ABSTRACT
Background
Post-viral pain syndrome, also known as post-viral syndrome, is a complex condition characterized by persistent pain, fatigue, musculoskeletal pain, neuropathic pain, neurocognitive difficulties, and sleep disturbances that can occur after an individual has recovered from a viral infection.
Aims
This narrative review provides a summary of the sequelae of post-viral syndromes, viral agents that cause it, and the pathophysiology, treatment, and future considerations for research and targeted therapies.
Methods
Medline, PubMed, and Embase databases were used to search for studies on viruses associated with post-viral syndrome.
Conclusion
Much remains unknown regarding the pathophysiology of post-viral syndromes, and few studies have provided a comprehensive summary of the condition, agents that cause it, and successful treatment modalities. With the COVID-19 pandemic continuing to affect millions of people worldwide, the need for an understanding of the etiology of post-viral illness and how to help individuals cope with the sequalae is paramount.
RÉSUMÉ
Contexte: Le syndrome de la douleur post-virale, également connu sous le nom de syndrome post-viral, est une affection complexe caractérisée par des douleurs persistantes, de la fatigue, des douleurs musculosquelettiques, des douleurs neuropathiques, des difficultés neurocognitives et des troubles du sommeil qui peuvent survenir après la guérison d’une infection virale.
Objectifs: Cette revue narrative présente un résumé des séquelles des syndromes post-viraux, des agents viraux qui les causent, ainsi que de la pathophysiologie, des traitements et des considérations futures pour la recherche et les traitements ciblés.
Méthodes utilisées: Les bases de données Medline, PubMed et Embase ont été utilisées pour rechercher des études sur les virus associés au syndrome post-viral.
Conclusion: La physiopathologie des syndromes post-viraux reste largement méconnue et peu d’études ont présenté un résumé complet de l’affection, des agents qui la provoquent et des modalités de traitement efficaces. Alors que la pandémie de COVID-19 continue d’affecter des millions de personnes dans le monde, il est primordial de comprendre l’étiologie de la maladie post-virale et de savoir comment aider les individus à faire face aux séquelles.
KEYWORDS:
Introduction
ost-viral pain syndrome, also known as post-viral syndrome (PVS), is a complex condition characterized by persistent pain, fatigue, musculoskeletal pain, neuropathic pain, neurocognitive difficulties, and sleep disturbancesCitation1,Citation2 that can occur after an individual has recovered from a viral infection. The condition can last for weeks, months, or even years and can significantly impact the individual’s quality of life. Cases of PVS have been documented after influenza B,Citation3 Ebola,Citation4 Chikungunya,Citation5 Dengue,Citation6 and many other infections; however, much remains unknown regarding the pathophysiology of PVSs, and few studies have provided a comprehensive summary of the condition, agents that cause it, and successful treatment modalities.
With the COVID-19 pandemic continuing to affect millions of people worldwide, the need for an understanding of the etiology of post-viral illness and how to help individuals cope with the sequalae is paramount.Citation2 A significant number of patients who were infected by SARS-CoV-2 continued to experience symptoms long after the acute phase of their disease.Citation7 Providing care for patients suffering from a PVS will place a significant burden on already-strained health care systems globally.Citation2,Citation8 For example, in Latvia, COVID-19 is predicted to increase the economic burden of chronic fatigue syndrome (CFS) by at least 15%.Citation9 To adequately cope with the numbers of patients who will present with post-COVID-19 syndromes, health care practitioners must familiarize themselves with the causes of PVS and how it can be effectively diagnosed and managed. This narrative review will provide a summary of the sequelae of PVSs, viral agents that cause it, and the pathophysiology and treatment.
Post-Viral Pain
Viral Agents Associated with Post-Viral Pain
Pain is one of the most common symptoms that can persist after a viral infection has been cleared. The pain can be either musculoskeletal in origin (e.g., myalgia, arthralgia)Citation5,Citation6,Citation10 or neuropathic (e.g., myelitis, allodynia, postherpetic neuralgia).Citation11,Citation12 In some cases, pain begins during the acute phase of the viral infection and continues into the postinfectious phase.Citation11 There are other cases in which new-onset pain develops weeks to months after recovery from infection.Citation6,Citation13 The presentation of the pain varies vastly depending on the viral agent that caused the infection ().
Table 1. Summary of viral agents associated with post-viral syndrome.
Chikungunya virus (CHIKV) is a single-stranded RNA virus that is mosquito-transmitted and was first isolated in Tanzania in 1952.Citation5,Citation14 Since then, there have been several outbreaks in Africa, Asia, Europe, and the Americas.Citation15 CHIKV causes chikungunya fever. The acute stage of the disease typically spans 21 daysCitation16 and consists of the triad of fever, rash, and arthralgias. After the acute stage, the articular symptoms either resolve on their own or persist for weeks to years. Patients may develop chronic periarticular pain or arthritis that mimics rheumatoid arthritis or spondyloarthritis.Citation17 It is estimated that approximately 52% of patients infected with CHIKV experience chronic articular pain.Citation5 Although less common, neuropathic pain can also develop after CHIKV infection. A Brazilian large-scale prospective study reported that 22% of patients had myelitis after infection,Citation18 which sometimes leads to central neuropathic pain at or below the level of the spinal cord lesion.Citation11
Varicella zoster virus (VZV) is an alpha herpesvirus that infects children and causes chickenpox, which presents as pruritic, fluid-filled vesicles on the skin.Citation19 After chickenpox resolves, VZV becomes latent in the dorsal root ganglia, cranial nerve ganglia, and autonomic ganglia.Citation11 As cell-mediated immunity declines with age, VZV may reactivate and lead to the development of herpes zoster (commonly known as shingles), a rash with a dermatomal distribution.Citation20 Approximately 10% of patients with herpes zoster experience postherpetic neuralgia (PHN) within a year of rash appearance.Citation21 PHN is the most well-known example of post-viral neuropathic pain. It is typically described as a burning sensation and allodynia in the same dermatomal distribution as the rash.Citation11
Human immunodeficiency virus (HIV) is the causative agent of acquired immunodeficiency syndrome.Citation22 HIV is most frequently transmitted sexually but can also be transmitted through injection drug use, via blood transfusions, and vertically from mother to child.Citation23 The virus progressively weakens the immune system by attacking CD4+ T cells, making patients more susceptible to opportunistic infections.Citation24 HIV is also able to attack the peripheral and central nervous system (CNS), causing sensory polyneuropathies.Citation11,Citation25 Some patients with HIV report allodynia and burning distally in the lower limbs,Citation26 and up to 50% of patients with HIV experience chronic pain in their lifetime.Citation11
Poliomyelitis is caused by poliovirus and was one of the most acutely debilitating viral infections in the 20th century.Citation27 The disease affected millions worldwide in the 1940s and 1950s, but due to widespread vaccination since the mid-1950s, polio is estimated to be 99% eradicated today, though it is still prevalent in some African, Asian, and South American countries.Citation11,Citation27 Permanent paralysis occurs in 1 of 200 infections,Citation11 and 30% to 80% of patients experience post-polio syndrome (PPS).Citation28 PPS presents with muscle weakness, fatigue, myalgia, arthralgia, neuropathic pain, and functional decline 10 to 15 years after poliovirus infection.Citation29 Myalgia and arthralgia have been found to be the most common and bothersome symptoms of PPS.Citation30
Severe acute respiratory syndrome (SARS) first emerged from Southeast Asia in 2003 and was caused by a novel SARS-associated coronavirus.Citation31 Acutely ill patients presented with fever, nonproductive cough, myalgia, and dyspnea.Citation32 After recovering from the infection, a proportion of patients experienced persisting symptoms, namely, musculoskeletal pain, fatigue, shortness of breath, and sleep issues.Citation31 The 2020 COVID-19 pandemic is caused by a coronavirus (SARS-CoV-2), and experts hypothesized that COVID-19 could lead to a PVS similar to that experienced after SARS.Citation2 Those predictions were correct; post-COVID syndrome, also known as “long COVID,” has been reported in a significant subset of resolved cases.Citation33 In addition to chronic fatigue and insomnia, patients experienced muscle pain, joint pain, chest pain, and headaches.Citation34 Neuropathic pain can also persist in the post-viral stage due to the virus’s effects on the peripheral and central nervous systems.Citation11 COVID-19 infection increases risk for stroke and development of Guillain-Barre syndrome, which can lead to post-stroke painCitation35 and chronic neuropathic pain, respectively.Citation11
Ebolavirus outbreaks have been occurring in central Africa since 1976,Citation4 with the largest outbreak taking place from 2013 to 2016.Citation10 Ebola virus disease has a mortality rate that ranges from 25% to 90% depending on the species.Citation36 Despite the high mortality rate, there are many survivors (>17,000 from the 2013–2016 outbreak alone), a significant proportion of whom have experienced long-term symptoms. Myalgias, arthralgias, and headache are among the most frequent symptoms experienced by patients in the postinfectious stage.Citation4,Citation10 An observational cohort study found that out of 802 patients, 38% had long-term musculoskeletal pain and 35% had long-term headaches.Citation4
Post-viral pain has also been reported in dengueCitation6,Citation13 and influenza BCitation3 cases. There is a report on a rare case of longitudinally extensive transverse myelitis in a 15-year-old male following dengue fever.Citation6 The patient presented with intense, debilitating pain in the lumbar region. Another case report on a patient with dengue discussed a 14-year-old female who presented with right-sided hip and buttock pain from sacroiliitis that began 10 days after developing dengue fever.Citation13 Influenza B was linked to multifocal neuropathy and local myositis in a case report of a 47-year-old male who presented with aching and dysesthesia in the right arm and left leg weeks after influenza B infection.Citation3
Pathophysiology of Post-Viral Pain
The pathogenesis of post-viral pain is poorly understood, and laboratory studies investigating molecular pathways that cause the phenomenon are scarce. The causes of chronic pain after CHIKVCitation15 and herpes zosterCitation37–39 have been the most researched of the conditions discussed herein, although the mechanisms remain undefined in CHIKV. There are fewer studies on pathogenesis for other diseases, such as Ebola virus disease,Citation40 COVID-19,Citation41–43 and West Nile virusCitation44; however, some researchers have postulated various theories.
Chronic articular pain is a common outcome in patients who have been infected with CHIKV, Ebola virus, and West Nile virus.Citation5,Citation40,Citation44 Immune activation is a natural response to viral infections whereby the immune system is activated to help fight the virus. However, prolonged or excessive immune activation can lead to the release of inflammatory molecules, such as cytokines, which can sensitize nerves and contribute to the development of pain. The arthralgia experienced by patients is characterized by tissue destruction and the presence of inflammatory cytokines, such as interleukin (IL)-1β, IL-6, and tumor necrosis factor alpha.Citation15,Citation44 Infected fibroblasts produce interferon, which increases expression of prostaglandins in the cells. This pathway is thought to contribute to nociceptor activation and sensitization in arthritic joints. When this pro-inflammatory reaction continues without cessation, it may lead to the joint pain that persists long after recovery from the acute phase.Citation15 Another cell type that can be infected by viruses are osteoclasts. Patients with CHIKV have been found to express high levels of RANKL/osteoprotegerin, which can indicate the presence of macrophage-derived osteoclasts, or cells that cause bone destruction.Citation15 Tissue damage may also be caused by cross-reactive antibodies to host proteins.Citation40
Post-viral pain can also be neuropathic in nature. The most common example of this is herpes zoster. When dormant VZV reactivates, it causes acute herpes zoster, also known as shingles.Citation20,Citation37 The virus then replicates and triggers an inflammatory immune response, which damages peripheral and central sensory neurons, leading to generalized necrosis in the skin, nerves, and ganglia.Citation38,Citation39 The damaged peripheral nerves may become unable to inhibit nociceptive signals and, as a result, the threshold for nociceptive pain is decreased and ectopic discharges are produced. This creates disproportionate pain response to nonpainful stimuli, a phenomenon called peripheral sensitization.Citation39 Inflammation triggered by the active virus also causes central sensitization by impairing descending inhibitory pain pathways. The combination of damaged peripheral nerves and changes in the CNS is what causes the pain experienced in PHN.Citation39 Neuropathic pain that persists post-COVID is also thought to be the result of virus-induced damage to the peripheral and central nervous systems.Citation11
The pathophysiology of the pain related to immune activation and reactive oxygen species (ROS) in post-viral infections is complex and multifaceted. It involves a range of cellular and molecular mechanisms that can interact and amplify each other, leading to persistent pain and inflammation. Elevated ROS levels in the spinal cord can cause hyperexcitability in the nervous system and subsequent hyperalgesia without inflicting any nerve damage or tissue inflammation.Citation45 In patients with chronic pain syndromes, production of ROS has been postulated to account for pain in the chronic phase, and inflammatory processes lead to pain in the acute phase. Therefore, the increased production of ROS in viral infections, such as influenza and COVID-19, may cause alterations in pain processing pathways and lead to chronic post-viral pain.Citation46,Citation47
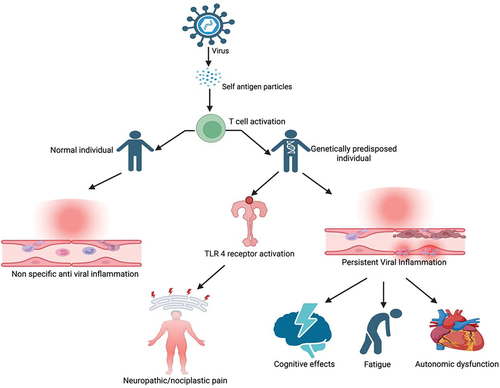
More recently, it has been identified that viral antigens can present with a structure similar to self-antigen or cause release of self-antigens that trigger activation of autoimmune T cells. These autoimmune cells instead lead to the activation of Toll-like receptors (TLRs), which are involved in the innate immune response.Citation48 TLRs are typically found in immune and glial cells of CNS. TLR activation can trigger neuroinflammation with the release of cytokines and chemokines, leading to altered nociception and inflammation in the absence of tissue damage or lesions in the somatosensory system.Citation49 After infection control, neuroinflammation typically subsides in healthy individuals. However, in genetically vulnerable individuals, the inflammatory process continues to progress, resulting in persistent chronic pain. Moreover, it can lead to the disruption of neurotransmitter systems, including those involved in autonomic regulation resulting in autonomic dysfunction ().Citation50 This phenomenon is now recognized as nociplastic pain, which is different from nociceptive and neuropathic pain.Citation51 Nociplastic pain is involved in a multitude of chronic widespread pain conditions like fibromyalgia and is likely implicated in post-viral pain.
Diagnosis and Treatment of Post-Viral Pain
Existing literature on the diagnosis and treatment of post-viral pain after viral infections is scant. With the exception of PHN, which can be diagnosed when neuropathic pain persists beyond 3 months in the same distribution as the preceding dermatomal rash, there are no clear guidelines on the diagnosis of post-viral pain.Citation39 The diagnosis is made when pain is preceded by a viral illness and cannot be explained by other conditions, such as rheumatoid arthritis, radiculopathy, and cancer.
Pain syndromes without a viral etiology are typically treated similarly to chronic pain.Citation11 However, the French Infectious Diseases Society has published guidelines for the management of patients with CHIKV at various disease stages.Citation16 In the postacute phase, analgesics and nonsteroidal anti-inflammatory drugs (NSAIDs) are used to relieve pain and reduce inflammation, with systemic corticosteroids such as prednisone reserved for exceptional cases. The choice of NSAID depends on the patient’s presentation and the clinician’s expertise as no specific class of NSAID has demonstrated superiority in treating post-CHIK symptoms.Citation16 In addition, physiotherapy may also be used to improve range of motion, prevent muscle atrophy, and provide pain relief. In the chronic phase, the goal is to prevent further damage and enhance quality of life through the use of DMARDs (e.g., methotrexate).Citation16 In an animal model, antiviral prophylactic drug research has yielded promising results but is yet to be replicated in human models.Citation52
In the case of PHN and other neuropathic pains post-viral illness, the first-line treatments are typically alpha-2 delta ligands (e.g., pregabalin, gabapentin), tricyclic antidepressants (TCAs), or serotonin–norepinephrine reuptake inhibitors, with opioids and cannabinoids used as supplementary treatment if necessary.Citation39 TCAs belong in one of two categories: secondary amines and tertiary amines. Tertiary amines, such as amitriptyline and clomipramine, have been shown to have greater efficacy in reducing PHN than secondary amines, such as nortriptyline and protriptyline.Citation39 In cases of herpes zoster, antiviral medications and vaccination are effective in reducing the intensity of acute pain and preventing the development of PHN.Citation39 However, there is no evidence to support the use of antiviral medications to prevent or treat post-viral pain and fatigue at this time.
There is also developing evidence for the efficacy of behavioral interventions in management of post-viral pain.Citation53 A recent trial comparing pregabalin alone and pregabalin+cognitive behavioral therapy (CBT) for management of PHN showed improvement in neuropathic symptoms of burning and allodynia as well as pain-related catastrophizing in the CBT group. Moreover, those who completed CBT while taking pregabalin showed downregulation of mRNA expression of IL-6.Citation54 Behavioral approaches, such as CBT or mindfulness-based interventions, are an important component of symptom management in post viral pain. In Sweden, intravenous immunoglobulin has been studied for post-polio pain, with 69% of patients reporting positive results.Citation55 In a case study involving peripheral neuropathy caused by COVID-19, a combination of intravenous immunoglobulin (IVIG), steroids, gabapentinoids, antidepressants, tramadol, and topical agents were used for pain management, resulting in total pain relief.Citation56 Though conventional analgesics and NSAIDs may provide temporary relief, the most effective treatments for neuropathic pain include gabapentinoids, antidepressants, tramadol, and topical agents.Citation11
Post-Viral Fatigue
Viral Agents Associated with Post-Viral Fatigue
Fatigue is another common symptom that can persist after a viral infection. In some cases, the fatigue occurs after activity, whereas in other cases it can persist even at rest.Citation1 There is also a difference between muscle weakness and brain fatigue that is associated with attention and cognitive deficits.Citation57 Fatigue can impair patients’ ability to work or fulfill their roles in other areas and may drastically reduce quality of life. When the fatigue continues for more than 6 months, CFS may be diagnosed.Citation58
In addition to causing post-viral pain, Ross River virus (RRV) has been found to be associated with post-viral fatigue.Citation58 In a prospective cohort study, 13 out of 60 patients with confirmed RRV infection developed postinfectious fatigue syndrome.Citation1 In the same study, 5 out of 68 patients with confirmed Epstein-Barr virus infection also developed postinfectious fatigue syndrome. With both infections, greater severity of acute illness was the strongest predictor of the severity of postinfectious fatigue.Citation1
Fatigue is the most common symptom of PPS; over 90% of polio survivors have reported new-onset or increased fatigue, and 41% reported that fatigue interfered with their ability to be productive at work.Citation57 More recently, COVID-19 has also been found to be associated with post-viral fatigue because a significant minority of patients have experienced continued fatigue and anhedonia after surviving the disease.Citation43,Citation59
Pathophysiology of Post-Viral Fatigue
Much remains unknown about the pathophysiology of post-viral fatigue, though several studies have proposed mechanisms that may lead to the syndrome. Proposed mechanisms include persistence of virus in the body causing autoimmunity, immune dysfunction, and autonomic dysregulation.
RRV has been thought to cause post-viral fatigue by evading the immune system through antibody-dependent enhancement (ADE), inflammatory cytokine dysregulation, and mitochondrial disruption.Citation60 ADE is a phenomenon in which preexisting antibodies facilitate viral entry into host cells rather than aiding antiviral immunity.Citation61 This process leads to increased virulence. RRV-ADE was first observed in the 1990s.Citation60 Once RRV enters macrophages, it disrupts cytokine expression, causing downregulation of pro-inflammatory cytokines, such as tumor necrosis factor, Interferon gamma inducible protein 10, while upregulating anti-inflammatory cytokines, such as IL-10. Dysregulation of the inflammatory cytokines has implications for the immune response to RRV and may undermine long-term immunity. The disequilibrium of cytokine expression also has potential to reduce the rate of ATP synthesis by mitochondria in lymphoblasts.Citation60 The alteration in mitochondrial function may account for the long-term fatigue often experienced after RRV.
Recent studies have also suggested that immune activation by viral infections may contribute to abnormalities of the autonomic nervous system, contributing to post-viral fatigue syndrome. One potential mechanism is through the production of secondary autoantibodies, by molecular mimicry of the immune system, against the autonomic nervous system, leading to its dysregulation and a host of neurological symptoms.Citation62 In addition, autoantibodies against α/β adrenoceptors and muscarinic receptors post-viral infection frequently lead to development of postural orthostatic tachycardia syndrome (POTS) and orthostatic intolerance (OH). Other mechanisms include cytokine storms.Citation63,Citation64
The chronic fatigue experienced as part of PPS has been thought to be due to poliovirus’s effect on the brain.Citation57 Since the 1950s, histopathology reports have shown lesions caused by poliovirus in the hypothalamus, thalamus, caudate nucleus, putamen, and substantia nigra.Citation57 Damage to these brain areas responsible for cortical activation may be responsible for the drowsiness and lethargy experienced in PPS. Magnetic resonance imaging studies of the brain have also found poliovirus lesions in the basal ganglia and reticular-activating system. Presence of hyperintense signal was correlated with fatigue severity and difficulty with attention and concentration.Citation57 Lesions in the hypothalamus may impair the paraventricular nucleus’s ability to secrete corticotropin-releasing hormone, thereby reducing production of adrenocorticotropic hormone and hypothalamus-pituitary-adrenal (HPA) axis activity overall. The HPA axis plays an important role in cortical stimulation, and decreased HPA axis activity has been documented in patients with CFS.Citation57 Thus, damage to the paraventricular nucleus and subsequent impairment of corticotropin-releasing hormone and adrenocorticotropic hormone production may be another mechanism through which poliovirus causes post-viral fatigue.Citation57
Diagnosis and Treatment of Post-Viral Fatigue
There are no agreed-upon diagnostic criteria for post-viral fatigue. The diagnosis is typically made when fatigue is preceded by a viral illness and cannot be explained by other conditions, such as anemia, adrenal insufficiency, and cancer.Citation65 Similarly, there is no standardized protocol for the treatment of post-viral fatigue. Management depends on the individual patient’s illness experience and is typically focused on energy conservation.Citation66 Lifestyle modifications, such as reducing daily activity and low-intensity aerobic graduated exercise, are commonly prescribed. CBT has also been suggested.Citation67 Indeed, a recent trial of CBT for management of fatigue in post-COVID syndrome found a medium effect size improvement in fatigue over treatment as usual, which was maintained 6 months after treatment.Citation68 Autonomic dysregulation (POTS and OH) is a major contributor of fatigue post-viral infection and should be evaluated in individuals experiencing fatigue in PVS. Treatment of POTS/OH with pharmacological (e.g., propranolol, ivabradine, midodrine) and nonpharmacological measures including graduated aerobic activity with mild resistance training can help alleviate fatigue symptoms.Citation69 One case report from England demonstrated the successful use of electroacupuncture in a patient who experienced post-viral fatigue following an “influenza-like” illness,Citation70 although larger-scale studies have not evaluated its efficacy and the case report was anecdotal.
Post-Viral Sleep Disorders
Viral Agents Associated with Post-Viral Sleep Disorders
In addition to pain and persistent fatigue, sleep disorders can develop after acute viral infection. Sleep abnormalities have been observed in SARS survivors who struggled with daily fatigue. When compared to healthy controls, patients post-SARS had more arousal disturbances and abnormal appearance of the electroencephalogram alpha frequency in approximately 50% of sleep on polysomnography.Citation31 Sleep disorders have also been documented following infection with the newer coronavirus, COVID-19.Citation7 After recovering from COVID-19, some previously healthy patients have developed sleep apnea, reducing the quality and restorative effects of their sleep.Citation71 One small study found that out of 11 patients post-COVID with suspected sleep disorders, 4 showed rapid eye movement (REM) sleep without atonia (RWA) on video polysomnography.Citation72 RWA is known to be a prodromal stage of REM sleep behavior disorder, which is characterized by abnormal body movement during REM sleep.Citation73
Tick-borne encephalitis (TBE) is caused by TBE virus, which infects the CNS and typically causes inflammation in the spinal cord, brain stem, basal ganglia, thalamus, and cerebellum.Citation74 One of the long-term sequelae of TBE is fatigue, and patients with TBE have been found to score their sleep-related quality of life lower than healthy controls did.Citation74 Pneumonia-associated viral encephalitis was also linked to disordered sleep in the case report of a 70-year-old man who developed parkinsonism and lethargy. Polysomnography revealed a fragmented sleep–wake cycle and a fragmented non-REM–REM ultradian cycle, leading the authors to suggest that encephalitis may lead to sleep disorders and parkinsonism.Citation75
Pathophysiology of Post-Viral Sleep Disorders
Sleep disturbance is a common symptom experienced by adults living with HIV. Difficulty with sleep maintenance, short sleep duration, and disturbed sleep–wake cycle have been reported by patients with HIV.Citation76 It has been suggested that these sleep issues may be the result of polymorphisms in the genes that regulate circadian rhythm. Genotyping showed that patients with HIV had polymorphisms in the CLOCK, CRY1, PER1, PER2, and PER3 genes, which have been associated with sleep outcomes in prior research.Citation76 The results indicated that longer HIV exposure may impact the effects that the polymorphisms have on circadian rhythm strength.Citation76 It is possible that genetic modifications play a role in the development of sleep disorders in other viral illnesses as well.
Sleep disorders seen in patients post-SARS and post-COVID-19 have been believed to be caused in part by CNS infiltration and inflammation.Citation31,Citation72 Both coronaviruses have the potential to be neuroinvasive through an olfactory route. The discovery of RWA in a group of patients post-COVID-19 led researchers to hypothesize that the virus caused changes in the brain stem, which maintains atonia during physiologic REM sleep.Citation72 This theory is supported by neuropathological studies that found that neuroinflammatory changes were most prominent in the brain stem.Citation72
Diagnosis and Treatment of Post-Viral Sleep Disorders
Similar to post-viral pain and post-viral fatigue, there are no specific diagnostic criteria for post-viral sleep disorders. There are also no treatment guidelines specific to post-viral sleep disorders, so patients are managed based on their presentation. For example, a patient with PVS experiencing sleep apnea would be treated much like a regular patient with sleep apnea. In a case series of patients post-COVID-19 who experienced new-onset sleep apnea, automated positive airway pressure therapy was successfully used to treat their condition.Citation71 The authors suggested that sleep apnea should be part of the differential diagnosis in all cases of post-COVID-19 fatigue syndrome. Behavioral sleep management interventions, such as CBT for insomnia (CBT-I), and brief behavioral treatment for insomnia (BBTI) also hold promise for treatment of post-viral sleep disorders. These brief interventions focus on changing behaviors associated with poor sleep and, in the case of CBT-I, changing cognitions that interfere with sleep. Efficacy of both CBT-I and BBTI is well established. Moreover, these interventions are effective for management of disordered sleep for individuals with complex medical concerns, including chronic pain.Citation77,Citation78 More research is needed to reveal special considerations required when managing sleep issues in patients with various PVSs.
Post-Viral Mental Health Sequelae
Viral Agents Associated with Mental Health Sequelae
Development of significant mental health concerns following viral infection has been commonly noted in PVS. However, this area has received only limited attention prior to the recent SARS-CoV-2 pandemic.Citation79–81 A recent meta-analysis examined rates of distress, depression, anxiety, and posttraumatic stress disorder (PTSD) following infection by SARS-CoV-1, H1N1, Middle East respiratory syndrome-CoV, H7N9, Ebola virus, or SARS-CoV-2. Rates of mental health problems following these infections were quite similar across virus populations and, pooled across mental health diagnoses and viruses, impacted 55% to 60% of individuals during acute illness and 11% to 22% of individuals after recovery from the virus. However, rates of specific mental health diagnoses were notably lower, with 5% to 10% of individuals reporting mild–moderate post-viral anxiety and/or depression, whereas 4% and 2% reported mild or moderate posttraumatic stress, respectively. There is no documented association between viral illness and severe mental illness.Citation82
Notably, rates of mental health concerns among individuals with HIV are higher than those described above. Among people living with HIV (PLWH), rates of mood and anxiety disorders are two to six times higher than in the general population.Citation83 In particular, rates of PTSD are much higher than has been reported in other post-viral populations, with some estimates placing the prevalence of PTSD among PLWH higher than 50%.Citation84 It is important to note that the social context and chronic nature of HIV infection also differ dramatically from those of the other viral agents described above.
Pathophysiology of Post-Viral Mental Health Concerns
The pathophysiology of post-viral mental health issues remains poorly understood. Putative mechanisms, physiological, socioenvironmental, or behavioral, are believed to be largely shared among viral illnesses. The immune response generated in response to a virus has been implicated as a causal generator of mental health problems.Citation85 Numerous studies have demonstrated dysregulation of inflammatory markers in mental health disorders, and it is possible that virally induced changes in pro-inflammatory molecules cause mental health problems.Citation86–90 Post-viral mental health problems may also be indirectly caused by other post-viral symptoms (i.e., pain, fatigue, sleep disruption) or by the impact of these symptoms on the ability to engage in meaningful behavior or social interaction. Indeed, social responses and stigmatization following infection with HIV or Ebola virus, such as loss of family relationships and friendships, can be extremely deleterious to mental health.Citation83,Citation91 Further research is needed to fully understand the multifactorial nature of this.
It is important to note that there is considerable concern among patients and providers regarding misattribution of causation for mental health problems following viral infection.Citation92,Citation93 Patients with PVS understandably stress biological causes for mental health concerns, given that symptoms are often met with invalidating responses from medical providers or other social relations. It is also possible that invalidation of other post-viral symptoms by providers, friends, or family contributes to distress.
Treatment of Post-Viral Mental Health Concerns
Research on treatment of post-viral mental health problems is limited. To the best of the authors’ knowledge, no interventions have been developed or tested specifically for post-viral mental health problems, with the exception of interventions for PLWH. In the context of PLWH, a range of mental health interventions have been deemed effective, with particular support for CBT-based interventions and those delivered by a psychologist.Citation84,Citation94 It is likely that similar adapted interventions would be beneficial for PVS originating from other viral illnesses. Alternatively, there has been some promising evidence for the use of anti-inflammatory treatments for mental health disorders.Citation95 Though this has not been investigated in the context of PVS, such approaches may hold potential as a mechanism-focused treatment.
Limitations of this Review
This review has some limitations. The main limitations of this review are the unsystematic methods in finding, selecting, and appraising the previous studies. Though it thoroughly addresses typical viral infections, it does not discuss the less common viral variants that can potentially lead to chronic pain. This review offers a comprehensive overview of the proposed pathophysiological mechanisms underlying PVSs, and it is imperative to acknowledge that empirical evidence supporting these mechanisms is still evolving and not yet fully elucidated. Furthermore, there exists a notable lack of consensus regarding universally accepted definitive classification and standardized diagnostic criteria for post-viral disorders. This review does not analyze the effectiveness and limitations of proposed therapeutic approaches for post-viral disorders. The usefulness and limitations of potential therapy options for post-viral diseases are not examined in this review. Finally, this study did not examine the severe influence of PVSs on patients’ quality of life, everyday functioning, and socioeconomic well-being. Expanding the scope of this review would undoubtedly facilitate a more comprehensive understanding of the complexities surrounding PVSs.
Future Considerations
The pathophysiology of post-viral pain syndromes is complex and remains unestablished. The COVID-19 pandemic demonstrated the prevalence of chronic pain in large population cohorts following viral infections.Citation8 There is an urgent need for research on the viral pain mechanisms, including their interactions with the immune pathways that cause chronic pain. In addition, it is essential to identify the epigenetic risk factors that contribute to the development of chronic persistent pain in some but not all individuals following a viral infection. This will aid in the development of targeted therapeutic approaches for the delivery of specific drug molecules designed to interrupt chronic pain pathways following viral infection. It is also important to note that specific PVS symptoms, such as pain, fatigue, or sleep disturbance, do not occur in isolation. Not only do these symptoms interact with each other but they are, in and of themselves, multifaceted, biopsychosocial constructs. Future research needs to take steps to consider this complexity in context to fully understand post-viral pain.
Conclusion
In conclusion, PVSs encompass persistent symptoms following viral infections, often including pain, fatigue, and sleep disturbances, which can significantly impact patients’ lives. Despite their prevalence, our understanding of the underlying mechanisms remains limited, with small-scale studies dominating the literature. Given the growing population of COVID-19 survivors facing long-term symptoms, urgent research is needed to unravel these mechanisms. It is worth noting that only a small fraction of patients achieve complete recovery from PVSs, emphasizing the need for targeted therapies. Enhanced pathophysiological insights may also lead to more efficient diagnosis and treatment, relieving health care system burdens. Ultimately, a deeper understanding of how viral illnesses interact with the CNS may pave the way for prevention strategies in the future.
Financial Benefits to the Authors
The authors confirm they did not receive any financial benefits as a result of conducting this study.
Previous Presentation of the Research/Manuscript
The authors confirm that this manuscript, in whole or in part, has not been previously presented or published.
Disclosure Statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hickie I, Davenport T, Wakefield D, Vollmer-Conna U, Cameron B, Vernon SD, Reeves WC, Lloyd A. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. 2006 Sep 16;333(7568):575. doi:10.1136/bmj.38933.585764.AE.
- Perrin R, Riste L, Hann M, Walther A, Mukherjee A, Heald A. Into the looking glass: post-viral syndrome post COVID-19. Med Hypotheses. 2020 Nov;144:110055. doi:10.1016/j.mehy.2020.110055.
- Kaida K, Kamakura K, Masaki T, Okano M, Nagata N, Inoue K. Painful small-fibre multifocal mononeuropathy and local myositis following influenza B infection. J Neurol Sci. 1997 Oct 3;151(1):103–13. doi:10.1016/s0022-510x(97)00085-3sss.
- Etard JF, Sow MS, Leroy S, Touré A, Taverne B, Keita AK, Msellati P, Magassouba N, Baize S, Raoul H, et al. Multidisciplinary assessment of post-Ebola sequelae in Guinea (Postebogui): an observational cohort study. Lancet Infect Dis. 2017 May;17(5):545–52. doi:10.1016/S1473-3099(16)30516-3.
- Edington F, Varjao D, Melo P. Incidence of articular pain and arthritis after chikungunya fever in the Americas: a systematic review of the literature and meta-analysis. Joint Bone Spine. 2018 Dec;85(6):669–78. doi:10.1016/j.jbspin.2018.03.019.
- Malik S, Saran S, Dubey A, Punj A. Longitudinally extensive transverse myelitis following dengue virus infection: a rare entity. Ann Afr Med. 2018 Apr-Jun;17(2):86–89. doi:10.4103/aam.aam_30_17.
- Sher L. Post-COVID syndrome and suicide risk. QJM. 2021 Apr 27;114(2):95–98. doi:10.1093/qjmed/hcab007.
- Rowbotham MC, Arendt-Nielsen L. A year like no other: introduction to a special issue on COVID-19 and pain. Pain Rep. 2021;6(1):e915. doi:10.1097/PR9.0000000000000915.
- Araja D, Berkis U, Lunga A, Murovska M. Shadow burden of undiagnosed Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS) on society: retrospective and prospective-in light of COVID-19. J Clin Med. 2021 Jul 6;10(14):3017. doi:10.3390/jcm10143017.
- Clark DV, Kibuuka H, Millard M, Wakabi S, Lukwago L, Taylor A, Eller MA, Eller LA, Michael NL, Honko AN, et al. Long-term sequelae after Ebola virus disease in Bundibugyo, Uganda: a retrospective cohort study. Lancet Infect Dis. 2015 Aug;15(8):905–12. doi:10.1016/S1473-3099(15)70152-0.
- Attal N, Martinez V, Bouhassira D. Potential for increased prevalence of neuropathic pain after the COVID-19 pandemic. Pain Rep. 2021 Jan–Feb;6(1):e884. doi:10.1097/PR9.0000000000000884.
- Kinchington PR, Goins WF. Varicella zoster virus-induced pain and post-herpetic neuralgia in the human host and in rodent animal models. J Neurovirol. 2011 Dec;17(6):590–99. doi:10.1007/s13365-011-0069-7.
- Jayamali WD, Herath H, Kulatunga A. A young female presenting with unilateral sacroiliitis following dengue virus infection: a case report. J Med Case Rep. 2017 Nov 1;11(1):307. doi:10.1186/s13256-017-1483-0.
- Caglioti C, Lalle E, Castilletti C, Carletti F, Capobianchi MR, Bordi L. Chikungunya virus infection: an overview. New Microbiol. 2013 Jul;36(3):211–27.
- Gasque P, Couderc T, Lecuit M, Roques P, Ng LF. Chikungunya virus pathogenesis and immunity. Vector Borne Zoonotic Dis. 2015 Apr;15(4):241–49. doi:10.1089/vbz.2014.1710.
- Simon F, Javelle E, Cabie A, Bouquillard E, Troisgros O, Gentile G, Leparc-Goffart I, Hoen B, Gandjbakhch F, Rene-Corail P, et al. French guidelines for the management of chikungunya (acute and persistent presentations). November 2014. Med Mal Infect. 2015 Jul;45(7):243–63. doi:10.1016/j.medmal.2015.05.007.
- Rodriguez-Morales AJ, Villamil-Gomez W, Merlano-Espinosa M, Simone-Kleber L. Post-chikungunya chronic arthralgia: a first retrospective follow-up study of 39 cases in Colombia. Clin Rheumatol. 2016 Mar;35(3):831–32. doi:10.1007/s10067-015-3041-8.
- Brito Ferreira ML, de Albuquerque Mfp M, de Brito CAA, de Oliveira França RF, Porto Moreira ÁJ, de Morais Machado MÍ, da Paz Melo R, Medialdea-Carrera R, Dornelas Mesquita S, Lopes Santos M, et al. Neurological disease in adults with Zika and chikungunya virus infection in Northeast Brazil: a prospective observational study. Lancet Neurol. 2020 Oct;19(10):826–39. doi:10.1016/s1474-4422(20)30232-5.
- Moshayedi P, Thomas D, Rinaldo CR, Moossy JJ, Maroon JC, Murdoch GH, Hamilton RL, Homayoun H. Subacute histopathological features in a case of varicella zoster virus myelitis and post-herpetic neuralgia. Spinal Cord Ser Cases. 2018;4:33. doi:10.1038/s41394-018-0068-5.
- Gershon AA, Breuer J, Cohen JI, Cohrs RJ, Gershon MD, Gilden D, Grose C, Hambleton S, Kennedy PGE, Oxman MN, et al. Varicella zoster virus infection. Nat Rev Dis Primers. 2015 Jul 2;1:15016. doi:10.1038/nrdp.2015.16.
- Johnson RW, Rice AS. Clinical practice. Postherpetic neuralgia. N Engl J Med. 2014 Oct 16;371(16):1526–33. doi:10.1056/NEJMcp1403062.
- Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annu Rev Pathol. 2011;6:223–48. doi:10.1146/annurev-pathol-011110-130254.
- Hurst SA, Appelgren KE, Kourtis AP. Prevention of mother-to-child transmission of HIV type 1: the role of neonatal and infant prophylaxis. Expert Rev Anti Infect Ther. 2015 Feb;13(2):169–81. doi:10.1586/14787210.2015.999667.
- Douek DC, Brenchley JM, Betts MR, Ambrozak DR, Hill BJ, Okamoto Y, Casazza JP, Kuruppu J, Kunstman K, Wolinsky S, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002 May 2;417(6884):95–98. doi:10.1038/417095a.
- Phillips TJC, Brown M, Ramirez JD, Perkins J, Woldeamanuel YW, Williams ACDC, Orengo C, Bennett DLH, Bodi I, Cox S, et al. Sensory, psychological, and metabolic dysfunction in HIV-associated peripheral neuropathy: a cross-sectional deep profiling study. Pain. 2014 Sep;155(9):1846–60. doi:10.1016/j.pain.2014.06.014.
- Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, et al. Neuropathic pain. Nat Rev Dis Primers. 2017;3(1):17002. doi:10.1038/nrdp.2017.2.
- Li Hi Shing S, Chipika RH, Finegan E, Murray D, Hardiman O, Bede P. Post-polio syndrome: more than just a lower motor neuron disease. Front Neurol. 2019;10:773. doi:10.3389/fneur.2019.00773.
- Pastuszak Ż, Stępień A, Tomczykiewicz K, Piusińska-Macoch R, Galbarczyk D, Rolewska A. Post-polio syndrome. Cases report and review of literature. Neurol Neurochir Pol. 2017 Mar–Apr;51(2):140–45. doi:10.1016/j.pjnns.2017.01.009.
- Kay L, Nielsen NM, Wanscher B, Jennum P. Neurological symptoms in danes with a history of poliomyelitis: lifelong follow-up of late symptoms, their association with initial symptoms of polio, and presence of postpolio syndrome. Eur Neurol. 2018;80(5–6):295–303. doi:10.1159/000497483.
- Klebek L, Sunnquist M, Jason LA. Differentiating post-polio syndrome from myalgic encephalomyelitis and chronic fatigue syndrome. Fatigue. 2019;7(4):196–206. doi:10.1080/21641846.2019.1687117.
- Moldofsky H, Patcai J. Chronic widespread musculoskeletal pain, fatigue, depression and disordered sleep in chronic post-SARS syndrome; a case-controlled study. BMC Neurol. 2011 Mar 24;11:37. doi:10.1186/1471-2377-11-37.
- Avendano M, Derkach P, Swan S. Clinical course and management of SARS in health care workers in Toronto: a case series. Cmaj. 2003 Jun 24;168(13):1649–60.
- Korompoki E, Gavriatopoulou M, Hicklen RS, Ntanasis-Stathopoulos I, Kastritis E, Fotiou D, Stamatelopoulos K, Terpos E, Kotanidou A, Hagberg CA, et al. Epidemiology and organ specific sequelae of post-acute COVID19: a narrative review. J Infect. 2021 Jul;83(1):1–16. doi:10.1016/j.jinf.2021.05.004.
- Salamanna F, Veronesi F, Martini L, Landini MP, Fini M. Post-COVID-19 syndrome: the persistent symptoms at the post-viral stage of the disease. A systematic review of the current data. Front Med (Lausanne). 2021;8:653516. doi:10.3389/fmed.2021.653516.
- Klit H, Finnerup NB, Jensen TS. Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 2009 Sep;8(9):857–68. doi:10.1016/s1474-4422(09)70176-0.
- Feldmann H, Geisbert TW. Ebola haemorrhagic fever. Lancet. 2011 Mar 5;377:9768):849–62. doi:10.1016/S0140-6736(10)60667-8.
- Wu S, Yang S, Ou M, Chen J, Huang J, Xiong D, Sun W, Xiao L. Transcriptome analysis reveals the role of cellular calcium disorder in Varicella Zoster virus-induced post-herpetic neuralgia. Front Mol Neurosci. 2021;14:665931. doi:10.3389/fnmol.2021.665931.
- Oaklander AL. The pathology of shingles: Head and Campbell’s 1900 monograph. Arch Neurol. 1999 Oct;56(10):1292–94. doi:10.1001/archneur.56.10.1292.
- Hadley GR, Gayle JA, Ripoll J, Jones MR, Argoff CE, Kaye RJ, Kaye AD. Post-herpetic neuralgia: a review. Curr Pain Headache Rep. 2016 Mar;20(3):17. doi:10.1007/s11916-016-0548-x.
- Carod-Artal FJ. Post-ebolavirus disease syndrome: what do we know? Expert Rev Anti Infect Ther. 2015;13(10):1185–87. doi:10.1586/14787210.2015.1079128.
- Guedj E, Campion JY, Dudouet P, Kaphan E, Bregeon F, Tissot-DuPont H, Guis S, Barthelemy F, Habert P, Ceccaldi M, et al. (18)F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021 Aug;48(9):2823–33. doi:10.1007/s00259-021-05215-4.
- Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol. 2020 Aug 1;77(8):1018–27. doi:10.1001/jamaneurol.2020.2065.
- Wostyn P. COVID-19 and chronic fatigue syndrome: is the worst yet to come? Med Hypotheses. 2021 Jan;146:110469. doi:10.1016/j.mehy.2020.110469.
- Leis AA, Grill MF, Goodman BP, Sadiq SB, Sinclair DJ, Vig PJS, Bai F. Tumor necrosis factor-alpha signaling may contribute to chronic West Nile virus post-infectious proinflammatory state. Front Med (Lausanne). 2020;7:164. doi:10.3389/fmed.2020.00164.
- Hendrix J, Nijs J, Ickmans K, Godderis L, Ghosh M, Polli A. The interplay between oxidative stress, exercise, and pain in health and disease: potential role of autonomic regulation and epigenetic mechanisms. Antioxidants (Basel). 2020 Nov 23;9(11). doi:10.3390/antiox9111166.
- Forcados GE, Muhammad A, Oladipo OO, Makama S, Meseko CA. Metabolic implications of oxidative stress and inflammatory process in SARS-CoV-2 pathogenesis: therapeutic potential of natural antioxidants. Front Cell Infect Microbiol. 2021;11:654813. doi:10.3389/fcimb.2021.654813.
- Liu M, Chen F, Liu T, Chen F, Liu S, Yang J. The role of oxidative stress in influenza virus infection. Microbes Infect. 2017 Dec;19(12):580–86. doi:10.1016/j.micinf.2017.08.008.
- Liu X, Yang W, Zhu C, Sun S, Wu S, Wang L, Wang Y, Ge Z. Toll-like receptors and their role in neuropathic pain and migraine. Mol Brain. 2022 Aug 20;15(1):73. doi:10.1186/s13041-022-00960-5.
- Lacagnina MJ, Watkins LR, Grace PM. Toll-like receptors and their role in persistent pain. Pharmacol Ther. 2018 Apr;184:145–58. doi:10.1016/j.pharmthera.2017.10.006.
- Li H, Yu X, Liles C, Khan M, Vanderlinde‐Wood M, Galloway A, Zillner C, Benbrook A, Reim S, Collier D, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014 Feb 26;3(1):e000755. doi:10.1161/jaha.113.000755.
- Bailly F, Cantagrel A, Bertin P, Perrot S, Thomas T, Lansaman T, Grange L, Wendling D, Dovico C, Trouvin A-P, et al. Part of pain labelled neuropathic in rheumatic disease might be rather nociplastic. RMD Open. 2020;6(2):e001326. doi:10.1136/rmdopen-2020-001326.
- Dagley A, Ennis J, Turner JD, Rood KA, Van Wettere AJ, Gowen BB, Julander JG. Protection against Chikungunya virus induced arthralgia following prophylactic treatment with adenovirus vectored interferon (mDEF201). Antiviral Res. 2014 Aug;108:1–9. doi:10.1016/j.antiviral.2014.05.004.
- Zhu X, Hu P, Fan Z, Zhan W, Wang H, Yang Y, Zhou Z, Ma L, Gao H. Effects of mindfulness-based stress reduction on depression, anxiety, and pain in patients with postherpetic neuralgia. J Nerv Ment Dis. 2019 Jun;207(6):482–86. doi:10.1097/NMD.0000000000000998.
- Saxena AK, Bhardwaj N, Chilkoti GT, Malik A, Thakur GK, Bajaj M, Banerjee A, Banerjee BD, Singal A. Modulation of mRNA expression of IL-6 and mTORC1 and efficacy and feasibility of an integrated approach encompassing cognitive behavioral therapy along with pregabalin for management of neuropathic pain in postherpetic neuralgia: a pilot study. Pain Med. 2021 Oct 8;22(10):2276–82. doi:10.1093/pm/pnab142.
- Werhagen L, Borg K. Effect of intravenous immunoglobulin on pain in patients with post-polio syndrome. J Rehabil Med. 2011 Nov;43(11):1038–40. doi:10.2340/16501977-0884.
- Bureau BL, Obeidat A, Dhariwal MS, Jha P. Peripheral neuropathy as a complication of SARS-Cov-2. Cureus. 2020 Nov 12;12(11):e11452. doi:10.7759/cureus.11452.
- Bruno RL, Creange SJ, Frick NM. Parallels between post-polio fatigue and chronic fatigue syndrome: a common pathophysiology? Am J Med. 1998 Sep 28;105(3a):66s–73s. doi:10.1016/s0002-9343(98)00161-2.
- Rasa S, Nora-Krukle Z, Henning N, Eliassen E, Shikova E, Harrer T, Scheibenbogen C, Murovska M, Prusty BK. Chronic viral infections in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). J Transl Med. 2018;16(1). doi:10.1186/s12967-018-1644-y.
- El Sayed S, Shokry D, Gomaa SM. Post‐COVID‐19 fatigue and anhedonia: a cross‐sectional study and their correlation to post‐recovery period. Neuropsychopharmacol Rep. 2021;41(1):50–55. doi:10.1002/npr2.12154.
- Lidbury BA. Ross river virus immune evasion strategies and the relevance to post-viral fatigue, and myalgic encephalomyelitis onset. Front Med (Lausanne). 2021;8:662513. doi:10.3389/fmed.2021.662513.
- Bournazos S, Gupta A, Ravetch JV. The role of IgG Fc receptors in antibody-dependent enhancement. Nat Rev Immunol. 2020;20(10):633–43. doi:10.1038/s41577-020-00410-0.
- Guilmot A, Maldonado Slootjes S, Sellimi A, Bronchain M, Hanseeuw B, Belkhir L, Yombi JC, De Greef J, Pothen L, Yildiz H, et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol. 2021 Mar;268(3):751–57. doi:10.1007/s00415-020-10108-x.
- Gunning WT, Kvale H, Kramer PM, Karabin BL, Grubb BP. Postural orthostatic tachycardia syndrome is associated with elevated G-protein coupled receptor autoantibodies. J Am Heart Assoc. 2019;8(18):e013602. doi:10.1161/JAHA.119.013602.
- Mallick D, Goyal L, Chourasia P, Zapata MR, Yashi K, Surani S. COVID-19 induced postural orthostatic tachycardia syndrome (POTS): a review. Cureus. 2023 Mar 31;15(3):e36955. doi: 10.7759/cureus.36955.
- Maisel P, Baum E, Donner-Banzhoff N. Fatigue as the chief complaint-epidemiology, causes, diagnosis, and treatment. Dtsch Arztebl Int. 2021 Aug 23;118(33–34):566–76. doi:10.3238/arztebl.m2021.0192.
- Ho-Yen DO. Patient management of post-viral fatigue syndrome. Br J Gen Pract. 1990 Jan;40(330):37–39.
- Wessely S, David A, Butler S, Chalder T. Management of chronic (post-viral) fatigue syndrome. J R Coll Gen Pract. 1989 Jan;39(318):26–29.
- Kuut TA, Muller F, Csorba I, Braamse A, Aldenkamp A, Appelman B, Assmann-Schuilwerve E, Geerlings SE, Gibney KB, Kanaan RAA, et al. Efficacy of cognitive behavioral therapy targeting severe fatigue following COVID-19: results of a randomized controlled trial. Clin Infect Dis. 2023 May 8;77:687–95. doi:10.1093/cid/ciad257.
- Raj SR, Fedorowski A, Sheldon RS. Diagnosis and management of postural orthostatic tachycardia syndrome. Can Med Assoc J. 2022;194(10):E378–E385. doi:10.1503/cmaj.211373.
- Mears T. Acupuncture in the treatment of post viral fatigue syndrome–a case report. Acupunct Med. 2005 Sep;23(3):141–45. doi:10.1136/aim.23.3.141.
- Koczulla AR, Stegemann A, Gloeckl R, Winterkamp S, Sczepanski B, Boeselt T, Storre J, Dreher M. Newly detected rapid eye movement associated sleep apnea after coronavirus disease 2019 as a possible cause for chronic fatigue: two case reports. J Med Case Rep. 2021 Apr 22;15(1):211. doi:10.1186/s13256-021-02819-0.
- Heidbreder A, Sonnweber T, Stefani A, Ibrahim A, Cesari M, Bergmann M, Brandauer E, Tancevski I, Löffler-Ragg J, Högl B, et al. Video-polysomnographic findings after acute COVID-19: REM sleep without atonia as sign of CNS pathology? Sleep Medicine. 2021;80:92–95. doi:10.1016/j.sleep.2021.01.051.
- Dauvilliers Y, Schenck CH, Postuma RB, Iranzo A, Luppi P-H, Plazzi G, Montplaisir J, Boeve B. REM sleep behaviour disorder. Nat Rev Dis Primers. 2018 Aug 30;4(1):19. doi:10.1038/s41572-018-0016-5.
- Veje M, Studahl M, Thunström E, Stentoft E, Nolskog P, Celik Y, Peker Y. Sleep architecture, obstructive sleep apnea and functional outcomes in adults with a history of Tick-borne encephalitis. PLoS One. 2021;16(2):e0246767. doi:10.1371/journal.pone.0246767.
- Brunetti V, Testani E, Iorio R, Frisullo G, Luigetti M, Di Giuda D, Marca GD. Post-encephalitic parkinsonism and sleep disorder responsive to immunological treatment: a case report. Clin EEG Neurosci. 2016 Oct;47(4):324–29. doi:10.1177/1550059416645706.
- Lee KA, Gay C, Byun E, Lerdal A, Pullinger CR, Aouizerat BE. Circadian regulation gene polymorphisms are associated with sleep disruption and duration, and circadian phase and rhythm in adults with HIV. Chronobiol Int. 2015;32(9):1278–93. doi:10.3109/07420528.2015.1087021.
- Selvanathan J, Pham C, Nagappa M, Peng PWH, Englesakis M, Espie CA, Morin CM, Chung F. Cognitive behavioral therapy for insomnia in patients with chronic pain – a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2021 Dec;60:101460. doi:10.1016/j.smrv.2021.101460.
- Johnson JA, Rash JA, Campbell TS, Savard J, Gehrman PR, Perlis M, Carlson LE, Garland SN. A systematic review and meta-analysis of randomized controlled trials of Cognitive Behavior Therapy for Insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016 Jun;27:20–28. doi:10.1016/j.smrv.2015.07.001.
- Arciniegas DB, Anderson CA. Viral encephalitis: neuropsychiatric and neurobehavioral aspects. Curr Psychiatry Rep. 2004 Oct;6(5):372–79. doi:10.1007/s11920-004-0024-x.
- Dube B, Benton T, Cruess DG, Evans DL. Neuropsychiatric manifestations of HIV infection and AIDS. J Psychiatry Neurosci. 2005 Jul;30(4):237–46.
- Hinkin CH, Castellon SA, Atkinson JH, Goodkin K. Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol. 2001 Dec;54(Suppl 1):S44–52. doi:10.1016/s0895-4356(01)00446-2.
- Zurcher SJ, Banzer C, Adamus C, Lehmann AI, Richter D, Kerksieck P. Post-viral mental health sequelae in infected persons associated with COVID-19 and previous epidemics and pandemics: systematic review and meta-analysis of prevalence estimates. J Infect Public Health. 2022 May;15(5):599–608. doi:10.1016/j.jiph.2022.04.005.
- Remien RH, Stirratt MJ, Nguyen N, Robbins RN, Pala AN, Mellins CA. Mental health and HIV/AIDS: the need for an integrated response. AIDS. 2019 Jul 15;33(9):1411–20. doi:10.1097/QAD.0000000000002227.
- Beckerman NL, Auerbach C. Post-traumatic stress disorder and HIV: a snapshot of co-occurrence. Soc Work Health Care. 2010;49(8):687–702. doi:10.1080/00981389.2010.485089.
- Müller N. Infectious diseases and mental health. Comorb Mental Phys Disord. 2014;179:99–113.
- Michopoulos V, Powers A, Gillespie CF, Ressler KJ, Jovanovic T. Inflammation in fear- and anxiety-based disorders: PTSD, GAD, and beyond. Neuropsychopharmacology. 2017 Jan;42(1):254–70. doi:10.1038/npp.2016.146.
- Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, Allen NB, Stuart AL, Hayley AC, Byrne ML, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013 Sep 12;11:200. doi:10.1186/1741-7015-11-200.
- Felger JC. Imaging the role of inflammation in mood and anxiety-related disorders. Curr Neuropharmacol. 2018;16(5):533–58. doi:10.2174/1570159X15666171123201142.
- Mellon SH, Gautam A, Hammamieh R, Jett M, Metabolism WOM. Metabolomics, and inflammation in posttraumatic stress disorder. Biol Psychiatry. 2018 May 15;83(10):866–75. doi:10.1016/j.biopsych.2018.02.007.
- Al-Hakeim HK, Al-Rubaye HT, Al-Hadrawi DS, Almulla AF, Maes M. Long-COVID post-viral chronic fatigue and affective symptoms are associated with oxidative damage, lowered antioxidant defenses and inflammation: a proof of concept and mechanism study. Mol Psychiatry. 2023 Feb;28(2):564–78. doi:10.1038/s41380-022-01836-9.
- Lotsch F, Schnyder J, Goorhuis A, Grobusch MP. Neuropsychological long-term sequelae of Ebola virus disease survivors – a systematic review. Travel Med Infect Dis. 2017 Jul-Aug;18:18–23. doi:10.1016/j.tmaid.2017.05.001.
- Rushforth A, Ladds E, Wieringa S, Taylor S, Husain L, Greenhalgh T. Long COVID – the illness narratives. Soc Sci Med. 2021 Oct;286:114326. doi:10.1016/j.socscimed.2021.114326.
- Callard F, Perego E. How and why patients made Long COVID. Soc Sci Med. 2021 Jan;268:113426. doi:10.1016/j.socscimed.2020.113426.
- van Luenen S, Garnefski N, Spinhoven P, Spaan P, Dusseldorp E, Kraaij V. The benefits of psychosocial interventions for mental health in people living with HIV: a systematic review and meta-analysis. AIDS Behav. 2018 Jan;22(1):9–42. doi:10.1007/s10461-017-1757-y.
- Fitton R, Sweetman J, Heseltine-Carp W, van der Feltz-Cornelis C. Anti-inflammatory medications for the treatment of mental disorders: a scoping review. Brain Behav Immun Health. 2022 Dec;26:100518. doi:10.1016/j.bbih.2022.100518.