, Zhao J, Taenzer P, Furlan AD. Promoting an interprofessional approach to chronic pain management in primary care using project ECHO. J Interprof Care. 2021;35(3):464–67. doi:10.1080/13561820.2020.1733502.
- Pearson AC, Moman RN, Moeschler SM, Eldrige JS, Hooten WM. Provider confidence in opioid prescribing and chronic pain management: results of the opioid therapy provider survey. J Pain Res. 2017;10:1395–400. doi:10.2147/JPR.S136478.
- Walton DM, Elliott JM. A new clinical model for facilitating the development of pattern recognition skills in clinical pain assessment. Musculoskelet Sci Pract. 2018;36:17–24. doi:10.1016/j.msksp.2018.03.006.
- Busse JW, Craigie S, Juurlink DN, Buckley DN, Wang L, Couban RJ, Agoritsas T, Akl EA, Carrasco-Labra A, Cooper L, et al. Guideline for opioid therapy and chronic noncancer pain. CMAJ. 2017;189(18):E659–E666. doi:10.1503/cmaj.170363.
- Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the opioid risk tool. Pain Med. 2005;6(6):432–42. doi:10.1111/j.1526-4637.2005.00072.x.
- Cleeland CS, Ryan KM. Pain assessment: global use of the brief pain inventory. Ann Acad Med Singapore. 1994;23:129–38.
- Broadbent E, Petrie KJ, Main J, Weinman J. The brief illness perception questionnaire. J Psychosom Res. 2006;60(6):631–37. doi:10.1016/j.jpsychores.2005.10.020.
- Sullivan MJL, Bishop SR, Pivik J. The pain catastrophizing scale: development and validation. Psychol Assess. 1995;7(4):524–32. doi:10.1037/1040-3590.7.4.524.
- Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the beck depression and anxiety inventories. Behav Res Ther. 1995;33(3):335–43. doi:10.1016/0005-7967(94)00075-U.
- Mayer TG, Neblett R, Cohen H, Howard KJ, Choi YH, Williams MJ, Perez Y, Gatchel RJ. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012;12(4):276–85. doi:10.1111/j.1533-2500.2011.00493.x.
- Bennett MI, Smith BH, Torrance N, Potter J. The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J Pain. 2005;6(3):149–58. doi:10.1016/j.jpain.2004.11.007.
- Miller RP, Kori SH, Todd DD. The Tampa scale: a measure of kinisophobia. Clin J Pain. 1991;7(1):51–52. doi:10.1097/00002508-199103000-00053.
- Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145–61. doi:10.36076/ppj.2011/14/145.
- Knoerl R, Lavoie Smith EM, Weisberg J. Chronic pain and cognitive behavioral therapy: an integrative review. West J Nurs Res. 2016;38(5):596–628. doi:10.1177/0193945915615869.
- IsHak WW, Wen RY, Naghdechi L, Vanle B, Dang J, Knosp M, Dascal J, Marcia L, Gohar Y, Eskander L, et al. Pain and depression: a systematic review. Harv Rev Psychiatry. 2018;26(6):352–63. doi:10.1097/HRP.0000000000000198.
- Henry SG, Paterniti DA, Feng B, Iosif A-M, Kravitz RL, Weinberg G, Cowan P, Verba S. Patients’ experience with opioid tapering: a conceptual model with recommendations for clinicians. J Pain. 2019;20(2):181–91. doi:10.1016/j.jpain.2018.09.001.
- Moulin D, Boulanger A, Clark AJ, Clarke H, Dao T, Finley GA, Furlan A, Gilron I, Gordon A, Morley-Forster PK, et al. Pharmacological management of chronic neuropathic pain: revised consensus statement from the Canadian Pain Society. Pain Res Manage. 2014;19(6):328–35. doi:10.1155/2014/754693.
- Wright A, Luedtke KE, Vandenberg C. Duloxetine in the treatment of chronic pain due to fibromyalgia and diabetic neuropathy. J Pain Res. 2010;4:1–10. doi:10.2147/JPR.S12866.
- Copay AG, Subach BR, Glassman SD, Polly DW Jr, Schuler TC. Understanding the minimum clinically important difference: a review of concepts and methods. Spine J. 2007;7(5):541–46. doi:10.1016/j.spinee.2007.01.008.
- Williams DA, Arnold LM. Measures of fibromyalgia: Fibromyalgia Impact Questionnaire (FIQ), Brief Pain Inventory (BPI), Multidimensional Fatigue Inventory (MFI-20), Medical Outcomes Study (MOS) sleep scale, and Multiple Ability Self-Report Questionnaire (MASQ). Arthritis Care Res (Hoboken). 2011;63(Suppl 11(0 11)):S86–S97. doi:10.1002/acr.20531.
- Sabourin S, Tram J, Sheldon BL, Pilitsis JG. Defining minimal clinically important differences in pain and disability outcomes of patients with chronic pain treated with spinal cord stimulation. J Neurosurg Spine. 2021;1–8. doi:10.3171/2020.11.SPINE201431.
- Canadian Interprofessional Health Collaborative. A national interprofessional competency framework 2010. https://drive.google.com/file/d/1Des_mznc7Rr8stsEhHxl8XMjgiYWzRIn/view.
