ABSTRACT
OBJECTIVE: The aim of this study is to compare the clinical characteristics, technology and outcomes between infants and older children using long-term NIV.
METHODS: In this 10-year retrospective review, 120 infants were matched to 240 older children in a 1:2 ratio based on sex and closest date of NIV initiation. Medical charts and sleep laboratory records were reviewed to extract demographic, NIV technology, polysomnography and clinic outcome data.
RESULTS: The results demonstrate a greater proportion of cardiorespiratory disease [16% vs. 6%, OR 3.04 (95% CI 1.47 to 6.31)] and a lower proportion of upper airway disorders [46% vs. 60%, OR 0.56 (95% CI 0.36 to 0.87)] in infants compared to older children. Infants had more comorbidities [4.0 (IQR 3.0) vs. 3.0 (IQR 2.0), p < 0.001] and used more additional technology [36% vs. 16%, OR 2.88 (1.73 to 4.78)] than older children. Improvements in respiratory parameters and NIV adherence were similar between groups. While NIV clinic discharge rates were similar, the reason for discharge differed with infants primarily ceasing NIV due to improvements in the underlying disease condition [42% vs. 30%, OR 2.04 (1.04 to 4.03)] or switching to invasive mechanical ventilation [10% vs. 1%, OR 11.43 (1.34 to 97.47)] while older children transferred to other services [9% vs. 35%, OR 0.17 (0.06 to 0.46)].
CONCLUSIONS: These results suggest infants are a distinct group with respect to NIV therapy and support the need for modifications in the approach to long-term NIV use in infants.
RÉSUMÉ
OBJECTIF: Le but de cette étude est de comparer les caractéristiques, la technologie et les résultats obtenus pour des nourrissons ayant recours à la ventilation non invasive (VNI) à long terme à ceux obtenus pour des enfants plus âgés.
MÉTHODES: Dans cet examen rétrospectif portant sur 10 ans, 120 nourrissons ont été jumelés à 240 enfants plus âgés selon un ratio de 1 : 2 en fonction de leur sexe et de la date la plus récente à laquelle la VNI a débuté. Les dossiers médicaux et les registres de laboratoires du sommeil ont été examinés pour en tirer des données démographiques, ainsi que des données relatives à la technologie de VIN, à la polysomnographie et aux résultats cliniques.
RéSULTATS: Les résultats démontrent une plus grande proportion de maladies cardiorespiratoires [16 % comparativement à 6 % RC 3,04 (IC 95 % 1,47 à 6,31)] et une plus faible proportion de maladies des voies aÉriennes supérieures [46 % comparativement à 60 %, RC 0,56 % (IC 95 % 0,36 à 0,87)] chez les nourrissons comparativement aux enfants plus âgés. Les nourrissons avaient davantage de comorbidités [4,0 (IIQ 3,0) comparativement à 3,0 (IIQ 2,0), p < 0,001] et faisaient davantage usage de technologie supplémentaire [36 % comparativement à 16 %, RC 2,88 (1,73 à 4,78)] que les enfants plus âgés. Les améliorations dans les paramètres respiratoires et l'adhésion à la VIN étaient similaires dans les deux groupes. Bien que les taux de sortie de la clinique étaient similaires, la raison pour la sortie différait : les nourrissons cessaient la VIN principalement en raison d'améliorations dans la maladie sous-jacente [42 % comparativement à 30 %, RC 2,04 (1,04 à 4,03)] ou du passage à la ventilation mécanique invasive [10 % comparativement à 1 %, RC 11,43 (1,34 à 97,47)], tandis que les enfants plus âgés étaient transférés dans d'autres services [9 % comparativement à 35 %, RC 0,17 (0,06 à 0.46)].
CONCLUSIONS: Ces résultats semblent indiquer que les nourrissons constituent un groupe distinct en ce qui a trait au traitement par VIN et viennent appuyer la nécessité d'apporter des modifications dans la façon d'aborder l'utilisation de la VIN à long terme chez les nourrissons.
Introduction
The number of children requiring long-term respiratory support has increased greatly over the last two decades,Citation1 at least in part, due to improvements in the survival of children with complex medical illness.Citation2,Citation3 In addition, there has been a shift from using long-term invasive mechanical ventilation (IMV) to noninvasive ventilation (NIV) therapies, such as continuous positive airway pressure (CPAP) and bilevel positive airway pressure (BPAP).Citation4,Citation5 The use of NIV, a method of delivering positive pressure breathing support via an interface outside the airway, has become the standard of care for providing long-term respiratory support for infants and older children with sleep and breathing disorders.Citation5,Citation6 Reasons for the shift towards NIV therapies include improvements in ventilator technology,Citation4,Citation6 avoidance of complications associated with IMVCitation7,Citation8 and a growing recognition by clinicians and families of NIV as a viable alternative respiratory therapy.Citation4–6
Although the use of NIV for all children with sleep and breathing disorders has been increasing, there is less data on its use and outcomes in infants.Citation9–11 The available data suggests that the current treatment approach to NIV therapy is similar in infants and older children. However, there are key differences in the sleep and breathing physiology in infants that may impact their response to NIV.Citation12,Citation13 For example, both total sleep duration and the proportion of total sleep time spent in rapid eye movement (REM) sleep is higher in early life and decreases across infancy and childhood.Citation12,Citation13 Arousals are common after birth and decrease across the first year of life.Citation13,Citation14 The central control of breathing is immature at birth and matures in the months following birth, resulting in an initial high variability in the respirator pattern in infants.Citation13,Citation15 Respiratory events, including both central and obstructive events, commonly occur even in otherwise healthy infants and decrease with age.Citation16,Citation17 These key differences in sleep and breathing in early life contribute to an increase in the risk for consequences of breathing disruption, and potential differences in response to respiratory support in infants compared to older children.
If the use and outcomes differ between infants and older children, a distinct approach to the use of long-term NIV for infants may be needed. Recognizing distinct benefits for long-term NIV in infants would help tailor its use as well as the support to families using this therapy. The aim of this study is to compare the clinical characteristics, technology and outcomes between infants and older children using long-term NIV. We hypothesize that the unique sleep and breathing physiology in infants will contribute to differences in the underlying disease conditions, higher levels of adherence and greater improvements in the underlying condition in infants using long-term NIV when compared to older children. Preliminary results were published in abstract form.Citation18
Materials and methods
Study design and participants
This is a 10-year retrospective study comparing long-term NIV use for infants and older children. It is part of a larger study of all 622 children started on long-term NIV in the province of Alberta from January 2005 through December 2014.Citation19 In the current study, all infants from the larger cohort were identified for inclusion. Infants were defined as age < 2 years and older children as age 2–18 years based on guidelines from the Public Health Agency of Canada.Citation20 Long-term NIV was defined as use for a minimum of three months outside of an acute care setting. Infants were matched to older children in a 1:2 ratio based on sex and the closest date of NIV initiation (within a 6 month period) to ensure contemporary comparisons. The study protocol was approved by Health Research Ethics Board for both participating institutions.
Data collection
Data were collected from the medical charts and sleep laboratory records of all children using long-term NIV at the two children's hospitals in the province of Alberta. These two institutions house the only publicly funded pediatric sleep laboratories in the province and, therefore, represent the majority, if not all, children using long-term NIV in the province. Data were collected at baseline and two follow-up times (6–12 months after initiation and the most recent clinic visit) from medical and sleep laboratory charts. Data were entered and stored in an electronic REDCap research database.Citation21
Demographics collected from medical charts included age, sex, ethnicity, primary disease condition leading to the need for long-term NIV, comorbidities (defined as any chronic diagnosis aside from the primary underlying condition) and history of previous upper airway surgery. The primary disease conditions were grouped into five broad disease categories: upper airway, central nervous system, musculoskeletal and neuromuscular, cardio-respiratory (excludes upper airway) and unclassified conditions. Data collected on NIV technology included location of NIV start, NIV type, mask type, NIV pressures, adherence rates from NIV machine downloads (average hours of NIV used per night and % of days with NIV use for > 4 hours) and use of any additional technology. Additional outcomes included continuation of NIV, clinic discharge, reasons for clinic discharge (improvements in the underlying condition, patient/family decision to stop NIV, or transfer of services including transfer to adult services, another NIV provider or out-of-province care) and mortality.
Sleep studies included diagnostic polysomnography (PSG) studies, titration studies and split-night studies (including a diagnostic and titration component) and were performed using standard laboratory procedures. Data collection of PSGs was completed by an experienced technician following standard laboratory protocols. Studies collected before 2007 were sleep staged according to Anders et alCitation22 (infants <6 months of age) or Rechtschaffen and KalesCitation23 (≥6 months of age) with scoring of respiratory variables based on the American Thoracic Society and previously published data in children.Citation24,Citation25 The guidelines of the American Academy of Sleep MedicineCitation26 were applied to scoring starting in 2007. Apnea-hypopnea index (AHI) was calculated using the number of apneas and hypopneas that occurred during sleep divided by the total sleep time (TST). Obstructive AHI (OAHI) was defined as the sum of the obstructive apneas and obstructive hypopneas divided by the TSTCitation26,Citation27; OAHI >3 events/h in infants ≤6 months and >1 event/h >6 months of age was consider abnormal. Variables collected from sleep study charts included total sleep time, sleep efficiency (%), % time spent in REM sleep, arousal index, total AHI, OAHI, central AHI, % of total sleep time spent with oxygen saturations <90% and % of total sleep time spent with transcutaneous carbon dioxide levels >50 mmHg. To compare changes in sleep and respiratory parameters over time, parameters were compared between a diagnostic and titration PSG (full or split night studies) at baseline or between a baseline diagnostic and the first titration PSG after 3 months of NIV use. A minimum total sleep time of 120 minutes was required for both study types for inclusion in the analysis.
Analysis
Analyses were performed using IBM SPSS Statistics version 24.0 (SPSS, Inc., Chicago, IL). Summary statistics were reported as median (IQR) or frequencies (n, %) as appropriate. Chi-square test or Fisher's exact test, and Mann-Whitney U test were used to compare categorical and continuous variables between groups respectively. Wilcoxon Signed Ranks test was used to compare sleep and respiratory parameters between paired diagnostic and titration PSG studies. Odds ratios with 95% confidence interval (CI) or Mann-Whitney U test statistic were calculated to determine a measure of association for two dichotomous or continuous variables respectively.
Logistic regression was used for multivariable analysis of outcomes including continuation of ventilation, or clinic discharge due to improvement of the underlying condition, patient/family decision to stop NIV, transfer of services, switch to IMV, and mortality. Variables for inclusion were determined based on mechanistic plausibility in addition to demonstration of univariable association with the outcomes; these included demographics, sleep and respiratory parameters, NIV technology, and adherence variables. The 10-year study period was divided into three equal epochs to assess timing of NIV start in the regression model. Since our study is a comparison between infants and older children, age at NIV initiation was included in all models. A p-value of <0.05 was used to support statistically significant effects.
Results
The full data set included 622 children, of which 122 (20%) were infants; 120 infants were matched to 240 older children for the purpose of this study, with insufficient appropriate matches for 2 infants. The median age of NIV initiation in infants and older children was 9.0 (IQR 12.0 months) and 108.5 (IQR 91.8 months) respectively (). The primary disease category leading to the need for long-term NIV differed between groups, with older children having more upper airway conditions and infants having more cardiopulmonary disease. Infants also had a higher number of comorbidities compared to older children.
Table 1. Comparison of baseline clinical characteristics, including respiratory parameters, for infants and older children using long-term noninvasive ventilation.
Baseline sleep and respiratory parameters also differed between infants and older children. Infants had a lower median total sleep time compared to older children. As expected, infants spent a higher proportion of total sleep time in REM sleep, and had a high total AHI, OAHI and central AHI compared to older children.Citation13 Infants also spent a higher amount of TST with oxygen saturations < 90%.
There were differences between both groups with respect to NIV technology use. Infants were more likely to start NIV in the hospital (). Infants were more likely to use a nasal interface, BPAP ventilation and require more additional technology compared to older children. Recommended CPAP pressures were lower in infants, but BPAP pressures were comparable between both groups at the baseline visit. Infants received more home-care support compared to older children [67% vs. 26%, OR: 5.92 (3.46 to 10.14)].
Table 2. Comparison of technology use for infants and older children using long-term noninvasive ventilation.
Treatment response and outcomes after starting NIV therapy showed both similarities and differences between groups. The average time between diagnostic and treatment PSGs was lower for infants compared to older children [5.0 (IQR 5.5 mo) vs 10.0 (IQR 13.5 mo), Mann-Whitney U statistic: 9366.50]. Data for sleep and respiratory parameters from a diagnostic to titration PSG showed similar improvements for infants and older children (). This included a significant decrease in the total and obstructive AHI from diagnostic to titration study in both groups (). Infants had a greater decrease in the total sleep time with oxygen saturations < 90%. Infants were less likely to have their OAHI normalize on titration PSG compared to older children [9% vs. 23%, OR: 0.33 (0.13 to 0.89)]. The average hours of NIV used per night was similar between groups for the first follow-up visit, but differed in the second visit, with infants using NIV for more hours than older children (). The percentage of days with NIV use for >4 hours/24 hours was similar for infants and older children at both of the two follow-up visits. Post-hoc analysis with subjects split into three groups (<1 y, 1–2 y, >2 y of age) showed the average hours of NIV use per night, and the percentage of days with NIV use >4 hours, were similar between all groups at the first and second follow-up visits (data not shown).
Table 3. Comparison of treatment response and outcomes for infants and older children using long-term noninvasive ventilation.
Figure 1. Change from a diagnostic to titration polysomnography (PSG) in (a) the total apnea-hypopnea index (AHI) in infants; (b) the total AHI in older children; (c) the obstructive AHI in infants; (d) the obstructive AHI in older children. There was a similar decrease in total AHI (p = 0.23) and obstructive AHI (p = 0.36) from a diagnostic to titration PSG for both infants and older children.
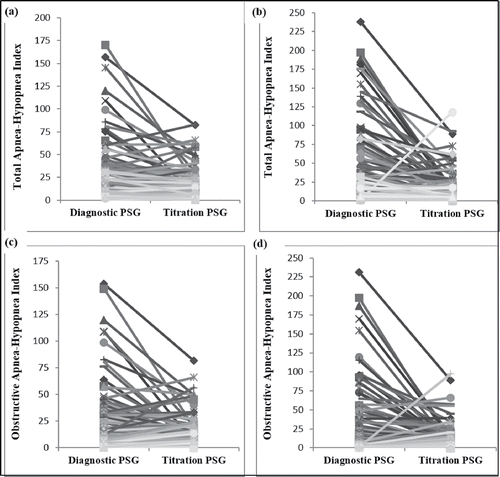
While clinic discharge rates were similar between infants and older children, the reasons for discharge differed between groups (). Infants predominantly discontinued because of improvements in the underlying condition or switch to IMV. By contrast, older children were primarily discharged from clinic because of transfer to other services. Mortality was higher in infants than older children. For those who discontinued long-term NIV during the follow-up period, duration of NIV use was 14.0 (IQR 20) months in infants and 22.5 (IQR 29) months in older children (Mann Whitney-U Statistic 5672.00, p < 0.01).
Covariates for multivariable analysis of the reasons for NIV clinic discharge and mortality included age, underlying disease category, number of comorbidities, total AHI, OAHI, central AHI, % TST with SpO2 <90%, % TST with TcCO2 > 50 mmHg, location of NIV start, NIV type, additional technology use and % of days with NIV use >4 hours (first and second follow-up visit). Increasing age and BPAP were significant predictors of continued use of NIV (). Younger age and CPAP ventilation were predictors of clinic discharge due to improvement in underlying conditions. Lower adherence at the second follow-up visit was predictive of patients/family decision to stop NIV. Increasing age and NIV initiation in a hospital setting were predictors of clinic discharge due to transfer of services. BPAP and use of a greater number of additional technologies were predictors of switch from NIV to IMV. Age was not a significant predictor of mortality; instead a higher number of comorbidities and a greater use of additional technology were independent predictors of mortality.
Table 4. Results of binomial logistic regression with continuation of noninvasive ventilation, reasons for clinic discharge, and mortality as outcome variables and population characteristics and technology use as predictors.
Discussion
To our knowledge, this is the first study comparing the use and outcomes of long-term NIV between infants and older children. Infants had more comorbidities and required more additional technology than older children, potentially making them a more medically complex group. Changes in most of the sleep and respiratory parameters were similar between groups highlighting the efficacy and viability of NIV as a method of providing long-term breathing support across childhood. Despite similar incidence of central nervous system disease and musculoskeletal/neuromuscular disease, the use of BPAP was more common in the infant population. Reasons for clinic discharge differed, demonstrating that infants may have a wider spectrum of outcomes, including improvement or switch to IMV. Somewhat surprisingly, adherence was similar for both infants and older children.
Differences in underlying clinical characteristics and technology use between infants and older children support the idea that infants represent a more medically complex group that may require separate treatment approaches. Medically complex children have been defined as those having multisystem disease, functional impairment of daily living, complex medical regiments and/or technology dependence.Citation2,Citation28 While our full cohort could be considered medically complex because of the need for long-term NIV, our results show that infants have a higher number of comorbidities and require more additional technology than older children. The impact of medical complexity on treatment strategies for children are not well understood, especially in infancy.Citation2,Citation28,Citation29 In our study, a higher number of comorbidities and greater use of additional technologies showed independent associations with mortality; this may simply be a marker of the underlying disease leading to the need for long-term NIV use. Further work is needed to determine appropriate treatment strategies to improve outcomes of long-term NIV use in infants which may include multidisciplinary care for high risk infants.
Improvements in sleep and respiratory parameters post-NIV initiation demonstrate that NIV is an effective therapy for improving sleep and breathing parameters in both infants and older children. Complications of disrupted sleep and breathing have been linked to abnormal growth, failure to thrive and cognitive impairment in infants;Citation30,Citation31 and cardiovascular,Citation32,Citation33 metabolicCitation34,Citation35 and adverse neurobehavioral outcomes in older children.Citation36,Citation37 Our analyses demonstrated improvements in sleep and respiratory parameters after starting NIV therapy, such as a higher percentage of REM sleep, decrease in the total and obstructive AHI and less total sleep time with oxygen saturations below 90%. These results are similar to those described in other infant studies reporting on improvement in PSG parameters with NIV for infants with obstructive sleep apnea and support benefit for NIV in other airway disorders.Citation38 With limited information on the impact of NIV on sleep and respiratory parameters outside upper airway disorders, additional work is needed to understand the relationship between improvements in sleep and respiratory parameters and subsequent health outcomes in other underlying disease categories for both infants and older children.
Although the most common type of NIV used in both groups was CPAP, more infants were using BPAP compared to older children. CPAP is commonly used in conditions where upper airway obstruction is present, while BPAP is beneficial for disorders resulting in abnormal central ventilatory drive or muscle weakness as it can help unload respiratory muscles and improve alveolar ventilation.Citation39,Citation40 Since upper airway disorders occurred most frequently for both age groups in our population, the higher rates of CPAP use seems appropriate. With the incidence of CNS and NMD being similar for both infants and older children, it is interesting that the rates of BPAP use were significantly higher in infants. Lack of infant-specific guidelines around the use of long-term NIV may be a factor for clinicians deciding whether to initiate an infant on CPAP or BPAP. More infants start NIV in an acute care setting without a prior PSG and, therefore, may be initiated and subsequently continued on BPAP therapy after being transferred home. Additionally, BPAP, but not CPAP, is funded by the provincial health system in Alberta, suggesting funding could be a factor influencing treatment decisions. Infant-specific guidelines around the use of long-term NIV would provide a standard for decision making.
Contrary to our hypothesis, the adherence was similar between infants and older children, despite expected longer sleep time and, therefore, longer opportunity to use NIV in infants. Previous studies have established that adherence is a common problem with children using NIV therapy;Citation41–43 however, there are few studies focusing on challenges to NIV adherence in infants.Citation44 Removal of NIV masks during sleep has remained a problem in the older child and adults; however, this problem should be less in infants as many infants will lack the manual dexterity to remove the NIV mask. This should, theoretically, improve the total time infants are using NIV, and, therefore, adherence measurement. It is important moving forward to work with parents/caregivers and children to understand barriers to adherence to help improve NIV use and resulting outcomes in both the infant and older child population.
There were some limitations to our study that must be acknowledged. As this was a retrospective chart review, information was collected from the medical charts and, therefore, we were limited in the available outcome data. We chose to limit outcomes to those treatment responses as measured by polysomnography, adherence from NIV machine downloads and clinic discharge as objective outcomes. Infants and children who started on NIV near the end of our data collection period had a shorter length of follow-up so had less time to experience outcomes. Lastly, infants were defined as age 0–2 years based on a previously established definition.Citation20 This cut-off may not reflect an important physiological marker and, therefore, may not represent the ideal cut-off-point to define important changes for long-term NIV use.
Conclusions
Our results demonstrate that infants represent a distinct group within the overall pediatric NIV population. Infants differed from older children with respect to the underlying disease category necessitating NIV. Infants had more comorbidities, required more additional technology and used more BPAP than older children. While infants primarily discharged from NIV clinic because of improvements in underlying conditions, older children were mostly transferred to other services. Based on differences in underlying population characteristics, NIV technology and outcomes on NIV therapy, it is reasonable to conclude that infants represent a distinct group within the overall pediatric NIV cohort. These differences suggest a need for infant-specific treatment strategies and guidelines around the use of long-term NIV.
Declaration of interest
The authors have no potential conflicts of interest to declare.
Acknowledgments
The authors would like to thank Dr. Maryna Yaskina (Women and Children's Health Research Institute, University of Alberta, Edmonton, AB, Canada) for her guidance and expertise in statistical analysis. Preliminary results for this manuscript were presented at the American Thoracic Society Meeting 2017, Washington, DC, USA.
Additional information
Funding
References
- Castro-Codesal ML, Dehaan K, Featherstone R, et al. Long-term non-invasive ventilation therapies in children: A scoping review. Sleep Med Rev. 2018;37:148–58. doi:10.1016/j.smrv.2017.02.005. PMID:28410811.
- Cohen E, Kuo DZ, Agrawal R, et al. Children with medical complexity: an emerging population for clinical and research initiatives. Pediatrics. 2011;127:529–38. doi:10.1542/peds.2010-0910. PMID:21339266.
- Edwards EA, Hsiao K, Nixon GM. Paediatric home ventilatory support: the Auckland experience. J Paediatr Child Health. 2005;41:652–8. doi:10.1111/j.1440-1754.2005.00753.x. PMID:16398869.
- Chatwin M, Tan HL, Bush A, Rosenthal M, Simonds AK. Long term non-invasive ventilation in children: impact on survival and transition to adult care. PLoS ONE [Electronic Resource]. 2015;10:e0125839. doi:10.1371/journal.pone.0125839.
- McDougall CM, Adderley RJ, Wensley DF, Seear MD. Long-term ventilation in children: longitudinal trends and outcomes. Arch Dis Child. 2013;98:660–5. doi:10.1136/archdischild-2012-303062. PMID:23838128.
- Amin R, Sayal P, Syed F, et al. Pediatric long-term home mechanical ventilation: twenty years of follow-up from one Canadian center. Pediatr Pulmonol. 2014;49:816–24. doi:10.1002/ppul.22868. PMID:24000198.
- Amin RS, Fitton CM. Tracheostomy and home ventilation in children. Semin Neonatol. 2003;8:127–35. doi:10.1016/S1084-2756(02)00220-8. PMID:15001149.
- Brochard L. Mechanical ventilation: invasive versus non-invasive. European Respiratory Journal. 2003;22:31s–7s. doi:10.1183/09031936.03.00050403.
- Castro-Codesal ML, Dehaan K, Featherstone R, et al. Long-term non-invasive ventilation therapies in children: A scoping review. Sleep Med Rev. 2018;37:148–158. doi:10.1016/j.smrv.2017.02.005. PMID:28410811.
- Kherani T, Sayal A, Al-Saleh S, Sayal P, Amin R. A comparison of invasive and noninvasive ventilation in children less than 1 year of age: A long-term follow-up study. Pediatr Pulmonol. 2016;51:189–95. doi:10.1002/ppul.23229. PMID:26079291.
- Robison JG, Wilson C, Otteson TD, Chakravorty SS, Mehta DK. Analysis of outcomes in treatment of obstructive sleep apnea in infants. Laryngoscope. 2013;123:2306–14. doi:10.1002/lary.23685. PMID:23804395.
- Galland BC, Taylor BJ, Elder DE, Herbison P. Normal sleep patterns in infants and children: A systematic review of observational studies. Sleep Med Rev. 2012;16:213–22. doi:10.1016/j.smrv.2011.06.001. PMID:21784676.
- MacLean JE, Fitzgerald DA, Waters KA. Developmental changes in sleep and breathing across infancy and childhood. Paediatr Respir Rev. 2015;16:276–84. PMID:26364005.
- Marcus CL. Sleep-disordered breathing in children. Am J Respir Crit Care Med. 2001;164:16–30. doi:10.1164/ajrccm.164.1.2008171. PMID:11435234.
- Al-Hathlol K, Idiong N, Hussain A, et al. A study of breathing pattern and ventilation in newborn infants and adult subjects. Acta Paediatr. 2000;89:1420–5. doi:10.1111/j.1651-2227.2000.tb02769.x. PMID:11195229.
- Kato I, Franco P, Groswasser J, et al. Frequency of obstructive and mixed sleep apneas in 1,023 infants. Sleep. 2000;23:487–92. doi:10.1093/sleep/23.4.1b. PMID:10875555.
- McNamara F, Issa FG, Sullivan CE. Arousal pattern following central and obstructive breathing abnormalities in infants and children. J Appl Physiol. 1996;81:2651–7. doi:10.1152/jappl.1996.81.6.2651. PMID:9018518.
- Bedi P, Dehaan K, Castro-Codesal M, MacLean J. The Use and Outcomes of Long-Term Non-Invasive Ventilation in Infants. Am J Respir Crit Care Med. 2017;195:A4105–A.
- Castro-Codesal ML, Dehaan K, Bedi PK, et al. Longitudinal changes in clinical characteristics and outcomes for children using long-term non-invasive ventilation. PLOS ONE. 2018;13:e0192111. doi:10.1371/journal.pone.0192111. PMID:29381756.
- Infancy (Birth – two years of age) 2017. (Accessed September 25, 2017). at http://www.phac-aspc.gc.ca/hp-ps/dca-dea/stages-etapes/childhood-enfance_0-2/index-eng.php.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi:10.1016/j.jbi.2008.08.010. PMID:18929686.
- Anders TER, Parmele A. A Manual of Standardized Terminology, Techniques and Criteria for Scoring States of Sleep and Wakefulness In Newborn Infants. Los Angeles: UCLA Brain Information Services; 1971.
- Rechtschaffen AKA. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages on Human Subjects. Washington DC: National Institutes of Health; 1968.
- Marcus CL, Omlin KJ, Basinki DJ, et al. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis. 1992;146:1235–9. doi:10.1164/ajrccm/146.5_Pt_1.1235. PMID:1443877.
- Standards and indications for cardiopulmonary sleep studies in children. American thoracic society. Am J Respir Crit Care Med. 1996;153:866–78. PMID:8564147.
- Iber C, Ancoli-Israel S, Chesson A, Quan S. The Aasm Manual for The Scoring of sleep and Associated Events American Academy of Sleep Medicine. Westchester: American Academy of Sleep Medicine; 2007.
- DeHaan KL, Seton C, Fitzgerald DA, Waters KA, MacLean JE. Polysomnography for the diagnosis of sleep disordered breathing in children under 2 years of age. Pediatr Pulmonol. 2015;50:1346–53. doi:10.1002/ppul.23169. PMID:25777054.
- Srivastava R, Stone BL, Murphy NA. Hospitalist care of the medically complex child. Pediatr Clin North Am. 2005;52:1165–87. doi:10.1016/j.pcl.2005.03.007. PMID:16009262.
- Burns KH, Casey PH, Lyle RE, et al. Increasing prevalence of medically complex children in US hospitals. Pediatrics. 2010;126:638–46. doi:10.1542/peds.2009-1658. PMID:20855383.
- Brouillette RT, Fernbach SK, Hunt CE. Obstructive sleep apnea in infants and children. J Pediatr. 1982;100:31–40. doi:10.1016/S0022-3476(82)80231-X. PMID:7057314.
- Guilleminault C, Pelayo R, Clerk A, Leger D, Bocian RC. Home nasal continuous positive airway pressure in infants with sleep-disordered breathing. J Pediatr. 1995;127:905–12. doi:10.1016/S0022-3476(95)70026-9. PMID:8523187.
- Kohyama J, Ohinata JS, Hasegawa T. Blood pressure in sleep disordered breathing. Arch Dis Child. 2003;88:139–42. doi:10.1136/adc.88.2.139. PMID:12538317.
- Marcus CL, Greene MG, Carroll JL. Blood pressure in children with obstructive sleep apnea. Am J Respir Crit Care Med. 1998;157:1098–103. doi:10.1164/ajrccm.157.4.9704080. PMID:9563725.
- Redline S, Storfer-Isser A, Rosen CL, et al. Association between metabolic syndrome and sleep-disordered breathing in adolescents. Am J Respir Crit Care Med. 2007;176:401–8. doi:10.1164/rccm.200703-375OC. PMID:17541017.
- Waters KA, Sitha S, O'Brien LM, et al. Follow-up on metabolic markers in children treated for obstructive sleep apnea. Am J Respir Crit Care Med. 2006;174:455–60. doi:10.1164/rccm.200401-110OC. PMID:16709938.
- Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102:616–20. doi:10.1542/peds.102.3.616. PMID:9738185.
- Marcus CL, Radcliffe J, Konstantinopoulou S, et al. Effects of positive airway pressure therapy on neurobehavioral outcomes in children with obstructive sleep apnea. Am J Respir Crit Care Med. 2012;185:998–1003. doi:10.1164/rccm.201112-2167OC. PMID:22323303.
- Bedi PK, Castro-Codesal ML, Featherstone R, et al. Long-term non-invasive ventilation in infants: A systematic review and meta-analysis. Front. 2018;6:13.
- Essouri S, Nicot F, Clement A, et al. Noninvasive positive pressure ventilation in infants with upper airway obstruction: comparison of continuous and bilevel positive pressure. Intensive Care Med. 2005;31:574–80. doi:10.1007/s00134-005-2568-6. PMID:15711977.
- Fauroux B, Leroux K, Desmarais G, et al. Performance of ventilators for noninvasive positive-pressure ventilation in children. Eur Respir J. 2008;31:1300–7. doi:10.1183/09031936.00144807. PMID:18321932.
- King MS, Xanthopoulos MS, Marcus CL. Improving positive airway pressure adherence in children. Sleep Med Clin. 2014;9:219–34. doi:10.1016/j.jsmc.2014.02.003. PMID:24910579.
- Machaalani R, Evans CA, Waters KA. Objective adherence to positive airway pressure therapy in an Australian paediatric cohort. Sleep Breath. 2016;20:1327–36. doi:10.1007/s11325-016-1400-6. PMID:27591801.
- Marcus CL, Rosen G, Davidson Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117:e442–e51. doi:10.1542/peds.2005-1634. PMID:16510622.
- Ramirez A, Khirani S, Aloui S, et al. Continuous positive airway pressure and noninvasive ventilation adherence in children. Sleep Med. 2013;14:1290–4. doi:10.1016/j.sleep.2013.06.020. PMID:24157098.
