ABSTRACT
The evaluation of a patient with fibrotic interstitial lung disease (ILD) includes assessment of clinical, radiological, and often histopathological data. There are currently no specific recommendations to guide the evaluation of a patient with fibrotic ILD within the context of the Canadian practice landscape. This position statement from a multidisciplinary panel of ILD experts provides guidance related to the diagnostic modalities commonly used in the evaluation of fibrotic ILD, including radiological studies, histopathologic sampling, assessment for rheumatologic disease and need for evaluation in a multi-disciplinary setting. Key messages are provided to guide clinical practice based on a thorough review of the scientific literature.
RÉSUMÉ
L’évaluation d'un patient atteint de fibrose pulmonaire interstitielle comprend l’évaluation de données cliniques, radiologiques et souvent histopathologiques. Il n'existe actuellement aucune recommandation pour guider l’évaluation d'un patient atteint de fibrose pulmonaire interstitielle dans le cadre de la pratique canadienne. Cet énoncé de position d'un comité multidisciplinaire d'experts en maladie pulmonaire interstitielle donne des conseils relativement aux modalités de diagnostic communément utilisées dans l’évaluation de la fibrose pulmonaire interstitielle, y compris les études radiologiques, l’échantillonnage histopathologique et l’évaluation pour un trouble rhumatologique, de même que la nécessité que l’évaluation soit menée dans une perspective multidisciplinaire. Afin de guider la pratique clinique, des messages clés fondés sur une revue approfondie de la littérature sont énoncés.
Introduction
The interstitial lung diseases (ILDs) are a heterogeneous group of disorders characterized by inflammation and/or fibrosis of the pulmonary interstitium.Citation1 ILDs are challenging to diagnose, with over 200 unique subtypes that frequently have overlapping clinical and radiological features. The fibrotic ILDs include those with radiological and/or pathological evidence of fibrosis and are typically characterized by progressive physiologic impairment, dyspnea, functional limitation and early mortality.
Current diagnostic guidelines are based on limited evidence and focus on idiopathic pulmonary fibrosis (IPF) or other idiopathic interstitial pneumonias (IIPs);Citation2–4 however, a more general approach is needed at the time of initial assessment. A recent survey of Canadian respirologists revealed heterogeneous practice patterns and deviations from international consensus recommendations.Citation5 These issues indicate the need for recommendations applicable to a broader variety of fibrotic ILDs.Citation6
The objectives of this position paper are to:
| 1) | Summarize the available literature on topics relevant to the evaluation of patients with fibrotic ILD. | ||||
| 2) | Provide evidence- and expertise-based key messages for the evaluation of patients with fibrotic ILD. | ||||
There are several essential components to the evaluation of fibrotic ILD that should be addressed in all patients. These include a thorough history identifying symptoms suggestive of connective tissue disease and relevant occupational and/or environmental exposures, a family history of ILD or autoimmune disease, a detailed physical examination and pulmonary function testing. These and other “good clinical practice” items were not reviewed as these should be evaluated in all patients with fibrotic ILD, and a comprehensive review of each of these topics is beyond the scope of the current work. In contrast, there are additional items that have insufficient data to support evidence-based key messages (eg, specific circulating antibodies in the evaluation of hypersensitivity pneumonitis, genetic testing in the routine evaluation of a patient with fibrotic ILD), and these topics will similarly not be reviewed.
The key messages provided herein are intended to guide evidence-based decision-making by clinical care providers (eg, general internists, respirologists, radiologists, pathologists, thoracic surgeons and any others initiating the evaluation for fibrotic ILD) and should be interpreted in the context of patient wishes and preferences. Other individuals, including family members and/or allied health care providers, should be involved in these discussions when appropriate to address the unique needs of each patient.
Methods
A working group was created within the Canadian Thoracic Society's Clinical Assembly on Interstitial Lung Disease. The group was co-chaired by two authors (KAJ and CJR), and included 9 adult respirologists with expertise in ILD patient care, 1 rheumatologist, 2 chest radiologists, 2 lung pathologists and 1 thoracic surgeon.
The document was developed in accordance with Canadian Thoracic Society (CTS) requirements for a position statement. The working group identified 7 clinically relevant questions pertaining to the diagnosis of fibrotic ILD and then conducted a narrative review of the scientific literature on these topics. Subcommittees produced a literature summary and identified key messages for each question, agreed upon through collaborative discussion by the working group. The completed document was reviewed by 2 ILD experts external to the CTS, 1 member of other CTS guideline committees, and members of the CTS Canadian Respiratory Guidelines Committee reviewed the completed document. One member completed the AGREE II checklist.Citation7 Original reviews and responses to reviews are posted along with the guideline and all author conflicts of interest, at respiratoryguidelines.ca. The CTS Executive approved the final document for publication. The position statement will be updated in accordance with the CTS Living Guideline Model (www.respiratoryguidelines.ca).
Summary of evidence and key messages
High resolution computed tomography
What are the appropriate specifications for computed tomography (CT) in the evaluation of fibrotic ILD?
High-resolution CT (HRCT) cross-sectional chest imaging permits visualization of the lungs in fine anatomic detail. HRCT is a critical component of the evaluation of the IIPsCitation2,Citation4 and should be performed in all patients with suspected fibrotic ILD, unless contra-indicated. The HRCT protocol should include thin-collimation axial scans or thin-section reconstruction of volumetric helically acquired data using multi-detector CT and narrow detector width (0.5-1.25░mm), image reconstruction with a high spatial frequency (sharp or edge-detecting) algorithm and sufficient radiation to keep image noise and patient exposure at acceptably low levels.Citation8
HRCT studies should be performed at full inspiration. Expiratory imaging should be considered, particularly when there is suspicion of diseases that can cause air trapping (eg, hypersensitivity pneumonitis, obliterative bronchiolitis). Prone imaging can help differentiate atelectasis from ILD in patients with mild subpleural changes in dependent parts of the lungs. Volumetric acquisition provides complete imaging of the lung, contiguous imaging of specific lesions (eg, nodules), multiplanar reformatting, precise comparison to prior exams and assessment of additional lung abnormalities. Contrast enhancement may be considered in cases where there is suspected concomitant thromboembolic disease, lymph node enlargement, mass lesions or pleural disease. Low dose chest CT for lung cancer screening or pulmonary nodule assessment can identify abnormalities suggestive of ILD but does not eliminate the need for further imaging with HRCT.
The diagnostic accuracy of HRCT depends on the experience of the interpreting radiologistCitation9–12 and whether interpretation is provided within the context of a multidisciplinary discussion (MDD).Citation2,Citation4,Citation13 The high inter-observer agreement among academic radiologists suggests the potential for greater diagnostic accuracy; however, the specific clinical implications of this finding are currently unknown. Academic radiologists have higher inter-observer agreement than community radiologists for the interpretation of HRCT for ILD, with the latter having a greater tendency to report a usual interstitial pneumonia (UIP) pattern.Citation14 Diagnostic accuracy can be as high as 90% when an experienced chest radiologist confidently identified a UIP pattern,Citation9 and this can eliminate the need for surgical lung biopsy in some patients.Citation2,Citation9
Evaluation for connective tissue disease
Which autoimmune serologic tests should be performed in the evaluation of fibrotic ILD?
Connective tissue diseases (CTDs) are systemic autoimmune disorders that typically affect the skin and musculoskeletal system. ILD is an important cause of morbidity and mortality in patients with CTD,Citation15,Citation16 commonly occurring in patients with systemic sclerosis, rheumatoid arthritis, idiopathic inflammatory myopathies, mixed CTD, Sjögren's syndrome and systemic lupus erythematosus.Citation17,Citation18 Consensus criteria have recently been proposed for interstitial pneumonia with autoimmune features (IPAF), defined as patients with ILD who have autoimmune features that do not meet criteria for a defined CTD.Citation19 Additional studies are required to determine whether it is appropriate to consider this population a distinct clinical entity.
It is important to identify CTD as an underlying cause of ILD given the different management and prognosis compared to other ILD subtypes. Most importantly, patients with CTD-ILD may benefit from immunosuppressive agents that carry an increased risk of death in patients with IPF.Citation20-23 Detailed evaluation for symptoms and signs of underlying CTD should be performed at baseline in all patients with fibrotic ILD, and should be repeated during follow-up since ILD can also develop prior to overt features of CTD.Citation24 No consensus exists on which autoimmune serology should be routinely performed when screening a broader ILD population for the presence of CTD; however, recent IPF guidelines recommend obtaining a rheumatoid factor (RF), antinuclear antibody (ANA) and anti-cyclic citrullinated peptide (CCP) antibody in patients with suspected IPF.Citation2 It is likely appropriate to perform these studies in all fibrotic ILD patients, with additional targeted serologic testing reserved for patients with a high pre-test probability of CTD. Indirect immunofluorescence is more sensitive than solid phase assays for detecting ANA, and should be used for the initial screening test.Citation25
When should a patient with fibrotic ILD be referred to a rheumatologist?
Circulating low-level autoantibodies have been reported in patients with IPF who lack clinical findings of CTD. An abnormal ANA is present in 25%-41% of IPF patients at a titre ≥1:40, and an elevated RF present in 6%-7%;Citation26-28 however, the significance of these autoantibodies is unclear given the similar frequencies seen in healthy age-matched control populations and the similar prognosis of serology-positive and serology-negative patients with IPF.Citation26–28 The mere presence of an autoantibody is therefore insufficient to provide a CTD-ILD diagnosis without additional confirmatory clinical data.
There are limited data to guide the need for assessment by a rheumatologist in a patient with fibrotic ILD. In the absence of additional CTD features, ILD patients with low-titre ANA and/or RF likely do not require referral to a rheumatologist, particularly if the onset of ILD is after the age of 65 years.Citation29 Conversely, referral to a rheumatologist should be considered in patients with suggestive clinical features, specific autoantibodies, or high titre ANA (≥1:320) and/or RF (≥60 IU/mL).Citation18,Citation19 Similarly, a rheumatology referral should be considered in patients that meet criteria for IPAF (see Reference 19 for IPAF criteria).Citation19 Some Canadian centres offer combined pulmonary-rheumatology ILD clinics for coordinated multidisciplinary evaluation of patients with suspected CTD-ILD.
Bronchoalveolar lavage
When should bronchoalveolar lavage be performed in the evaluation of fibrotic ILD?
Bronchoalveolar lavage (BAL) is a minimally invasive and generally well-tolerated method to collect cells, organisms and inhaled particles from the lower respiratory tract and alveolar spaces. The recommended techniques for obtaining and processing BAL fluid have been described elsewhere and are essential to ensure meaningful results.Citation30 BAL can be diagnostic in many pulmonary diseases in the appropriate clinical context, including infection, malignancy and some nonfibrotic ILDs (eg, pulmonary alveolar proteinosis, eosinophilic pneumonia).
There are limited data supporting the diagnostic utility of BAL in fibrotic ILD. BAL neutrophilia >5% is most consistent with IPF although can be found in other fibrotic ILDs, whereas lymphocytosis >25% suggests granulomatous disease and >50% is highly suggestive of hypersensitivity pneumonitis (HP) or idiopathic nonspecific interstitial pneumonia (NSIP).Citation30 BAL cellular analysis, and particularly a lymphocytosis >30%, resulted in a change in diagnosis for 8% of patients that had a confident diagnosis of IPF based on clinical and radiological features in a previous cohort study.Citation31 Other studies have conversely shown that significant lymphocytosis is less common in chronic (fibrotic) HP compared to acute or subacute forms.Citation32,Citation33 Some reports suggest an informative role of lymphocyte subset analysis (eg, CD4 to CD8 ratio) in sarcoidosis, but this test has no clear utility in fibrotic ILD.Citation30,Citation34,Citation35 There are no adequately designed studies evaluating whether BAL adds incremental diagnostic information beyond a thorough evaluation for alternative diagnoses in patients with fibrotic ILD.
The uncertain benefit of BAL should be balanced against the potential risks, including transient worsening of hypoxemia and, rarely, acute exacerbation of the underlying ILD.Citation36–40 Recent guidelines recommend against BAL in the evaluation of most IPF patients but acknowledge that it may be appropriate in a minority.Citation2 Given the absence of additional supportive data, the primary role of BAL in fibrotic ILD is to exclude infection in patients presenting with suggestive clinical and radiological features, especially if immunosuppressive therapy is being considered.
Transbronchial biopsy
When should transbronchial biopsy be performed in the evaluation of fibrotic ILD?
Transbronchial biopsy (TBBx) provides small (∼1-3mmCitation2) samples of parenchymal lung tissue taken through the working channel of a bronchoscope. TBBx may have a role in the evaluation of some nonfibrotic ILDs including sarcoidosis;Citation41,Citation42 however, its role in the evaluation of fibrotic ILD is less clear. In one study, 3/33 patients (9%) with bird fancier's disease had the classic triad of HP findings on TBBx (lymphocytic-histiocytic infiltrate, poorly formed granuloma, bronchiolitis obliterans), although poorly formed granulomas were identified in 21% of cases.Citation32 TBBx is less helpful in diagnosing other fibrotic ILDs, and can also provide misleading information. For example, TBBx showed an NSIP pattern in 9/18 patients (50%) with a usual interstitial pneumonia (UIP) pattern proven by surgical lung biopsy.Citation43
The role of TBBx in fibrotic ILD is further limited by the risk of significant bleeding (1%-4% of cases) and pneumothorax (9%), with half of the latter requiring chest tube drainage.Citation44-50 Based on the limited diagnostic utility and potential risks, a previous consensus statement recommended against TBBx in patients with suspected IPF,Citation3 suggesting that it primarily be used to diagnose infection or malignancy in appropriate settings.
Surgical lung biopsy
When should surgical lung biopsy be performed in the evaluation of fibrotic ILD?
Surgical lung biopsy (SLBx) is performed using single-lung ventilation under general anesthesia. SLBx was historically performed via open thoracotomy (ie, “open lung biopsy”), but is now typically performed using video-assisted thoracoscopic surgery (VATS), a technique associated with lower perioperative morbidity and mortality.Citation51,Citation52
SLBx for histopathologic sampling in the evaluation of fibrotic ILD should be considered when a definitive ILD diagnosis cannot be established using noninvasive data. A recent systematic review of 2148 patients from 23 studies showed SLBx had a median diagnostic yield of 95% (range 42–100) and changed management in 42%-90% of cases in the 8 studies that reported longitudinal data.Citation52 Two studies reported yields below 70%, although these outliers were limited by the lack of SLBx review in a MDD.Citation53,Citation54
The yield of SLBx can be optimized through several means.Citation3 Biopsies should have a depth ≥2 cm and maximum diameter ≥4 cm when gently inflated post-resection.Citation3,Citation55 Clinicians should clarify the optimal sampling locations with the surgeon as yield is highest for biopsies taken from more involved areas on HRCT, recognizing that areas with severe fibrosis and honeycombing frequently show nonspecific findings and should therefore be avoided.Citation56 Approximately 20% of patients have discordant pathology patterns (eg., UIP in one lobe and NSIP in another lobe),Citation55,Citation57 indicating the importance of sampling at least 2 lobes.Citation3,Citation55,Citation58 In two previous studies, the clinical behavior and prognosis of patients with discordant NSIP and UIP findings were similar to patients with UIP on both biopsies, emphasizing the need to identify a UIP pattern when present.Citation55,Citation57
SLBx for histopathologic sampling in the evaluation of fibrotic ILD should be pursued following a detailed discussion with the patient regarding potential benefits and risks. A recent systematic review and meta-analysis of 1319 patients from 16 studies showed pooled 30- and 90-day post-operative mortality of 2.2% (95%CI 1.0-4.0) and 3.4% (95%CI 1.8-5.5), respectively;Citation52 however, there was significant heterogeneity among studies. A recent retrospective analysis of over 32 000 patients undergoing SLBx showed an in-hospital mortality of 6.4% with significantly higher risk for nonelective compared to elective procedures (16.0% vs. 1.7%). SLBx has been associated with a very low risk of mortality in some cohorts, seemingly related to appropriate patient selection.Citation59 Nonfatal post-operative complications are reported in 8.4%-56% of patients undergoing SLBx, including acute exacerbation of ILD; pneumonia; pleural effusion; chronic chest pain; prolonged air leak; post-operative need for mechanical ventilation; and readmission to hospital within 1 month of discharge.Citation40,Citation58,Citation60-62 Common risk factors and relative contraindications to SLBx are listed in .Citation52,Citation59,Citation62
Table 1. Relative contraindications to surgical lung biopsy.
Transbronchial lung cryobiopsy
When should transbronchial lung cryobiopsy be performed in the evaluation of fibrotic ILD?
Transbronchial lung cryobiopsy (TBLC) has been proposed as a histopathologic sampling technique in the evaluation of ILD that potentially provides a balance between the higher yield of SLBx and the lower complication rate of TBBx. TBLC is based on the Joule-Thompson effect, whereby a compressed gas released at high flow expands quickly, leading to a very low temperature at the tip of a probe passed through the working channel of a bronchoscope.Citation63 This rapid freezing preserves lung architecture to provide samples with a cross-sectional area of 4–64 mm2,Citation64,Citation65 compared to ∼1-3 mm2 in TBBx.Citation64,Citation66 Both cryoprobe and bronchoscope must be removed simultaneously as each biopsy exceeds the size of the bronchoscope's working channel and patients typically undergo endotracheal intubation for the procedure.
The diagnostic yield and complication rates of TBLC for ILD have been reported in 10 publications from 8 distinct cohorts,Citation64–74 although there was significant heterogeneity among studies (eg, patient population, method of sedation, flexible vs. rigid bronchoscope, method of determining the diagnosis). The reported diagnostic yield ranges from 51%-98% when TBLC is guided by HRCT and real-time fluoroscopy, varying by whether the diagnostic yield is reported based on pathological findings assessed in isolation or whether biopsies were reviewed in a MDD. In the only prospective randomized controlled trial, 77 patients with suspected acute or chronic ILD were randomized to either conventional TBBx or TBLC.Citation66 The TBLC group had a higher percentage of definitive histologic pattern established compared to TBBx (74.4% vs. 34.1%) and a higher percentage of confident MDD diagnoses (51.4% vs. 29.1%). Confident MDD diagnoses were achieved less often than confident histologic diagnoses, which is likely due to discrepancies among clinical, radiological and histological findings, indicating the importance for review of TBLC findings in the context of a MDD. There were no MDD diagnoses of IPF in this study, and it is unknown whether IPF was under-represented in this cohort, or whether the absence of IPF reflects a lack of sensitivity of TBLC for a UIP pattern. Conversely, IPF was the most frequent MDD diagnosis after TBLC in a recent retrospective study of TBLC and SLBx in fibrotic ILD.Citation70
There is significant heterogeneity in the reported complications following TBLC with pneumothorax rates ranging from 0%-33%Citation64,Citation67,Citation70,Citation73 and moderate to severe bleeding ranging from 0%-78%.Citation66,Citation67,Citation69,Citation71 It is unclear whether this heterogeneity reflects differences in study populations, reporting standards, procedural techniques and/or operator expertise. A prospective study is needed to directly compare the yield and safety of TBLC to SLBx in the evaluation of fibrotic ILD prior to widespread adoption of TBLC into clinical practice.
Multidisciplinary discussion
Is multidisciplinary discussion required prior to initiation of antifibrotic or immunosuppressive pharmacotherapies in patients with fibrotic ILD?
MDD is the interactive dynamic dialogue among clinicians, radiologists and pathologists with expertise in ILD, with the goal of achieving a consensus diagnosis in patients with suspected ILD.Citation13 MDD was first recommended in the 2002 consensus statement describing the classification of the IIPsCitation3 and has since been considered the gold standard for ILD diagnosis.Citation3,Citation4 MDD improves diagnostic accuracy (measured as inter-observer agreement) in both community and academic settings compared to diagnoses made independently.Citation13 Inter-observer agreement is higher among academic compared to community physicians, with the latter group more likely to assign a diagnosis of IPF,Citation14 illustrating the importance of diagnosing ILD using a MDD among ILD experts. Inter-MDD agreement is acceptable overall for establishing a specific ILD diagnosis, and good for IPF.Citation75 MDD leads to greater confidence in a diagnosis of IPF, compared to a diagnosis established by individual clinicians or radiologists and may help avoid an unnecessary surgical lung biopsy in some patients.
An accurate ILD diagnosis is particularly important in the context of IPF-specific medications that are not indicated in other fibrotic ILDsCitation76,Citation77 and considering the harm caused by inappropriate use of immunosuppressive medications in IPF.Citation20 The strong rationale and improved diagnostic accuracy have prompted multiple groups to recommend that ILD diagnoses be established via MDD whenever possible,Citation2-4 including review of at least clinical, laboratory and radiological data. Patients that demonstrate unanticipated clinical changes or that subsequently undergo SLBx should be re-presented, illustrating the iterative nature of a MDD. A substantial proportion of ILD patients cannot be confidently classified with a specific ILD diagnosis despite detailed review of all available data;Citation78 however, a MDD can often help guide management decisions even in the absence of a confident ILD diagnosis. Patients with “unclassifiable ILD” should periodically be re-evaluated by an ILD expert and MDD group to evaluate new findings that may suggest a specific underlying diagnosis. This general approach to ILD diagnosis is summarized in , highlighting the iterative nature of the MDD with a proposed schematic framework to the evaluation of fibrotic ILD.
Figure 1. Approach to the evaluation of fibrotic interstitial lung disease. Abbreviations: CTD, connective tissue disease; HRCT, high resolution computed tomography; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; MDD, multidisciplinary discussion; UIP, usual interstitial pneumonia.
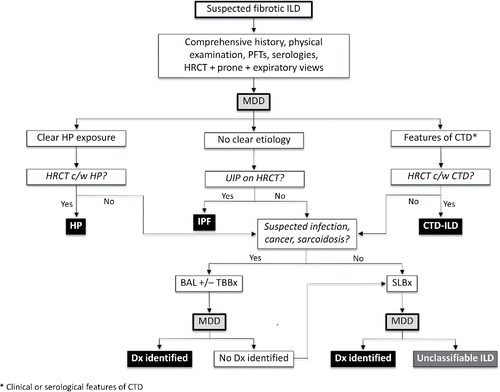
Access to a formal MDD is frequently restricted by time constraints, geographic barriers, lack of remuneration for participating physicians and the limited number of experienced ILD centres. Some Canadian ILD centres offer review of ILD cases through tele-health or other similar means, although it is unknown how this process compares to a standard MDD. Despite these potential barriers, it is appropriate to review ILD patients in a MDD setting whenever possible before initiating disease-specific pharmacotherapies, considering the costs and potential adverse effects of these medications.
Knowledge transfer and tools for practice
| • | The present document is available for download at www.respiratoryguidelines.ca and www.tandfonline.com. | ||||
| • | A slide deck for teaching and self-learning as well as a handout for health care professionals and students is available at www.respiratoryguidelines.ca. | ||||
| • | The CTS Clinical Assembly on Interstitial Lung Disease welcomes the opportunity to partner with other organizations and stakeholders in the development of educational tools and resources that support the implementation of the key messages described herein, with various targeted groups. | ||||
| • | Successful implementation of the clinical guidance in this position paper is integral to its aims, and this may be monitored through surveys of document end-users, as well as administrative database analyses of diagnostic testing in the evaluation of patients with suspected fibrotic ILD. | ||||
Conclusions and future directions
Fibrotic ILD represents a large heterogeneous group of disorders that are challenging to diagnose. The evaluation of patients with fibrotic ILD should include a thorough history, physical examination, basic serological testing, pulmonary function tests and HRCT of the chest. Specific serologic testing, BAL, TBBx and SLBx may be informative in select patients depending on the clinical presentation and diagnoses under consideration. Where feasible, fibrotic ILD cases should be reviewed in a MDD that includes clinicians, radiologists and pathologists with expertise in ILD. This comprehensive patient evaluation aims to provide adequate clinical data to enable accurate diagnoses, consistent with disease-specific diagnostic criteria and a standardized ontological framework.Citation79 There is a relative paucity of data to guide these recommendations in the broad category of fibrotic ILD and the key messages in this document represent the consensus opinion of ILD experts from a diversity of disciplines across Canada. In certain clinical contexts, there may be barriers to implementing these recommendations, including limited clinical experience or access to specific clinical resources. Future work should identify these barriers while testing the efficacy of specific interventions to improve the implementation of evidence-based recommendations. Further research is needed for each modality previously outlined to inform their performance characteristics and clinical utility in the evaluation of fibrotic ILD. We also note that we did not consider the views and preferences of the target population (patients with fibrotic ILD) in this consensus statement development process, and these valuable insights should be sought in future position statements or clinical practice guidelines.
Declaration of interest
The CTS Clinical Assembly on Interstitial Lung Disease is accountable to the CTS Respiratory Guidelines Committee and the CTS Board of Directors. The CTS Clinical Assembly on Interstitial Lung Disease is functionally and editorially independent from any funding sources of the CTS and does not receive any direct funding from external sources. The CTS receives unrestricted grants that are combined into a central operating account to facilitate the knowledge translation activities of the CTS Clinical Assemblies. No funders played a role in the collection, review, analysis or interpretation of the scientific literature or in any decisions regarding the key messages presented in this document.
Members of the CTS Clinical Assembly on Interstitial Lung Disease declared potential conflicts of interest at the time of appointment and these were updated throughout the process in accordance with the CTS Conflict of Interest Disclosure Policy. Individual member conflict of interest statements are posted at www.respiratoryguidelines.ca.
Acknowledgments
The authors would like to thank the CTS and the Executive Committee of the Canadian Respiratory Guidelines Committee for guidance throughout the process: Louis-Philippe Boulet, Samir Gupta and Christopher Licskai.
We would also like to acknowledge with deep appreciation our Expert Peer Reviewers who made valuable contributions to the manuscript: Harold R. Collard, MD, University of California, San Francisco, San Francisco, California; Vincent Cottin, MD, PhD, Centre Hospitalier Universitaire de Lyon, Groupement Hospitalier Est – Hôpital Louis Pradel, Lyon, France; and André Cantin, MD, Université de Sherbrooke, Faculté de Médecine et des Sciences de la Santé, Sherbrooke, Québec.
References
- Schwarz MI, King TE, Jr. Interstitial Lung Disease. 5 ed. Shelton, CT: People's Medical Publishing House; 2011.
- Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183(6):788–824.
- American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med. 2002;165(2):277–304.
- Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188(6):733–748.
- Weatherald JM, R; Fell C. D. Trends in diagnosis and management of idiopathic pulmonary fibrosis in Canada. Can J Respir Crit Care Sleep Med. 2017:1–6.
- Cox G, Ryerson C, Wilcox P, eds. Survey of management of IPF in Canada. International Colloquium on Airway and Lung Fibrosis; 2014; Mont Tremblant, QC.
- Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. Cmaj. 2010;182(18):E839–E842.
- Webb WR, Muller NL, Naidich DP. High-Resolution CT of the Lung. 5th ed. Kluwer W, ed.; Philadelphia, PA: Wolters Kluwer Health; 2014.
- Hunninghake GW, Zimmerman MB, Schwartz DA, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164(2):193–a96.
- Collins CD, Wells AU, Hansell DM, et al. Observer variation in pattern type and extent of disease in fibrosing alveolitis on thin section computed tomography and chest radiography. Clin Radiol. 1994;49(4):236–240.
- Daniloff EM, Lynch DA, Bartelson BB, Newell JD, Jr., Bernstein SM, Newman LS. Observer variation and relationship of computed tomography to severity of beryllium disease. Am J Respir Crit Care Med. 1997;155(6):2047–2056.
- Kazerooni EA, Martinez FJ, Flint A, et al. Thin-section CT obtained at 10-mm increments versus limited three-level thin-section CT for idiopathic pulmonary fibrosis: correlation with pathologic scoring. AJR Am J Roentgenol. 1997;169(4):977–983.
- Flaherty KR, King TE, Jr., Raghu G, et al. Idiopathic interstitial pneumonia: what is the effect of a multidisciplinary approach to diagnosis? Am J Respir Crit Care Med. 2004;170(8):904–910.
- Flaherty KR, Andrei AC, King TE, Jr., et al. Idiopathic interstitial pneumonia: do community and academic physicians agree on diagnosis? Am J Respir Crit Care Med. 2007;175(10):1054–1060.
- Rubio-Rivas M, Royo C, Simeon CP, Corbella X, Fonollosa V. Mortality and survival in systemic sclerosis: systematic review and meta-analysis. Semin Arthritis Rheum. 2014;44(2):208–219.
- Young A, Koduri G, Batley M, et al. Mortality in rheumatoid arthritis. Increased in the early course of disease, in ischaemic heart disease and in pulmonary fibrosis. Rheumatology (Oxford). 2007;46(2):350–357.
- Antoniou KM, Margaritopoulos G, Economidou F, Siafakas NM. Pivotal clinical dilemmas in collagen vascular diseases associated with interstitial lung involvement. Eur Respir J. 2009;33(4):882–896.
- Fischer A, Richeldi L. Cross-disciplinary collaboration in connective tissue disease-related lung disease. Semin Respir Crit Care Med. 2014;35(2):159–165.
- Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015; 46(4):976–987.
- Idiopathic Pulmonary Fibrosis Clinical Research N, Raghu G, Anstrom KJ, King TE, Jr., Lasky JA, Martinez FJ. Prednisone, azathioprine, and N-acetylcysteine for pulmonary fibrosis. N Engl J Med. 2012;366(21):1968–1977.
- Fischer A, Brown KK, Du Bois RM, et al. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol. 2013;40(5):640–646.
- Morisset J, Johannson KA, Vittinghoff E, et al. Use of Mycophenolate Mofetil or Azathioprine for the Management of Chronic Hypersensitivity Pneumonitis. Chest. 2017;151(3):619–625.
- Tashkin DP, Roth MD, Clements PJ, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4(9):708–719.
- Mittoo S, Gelber AC, Christopher-Stine L, Horton MR, Lechtzin N, Danoff SK. Ascertainment of collagen vascular disease in patients presenting with interstitial lung disease. Respir Med. 2009;103(8):1152–1158.
- Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis. 2010;69(8):1420–4122.
- Lee JS, Kim EJ, Lynch KL, et al. Prevalence and clinical significance of circulating autoantibodies in idiopathic pulmonary fibrosis. Respir Med. 2013;107(2):249–255.
- Fischer A, Pfalzgraf FJ, Feghali-Bostwick CA, et al. Anti-th/to-positivity in a cohort of patients with idiopathic pulmonary fibrosis. J Rheumatol. 2006;33(8):1600–1605.
- Vij R, Noth I, Strek ME. Autoimmune-featured interstitial lung disease: a distinct entity. Chest. 2011;140(5):1292–1299.
- Fell CD, Martinez FJ, Liu LX, et al. Clinical predictors of a diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2010;181(8):832–837.
- Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185(9):1004–1014.
- Ohshimo S, Bonella F, Cui A, et al. Significance of bronchoalveolar lavage for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179(11):1043–1047.
- Morell F, Roger A, Reyes L, Cruz MJ, Murio C, Munoz X. Bird fancier's lung: a series of 86 patients. Medicine (Baltimore). 2008;87(2):110–130.
- Vourlekis JS, Schwarz MI, Cherniack RM, et al. The effect of pulmonary fibrosis on survival in patients with hypersensitivity pneumonitis. Am J Med. 2004;116(10):662–668.
- Lee W, Chung WS, Hong KS, Huh J. Clinical usefulness of bronchoalveolar lavage cellular analysis and lymphocyte subsets in diffuse interstitial lung diseases. Ann Lab Med. 2015;35(2):220–225.
- Capelozzi VL, Faludi EP, Balthazar AB, Fernezlian Sde M, Filho JV, Parra ER. Bronchoalveolar lavage improves diagnostic accuracy in patients with diffuse lung disease. Diagn Cytopathol. 2013;41(1):1–8.
- Hiwatari N, Shimura S, Takishima T, Shirato K. Bronchoalveolar lavage as a possible cause of acute exacerbation in idiopathic pulmonary fibrosis patients. Tohoku J Exp Med. 1994;174(4):379–386.
- Kim DS, Park JH, Park BK, Lee JS, Nicholson AG, Colby T. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J. 2006;27(1):143–150.
- Sakamoto K, Taniguchi H, Kondoh Y, et al. Acute exacerbation of IPF following diagnostic bronchoalveolar lavage procedures. Respir Med. 2012;106(3):436–442.
- Simon-Blancal V, Freynet O, Nunes H, et al. Acute exacerbation of idiopathic pulmonary fibrosis: outcome and prognostic factors. Respiration. 2012;83(1):28–35.
- Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37(2):356–363.
- Gupta D, Dadhwal DS, Agarwal R, Gupta N, Bal A, Aggarwal AN. Endobronchial ultrasound-guided transbronchial needle aspiration vs conventional transbronchial needle aspiration in the diagnosis of sarcoidosis. Chest. 2014;146(3):547–556.
- Torre O, Harari S. The diagnosis of cystic lung diseases: a role for bronchoalveolar lavage and transbronchial biopsy? Respir Med. 2010;104 Suppl 1:S81–S85.
- Berbescu EA, Katzenstein AL, Snow JL, Zisman DA. Transbronchial biopsy in usual interstitial pneumonia. Chest. 2006;129(5):1126–1131.
- Du Rand IA, Blaikley J, Booton R, et al. British Thoracic Society guideline for diagnostic flexible bronchoscopy in adults: accredited by NICE. Thorax. 2013;68 Suppl 1:i1–i44.
- Zavala DC. Diagnostic fiberoptic bronchoscopy: Techniques and results of biopsy in 600 patients. Chest. 1975;68(1):12–19.
- Zavala DC. Pulmonary hemorrhage in fiberoptic transbronchial biopsy. Chest. 1976;70(5):584–588.
- Herf SM, Suratt PM, Arora NS. Deaths and complications associated with transbronchial lung biopsy. Am Rev Respir Dis. 1977;115(4):708–711.
- Andersen HA. Transbronchoscopic lung biopsy for diffuse pulmonary diseases. Results in 939 patients. Chest. 1978;73(5 Suppl):734–736.
- Descombes E, Gardiol D, Leuenberger P. Transbronchial lung biopsy: an analysis of 530 cases with reference to the number of samples. Monaldi Arch Chest Dis. 1997;52(4):324–329.
- Mitchell DM, Emerson CJ, Collins JV, Stableforth DE. Transbronchial lung biopsy with the fibreoptic bronchoscope: analysis of results in 433 patients. Br J Dis Chest. 1981;75(3):258–262.
- Nguyen W, Meyer KC. Surgical lung biopsy for the diagnosis of interstitial lung disease: a review of the literature and recommendations for optimizing safety and efficacy. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30(1):3–16.
- Han Q, Luo Q, Xie JX, et al. Diagnostic yield and postoperative mortality associated with surgical lung biopsy for evaluation of interstitial lung diseases: A systematic review and meta-analysis. J Thorac Cardiovasc Surg. 2015;149(5):1394–1401.e1.
- Blackhall V, Asif M, Renieri A, et al. The role of surgical lung biopsy in the management of interstitial lung disease: experience from a single institution in the UK. Interact Cardiovasc Thorac Surg. 2013;17(2):253–257.
- Qureshi RA, Ahmed TA, Grayson AD, Soorae AS, Drakeley MJ, Page RD. Does lung biopsy help patients with interstitial lung disease? Eur J Cardiothorac Surg. 2002;21(4):621–626; discussion 6.
- Flaherty KR, Travis WD, Colby TV, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med. 2001;164(9):1722–1727.
- Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax. 2008;63 Suppl 5:v1–58.
- Monaghan H, Wells AU, Colby TV, du Bois RM, Hansell DM, Nicholson AG. Prognostic implications of histologic patterns in multiple surgical lung biopsies from patients with idiopathic interstitial pneumonias. Chest. 2004;125(2):522–526.
- Lettieri CJ, Veerappan GR, Helman DL, Mulligan CR, Shorr AF. Outcomes and safety of surgical lung biopsy for interstitial lung disease. Chest. 2005;127(5):1600–1605.
- Raj R, Brown KK. Mortality Related to Surgical Lung Biopsy in Patients with Interstitial Lung Disease. The Devil Is in the Denominator. Am J Respir Crit Care Med. 2016;193(10):1082–1084.
- Kreider ME, Hansen-Flaschen J, Ahmad NN, et al. Complications of video-assisted thoracoscopic lung biopsy in patients with interstitial lung disease. Ann Thorac Surg. 2007;83(3):1140–1144.
- Carrillo G, Estrada A, Pedroza J, et al. Preoperative risk factors associated with mortality in lung biopsy patients with interstitial lung disease. J Invest Surg. 2005;18(1):39–45.
- Hutchinson JP, Fogarty AW, McKeever TM, Hubbard RB. In-hospital mortality following surgical lung biopsy for interstitial lung disease in the USA: 2000–2011. Am J Respir Crit Care Med. 2016;193(10):1161–1167.
- Poletti V, Casoni GL, Gurioli C, Ryu JH, Tomassetti S. Lung cryobiopsies: a paradigm shift in diagnostic bronchoscopy? Respirology. 2014;19(5):645–654.
- Kropski JA, Pritchett JM, Mason WR, et al. Bronchoscopic cryobiopsy for the diagnosis of diffuse parenchymal lung disease. PLoS One. 2013;8(11):e78674.
- Hernandez-Gonzalez F, Lucena CM, Ramirez J, et al. Cryobiopsy in the diagnosis of diffuse interstitial lung disease: yield and cost-effectiveness analysis. Arch Bronconeumol. 2015;51(6):261–267.
- Pajares V, Puzo C, Castillo D, et al. Diagnostic yield of transbronchial cryobiopsy in interstitial lung disease: a randomized trial. Respirology. 2014;19(6):900–906.
- Griff S, Schonfeld N, Ammenwerth W, et al. Diagnostic yield of transbronchial cryobiopsy in non-neoplastic lung disease: a retrospective case series. BMC Pulm Med. 2014;14:171.
- Fruchter O, Fridel L, El Raouf BA, Abdel-Rahman N, Rosengarten D, Kramer MR. Histological diagnosis of interstitial lung diseases by cryo-transbronchial biopsy. Respirology. 2014;19(5):683–688.
- Hagmeyer L, Theegarten D, Wohlschlager J, et al. The role of transbronchial cryobiopsy and surgical lung biopsy in the diagnostic algorithm of interstitial lung disease. Clin Respir J. 2016;10(5):589–595.
- Tomassetti S, Wells AU, Costabel U, et al. Bronchoscopic lung cryobiopsy increases diagnostic confidence in the multidisciplinary diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2016; 193(7):745–752.
- Ravaglia C, Bonifazi M, Wells AU, et al. Safety and diagnostic yield of transbronchial lung cryobiopsy in diffuse parenchymal lung diseases: a comparative study versus video-assisted thoracoscopic lung biopsy and a systematic review of the literature. Respiration. 2016;91(3):215–227.
- Casoni GL, Tomassetti S, Cavazza A, et al. Transbronchial lung cryobiopsy in the diagnosis of fibrotic interstitial lung diseases. PLoS One. 2014;9(2):e86716.
- Johannson KA, Marcoux VS, Ronksley PE, Ryerson CJ. Diagnostic yield and complications of transbronchial lung cryobiopsy for interstitial lung disease. A sstematic review and metaanalysis. Ann Am Thorac Soc. 2016;13(10):1828–1838.
- Ussavarungsi K, Kern RM, Roden AC, Ryu JH, Edell ES. Transbronchial cryobiopsy in diffuse parenchymal lung disease: retrospective analysis of 74 cases. Chest. 2017;151(2):400–408.
- Walsh SL, Calandriello L, Sverzellati N, Wells AU, Hansell DM. Interobserver agreement for the ATS/ERS/JRS/ALAT criteria for a UIP pattern on CT. Thorax. 2016;71(1):45–51.
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2071–2082.
- King TE, Jr., Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370(22):2083–2092.
- Ryerson CJ, Urbania TH, Richeldi L, et al. Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J. 2013;42(3):750–757.
- Ryerson CJ, Corte TJ, Lee JS, et al. A standardized diagnostic ontology for fibrotic interstitial lung disease: an international working group perspective. Am J Respir Crit Care Med. 2017. doi:10.1164/rccm.201702-0400PP. [Epub ahead of print]
