Abstract
Background
This asthma guideline update focuses on the management of individuals with asthma at the mild end of the spectrum. It applies to children 1 year of age and over and adults. This update was initiated to address new clinical trials in this patient group as well as changes in the recommendations from the Global Initiative for Asthma (GINA) asthma strategy group. This guideline applies the current evidence to the Canadian context.
Methods
A representative multidisciplinary panel of experts undertook a formal clinical practice guideline development process. A total of 9 key clinical questions were defined according to the Patient/problem, Intervention, Comparison, Outcome (PICO) approach. The panel performed an evidence-based, systematic literature review, assessed and graded the relevant evidence to synthesize 11 key recommendations. These recommendations were reviewed in the context of the existing Canadian Asthma Guidelines and changes from previous guidelines are highlighted.
Results
The updated evidence demonstrated that daily inhaled corticosteroids (ICS) + PRN short-acting beta-agonist (SABA) decrease exacerbations and improve asthma control compared to PRN SABA in individuals with very mild and mild asthma. There is new evidence in children ≥12 years of age and adults that PRN budesonide/formoterol (bud/form) decreases exacerbations in comparison to PRN SABA, with different levels of evidence in those with very mild versus mild asthma. Individuals with very mild asthma at higher risk of exacerbation should be given the option of switching from PRN SABA to daily ICS + PRN SABA (all ages) or PRN bud/form (≥12 years of age). In individuals with mild asthma, daily ICS + PRN SABA are still recommended as first line controller therapy. However, in individuals ≥12 years of age with poor adherence to daily medication despite substantial asthma education and support, PRN bud/form is an alternative. Intermittent use of very high dose ICS for acute loss of asthma control is not suggested in preschoolers given potential for harm.
Discussion
This guideline provides a detailed review of the evidence and provides recommendations for the treatment of very mild and mild asthma within the Canadian context for preschoolers, children and adults. The Canadian Thoracic Society 2021 Asthma Guideline update will amalgamate these recommendations with previous guidelines to provide a document that address diagnosis and management of asthma.
INTRODUCTION
Asthma has a national prevalence of 10.8% and affects 3.8 million Canadians over the age of 1 year.Citation1
The last Canadian Thoracic Society (CTS) asthma position statement focused on the treatment and management of those with severe asthma,Citation2 which is estimated to affect 5-10% of those with asthma. This guideline update focuses on those with asthma at the milder end of the spectrum, which represents approximately 28-41% of the asthma population in Canada.Citation3,Citation4 Although the per patient cost of asthma is 2.6 to 5 times higher in an individual with severe compared to mild asthma, given the high prevalence of mild asthma, the total cost of asthma care for these patients is substantial.Citation5 The majority of Canadians with asthma continue to have suboptimal control, with surveys finding that 53-90% of patients had 1 or more criteria for poorly-controlled asthma.Citation6,Citation7 They also continue to have severe asthma exacerbations with the rate of Emergency Department (ED) visits for asthma estimated at 19-21 per 1,000 patients with 6-11% of those presenting to the ED requiring admission to hospital.Citation8 Asthma specific mortality rates have decreased over time but there continue to be deaths from asthma at a rate of 6.2 per 100,000 asthma population.Citation9
The severity of an individual’s asthma is classified by the intensity of treatment needed to maintain asthma control. Accordingly, this is not a useful concept when deciding on initial treatment, as asthma severity can only be determined once treatment has been started and asthma control is or is not attained. In this guideline, recommendations refer to individuals who have well or poorly-controlled asthma on PRN short-acting beta-agonist (SABA) alone or no medication. We have also more clearly defined the severity classification, given that this terminology is often used by practitioners and patients. An unfortunate consequence of classifying asthma severity is that the term “very mild and mild asthma” suggests that there is a minimal amount of morbidity or mortality associated with it; whereas, it is known that these patients are still at risk for asthma exacerbations and subsequent asthma death.Citation10 Patients themselves identified that a common barrier to improved asthma care was the “perceived lack of seriousness of the condition”.Citation6
Our previous guidelines mentioned the importance of early initiation of daily inhaled corticosteroids (ICS) in individuals with symptoms “less than three times a week” (though the lower limit was not clearly defined), and in those with an exacerbation requiring oral corticosteroids.Citation11,Citation12 However, several practical issues with implementation of this prior guidance have been observed. For example, patients on PRN SABA who were well-controlled as per previous CTS criteria (i.e., who had symptoms as often as 3 times a week) were not typically escalated to ICS therapy. In addition, if a patient had not had an exacerbation requiring oral steroids since their last visit, they were often considered to be well-controlled and may have been weaned off their daily controller medication prematurely, which was not the intent of the recommendations in the guideline. Furthermore, patients commonly only take their controller medication when they feel that it is needed, and although in some studies only 14% report not taking medication as prescribed, adherence in clinical trials (in which patients know that adherence is being monitored) is only 56-75%; and may be considerably lower in a real-world setting.Citation6,Citation13–15 This leads to a pattern of intermittent ICS use that was specifically not recommended in the CTS 2012 update. In addition to symptom control and prevention of exacerbations, the use of daily ICS is also required to control airway inflammation and may reduce remodeling.Citation16,Citation17
OBJECTIVE
The overall objective of this CTS clinical practice guideline is to provide an update on the management of individuals with very mild or mild asthma, currently on PRN SABA alone or on no asthma therapy.
TARGET PATIENT POPULATION
The update applies to all individuals ≥1 year of age with a confirmed diagnosis of asthma who are currently on treatment with a SABA as needed or no asthma medication.
TARGET USERS
KEY DEFINITIONS
Preschool = refers to children ≥1 year of age to 5 years of age
Children = refers to children ≥6 years of age to 11 years of age
Adult = refers to individuals ≥12 years of age unless otherwise specified, individuals 12-18 years of age are included in this category because medication approval is often for patients ≥12 years of age; however, patients 12 to 18 years of age (particularly those who are prepubertal) are at higher risk for some medication side-effects such as growth suppression and should be monitored similarly to children
Controller = A medication taken daily to decrease airway inflammation, maintain asthma control and prevent exacerbations
Reliever = A medication taken only as needed for quick relief of symptoms (e.g., SABA, bud/form); use of >2 doses of reliever medication in a week is a sign of poorly-controlled asthma (the number of actuations in a dose is variable depending on the reliever medication but is often 1-2 actuations)
SABA = Short-acting beta-agonist (e.g., salbutamol, terbutaline)
LABA = Long-acting beta-agonist (e.g., salmeterol, formoterol, vilanterol)
FABA = Fast-acting beta-agonist which can either be a short-acting beta-agonist or a long-acting beta-agonist with rapid onset of action. In Canada, formoterol in a single inhaler with budesonide is approved for use as a fast-acting beta-agonist. The term is used in this document in reference to previous CTS guidelines, however for clarity the terms SABA and bud/form will be used when appropriate
bud/form = Single inhaler of budesonide and formoterol
PRN ICS-SABA = As needed use of an inhaled corticosteroid each time a short-acting beta-agonist is taken; in Canada, this would be in 2 separate inhalers as there is not currently a single inhaler containing ICS and SABA
Severe exacerbation: an exacerbation requiring any of the following:
systemic steroids
emergency department visit; or
hospitalization
Mild exacerbation: an increase in asthma symptoms from baseline that does not require systemic steroids, an emergency department visit or a hospitalization. Differentiating this from chronic poorly-controlled asthma may only occur retrospectively.
Non-severe exacerbation: one of the outcome measures assessed in the systematic review of evidence, this was defined as an exacerbation in the clinical trial that did not meet criteria for severe exacerbation (defined previously)
Higher risk of exacerbation is defined by presence of any of the following:
any history of a previous severe asthma exacerbation (requiring either systemic steroids, ED visit or hospitalization)
poorly-controlled asthma as per CTS criteria
overuse of SABA (using more than 2 inhalers of SABA in 1 year); or
being a current smoker
Individuals without any of these features have a lower risk of exacerbation.
Well-controlled asthma: Asthma in which all criteria for well-controlled asthma are met ()
Table 1. Severity classification.
Table 2. Comparative inhaled corticosteroids (ICS) dosing categories in preschoolers, children and adults.
Table 3. Well-controlled asthma criteria.
Poorly-controlled asthma: Asthma in which any 1 of the criteria for well-controlled are not met ()
SUMMARY OF NEW FEATURES COMPARED TO THE 2012 GUIDELINE
Change in control criteria for daytime symptoms and frequency of reliever need. Those with well-controlled asthma should have daytime symptoms ≤2 days per week and need for reliever (SABA or PRN bud/form) ≤2 doses per week, representing a decrease from the 2012 guideline,Citation12 in which it was <4 days per week of daytime symptoms or <4 doses per week of FABA (, further details in section Revisions to Asthma Control Criteria and Assessment of Exacerbation Risk, p. 8).
Assessing risk of exacerbation in addition to asthma control. When deciding on optimal treatment, in addition to evaluating asthma control, risk of asthma exacerbation should be assessed. A higher risk for an exacerbation is defined by any of the following criteria: 1) history of a previous severe asthma exacerbation (requiring any of: systemic steroids; ED visit; or hospitalization); 2) poorly-controlled asthma as per CTS criteria; 3) overuse of SABA (defined as use of more than 2 inhalers of SABA in a year); or 4) current smoker (further details in section Revisions to Asthma Control Criteria and Assessment of Exacerbation Risk, p. 8).
Clarification for criteria of mild versus severe asthma exacerbation. A severe asthma exacerbation is one that requires systemic steroids, an ED visit, or hospitalization. A mild exacerbation is an increase in asthma symptoms from baseline that does not require systemic steroids, an ED visit or a hospitalization.
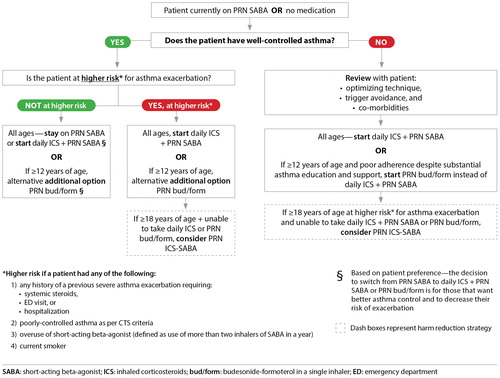
Clarification of criteria for initiating daily ICS. Patients should be started on daily ICS if they are on PRN SABA and have poorly-controlled asthma as per the updated CTS control criteria or have well-controlled asthma but are at higher risk for asthma exacerbation (). Daily ICS is also an option for patients on PRN SABA with well-controlled asthma who are not at higher risk for exacerbation, if they prefer to have better asthma control and to decrease their risk of asthma exacerbation.
Previous CTS guidelines recommended that individuals with very mild intermittent asthma may be treated with PRN SABA but that ICS should be prescribed for those with symptoms even “less than three times a week,” those with mild loss of control, or those presenting with an asthma exacerbation requiring systemic steroids.Citation11 We have now clarified definitions for these criteria. For preschoolers, daily controller therapy was recommended for children with symptoms ≥8 days/month and/or those with an exacerbation requiring oral steroids or a hospital admission.Citation18 To align with the criteria in older children, daily controller therapy is recommended for preschool children with symptoms >8 days/month.
Addition of new treatment option for very mild asthma in individuals ≥12 years of age. PRN bud/form is a treatment option for individuals ≥12 years of age who are well-controlled on PRN SABA, but at higher risk for asthma exacerbation, or for those ≥12 years of age with poorly-controlled asthma on PRN SABA who have poor adherence to daily ICS despite substantial asthma education and support. PRN bud/form is also an option for individuals with well-controlled asthma on PRN SABA who are not at higher risk for exacerbation, if they prefer to have better asthma control and to decrease their risk of asthma exacerbation ().
Previous CTS guidelines did not recommend the use of an ICS/LABA combination as a reliever in lieu of a fast-acting beta-agonist (FABA) alone.Citation12
Update of severity classification last referenced in the 1999 Guideline. Reclassification of asthma severity to remove the very severe category to align with the Recognition and Management of Severe Asthma Position Statement,Citation2 and to include other asthma therapies. Although categories such as “mild intermittent” and “mild persistent” asthma were referred to in previous guidelines, these categories are not included in the updated severity classification as these are not felt to be clinically useful. More importantly, the terminology “mild intermittent asthma” can lead to a misunderstanding of the underlying pathophysiology of asthma as “mild intermittent” may suggest to individuals that there are times when they do not have asthma when in fact, asthma is a chronic condition and it is only the symptoms that can be intermittent.
Revised ICS dosing table. Low dose beclomethasone in adult dosing table changed to ≤200 mcg from ≤250 mcg for implementability (beclomethasone metered-dose inhaler (MDI) available in 50 mcg and 100 mcg doses, as opposed to a 250 mcg dose), and consistency across age groups.
Asthma continuum. ICS in the continuum has been changed from beclomethasone HFA equivalents to fluticasone propionate equivalents. Historically, asthma guidelines used beclomethasone equivalents; however, this can lead to confusion when comparing to other guidelines and reviewing clinical trials as there are 2 forms of beclomethasone available in other countries. One form is the beclomethasone available in Canada (e.g., QVAR) and the other is beclomethasone available in other countries (e.g., Clenil) that is half as potent as the formulation licensed in Canada. In this guideline, the less potent beclomethasone is referred to as beclomethasoneEUR to avoid confusion and ex-valve doses (not ex-actuator doses) are reported (e.g., beclomethasone 50 mcg ex-valve dose is equivalent to beclomethasone 40 mcg ex-actuator dose).
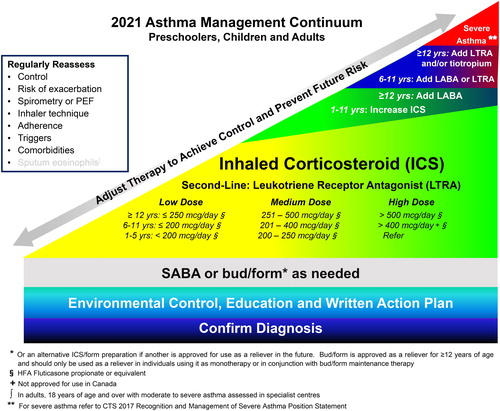
SABA or bud/form as needed has been extended across the bottom of the continuum and dosing categories and treatment have been added to include children 1-5 years of age. Evaluating the risk of exacerbation has been added to the list of items to regularly reassess.
Figure 1. 2021 Asthma management continuum preschoolers, children and adults.
Management relies on an accurate diagnosis of asthma and regular reassessment of control and risk of exacerbation. All individuals with asthma should be provided with self-management education, including a written action plan. Adherence to treatment, inhaler technique, exposure to environmental triggers, and the presence of comorbidities should be reassessed at each visit and optimized.
Individuals with well controlled asthma on no medication or PRN SABA at lower risk of exacerbation can use PRN SABA, daily ICS + PRN SABA, and if ≥ 12 years of age PRN bud/form*.
Individuals at higher risk of exacerbation even if well-controlled on PRN SABA or no medication, and those with poorly-controlled asthma on PRN SABA or no medication should be started on daily ICS + PRN SABA. In individuals ≥ 12 years old with poor adherence despite substantial asthma education and support, PRN bud/form* can be considered. LTRA are second-line monotherapy for asthma. If asthma is not adequately controlled by daily low doses of ICS with good technique and adherence, additional therapy should be considered. In children 1-11 years old, ICS should be increased to medium dose and if still not controlled in children 6-11 years old, the addition of a LABA or LTRA should be considered. In individuals 12 years of age and over, a LABA in the same inhaler as an ICS is first line adjunct therapy. If still not controlled, the addition of a LTRA or tiotropium should be considered.
In children who are not well-controlled on medium dose ICS, a referral to an asthma specialist is recommended. After achieving asthma control, including no severe exacerbations, for at least 3-6 months, medication should be reduced to the minimum necessary dose to maintain asthma control and prevent future exacerbations.
HFA: hydrofluoroalkane; SABA: short-acting beta-agonist, LABA: long-acting beta-agonist, ICS: inhaled corticosteroid, LTRA: leukotriene receptor antagonist, bud/form: budesonide-formoterol in a single inhaler.
METHODOLOGY
Guideline panel composition
The asthma guideline panel comprised 9 experts: 6 respirologists (3 pediatric respirologists and 3 adult respirologists) with experience in asthma management, research and research methodology (including 1 epidemiologist); 1 primary care physicians appointed by the College of Family Physicians of Canada; 1 pharmacist who is a certified respiratory educator (CRE); and 1 nurse practitioner who is also a CRE. All author conflicts of interests are available at https://cts-sct.ca/guideline-library/. Patient and caregiver input was not sought in the development of this current guideline; this will be addressed in the next update.
This guideline was developed in accordance with the CTS guideline development process (https://cts-sct.ca/guideline-library/methodology/). The panel used the AGREE II checklist to guide the development of the guideline.Citation19
Formulation of key clinical questions
The PICO method was used, taking into consideration the Patient group or groups that should be addressed, the Intervention or interventions that should be examined, the Comparison groups that should be part of the studies of the various interventions and the Outcome or outcomes of interest. The panel initially selected 2 PICO questions to find the best management strategy for 2 patient groups: 1) Individuals with well-controlled asthma on PRN SABA (very mild asthma) and 2) Individuals with poorly-controlled asthma on PRN SABA or well-controlled asthma on daily low dose ICS + PRN SABA (mild asthma). After evaluating the results of the systematic review for these patient groups, the PICO questions were further refined to 9 PICO questions. For individuals with well-controlled asthma on PRN SABA, the interventions of daily ICS + PRN SABA, PRN bud/form and PRN ICS-SABA were compared to PRN SABA. In addition, PRN bud/form was also compared to daily ICS + PRN SABA in this patient group. For individuals with poorly-controlled asthma on PRN SABA or well-controlled asthma on daily low dose ICS + PRN SABA, the interventions of PRN SABA, PRN bud/form and PRN ICS-SABA were compared to daily ICS + PRN SABA.
During the systematic review, a study comparing daily SABA taken with daily ICS + PRN SABA to daily ICS + PRN SABA was identified, and this question was included because it was felt to represent a potential knowledge to practice gap given that this regimen is prescribed to patients and previous guidelines have not addressed this. The question was also felt to be particularly relevant for this focused update given that frequent refill of SABA inhalers is a risk factor for asthma exacerbations, and the practice of prescribing regular use of SABAs would lead to frequent SABA refills.
In individuals with well- or poorly-controlled asthma on PRN SABA, the comparison of intermittent short courses of ICS + PRN SABA compared to PRN SABA was chosen because this is a common practice and particularly relevant in individuals with very mild and mild asthma. The comparison of intermittent short courses of ICS compared to daily ICS was assessed in the 2012 CTS Asthma Guideline and was therefore not chosen as a PICO question for this focused update.
PICO questions were selected based on the availability of new evidence for the management of individuals with very mild and mild asthma, the recent changes in the Global Initiative for Asthma (GINA) strategy,Citation20 and the potential for new evidence to significantly change current management recommendations.
A priori, through a consensus, the panel identified the following outcomes which would take priority in guideline decision-making, and therefore, included in the GRADE evidence table: severe exacerbations (defined as per Key Definitions), non-severe exacerbations (defined as an exacerbation that did not meet criteria for a severe exacerbation), asthma control, lung function, markers of inflammation, and safety-mortality. These were prioritized based on the panel's opinion on the importance to patients and their impact on patient quality of life. Thus, all of the chosen outcomes were considered critical except for markers of inflammation and lung function which although important, were considered indirectly relevant to patients.
Literature search and screening of abstracts
An initial literature search for randomized controlled trials (RCTs) or systematic reviews of RCTs was conducted from January 1, 2014 to September 1, 2019 for <6 years of age and from January 1, 2010 to September 1, 2019 for >6 years old using MEDLINE (OVID); Embase (OVID); OVID Medline Epub Ahead of Print, In-Process & Other Non-Indexed Citations; and the Cochrane Library. A second literature search was conducted to expand the search for <6 years of age back to January 1, 2010 and refined the search terms for both age groups to include names of medication as well as terms associated with the population such as mild persistent asthma and Step 2 treatment for PICO 2 and Step 1 treatment and mild intermittent asthma for PICO 1. The reference lists from recently published guidelinesCitation20–22 and relevant studies were hand-searched to identify further articles. The title and abstracts of each article were scrutinized by 2 panel members (PM/EAH-very mild asthma population; CY/OK-mild asthma population) to decide whether each article was relevant. Where there was a difference of opinion, the panel members endeavored to reach consensus. When a consensus was reached on the list of relevant abstracts, copies of the articles of all relevant and possibly relevant articles were obtained and reviewed by 2 panel members. Details of the flow of citations and articles and study inclusion and exclusion criteria are detailed in Appendix 1.
Study selection criteria
We included only RCTs and systematic reviews for further review and inclusion. Other study designs and studies published in a language other than English were excluded. Each abstract and full text article was assessed by 2 reviewers (PM/EAH/CY/OK) to determine if they were eligible (Appendix 1).
Risk of bias and critical appraisal of identified studies
Two panel members per area of focus were assigned to critically appraise and assess studies for risk of bias: PICO 1, 2 (OK/KH), PICO 3, 4, 5 (CR/DP) and PICO 6 (JR/MW). The Cochrane Risk of Bias Tool for RCTs was used to assess the risk of bias in individual studies.Citation23 The Documentation and Appraisal Review (DART) tool was used to assess the quality of systematic reviews addressing a variety of research designs.Citation24 We compiled data from all articles relevant to each PICO question into GRADE evidence tables, which are available on the CTS website. These GRADE evidence tables were developed by CY. The entire panel then discussed each PICO question via webinars in February and March 2020, at which time all evidence tables were reviewed and agreed upon by the whole group. Where possible, the number needed to treat was calculated using a random-effects model.
Grading the evidence and formulation of recommendations
GRADE evidence profilesCitation25 were developed to rate the certainty of evidence for each outcome as high, moderate, low or very low. Evidence originating from RCTs was considered to be high-quality evidence as a starting point, but could be downgraded due to risk of bias. The quality of evidence across studies was assessed for methodological limitations, inconsistency, indirectness, imprecision and publication bias. If results were downgraded by 1 or 2 levels (serious or very serious), the lead authors added an explanation.
The panel drafted recommendations for each PICO question by working through the GRADE evidence to decision framework.Citation26 This framework considers the quality of evidence, balance of desirable and undesirable effects, patient values, preferences, resource use, health equity, acceptability of an intervention and feasibility of implementation (these factors are explicated along with recommendations, where applicable). For each recommendation, the panel established a consensus on the strength of the recommendation based on the above framework (either conditional/weak or strong) and a rating of the overall quality of the body of evidence. The recommendations were then vetted by the CTS Canadian Respiratory Guidelines Committee (CRGC) Chair to optimize the language of each recommendation to ensure implementability. The recommendation consensus process was completed by electronic survey using a 6-point voting scale, whereby it was defined a priori that a recommendation would only be accepted if each panel member voted for option 1, 2 or 3 (wholeheartedly agree, agree or can support). For a recommendation to be accepted, it had to be voted on by 75% of the eligible panel members and achieve ratings of 1, 2 or 3 by 80% of the voting panelists. No panel member was excluded from voting. In the event of a failure to reach 80% of votes with ratings of 1, 2 or 3, another period of discussion ensued, whereby dissenting opinions were heard and considered. The recommendation was revised and followed by a second round of voting by electronic survey using a 3-point scale, for which acceptance of a recommendation required a 80% of panelists to choose option 1 (Agree) or 2 (Can Support). Throughout this process, all recommendations achieved acceptance, with no recommendation requiring a second round of voting.
Implications of Strong and Conditional RecommendationsCitation27,Citation28
The implications of a strong recommendation are:
For patients – most people in your situation would want the recommended course of action and only a small proportion would not; request discussion if the intervention is not offered
For clinicians – most patients should receive the recommended course of action
For policy makers – the recommendation can be adopted as a policy in most situations
For patients – most people in your situation would want the recommended course of action, but many would not
For clinicians – you should recognize that different choices might be appropriate for different patients and that you must help each patient to arrive at a management decision consistent with her or his values and preferences
For policy makers – policy making will require substantial debate and involvement of many stakeholders
We also included informed clinical remarks with PICO clinical questions and recommendations, in an effort to complement recommendations with practical clinical advice. Some of these remarks are not based on strong evidence but represent the consensus opinions of panel members, based on expertise.
Good practice points are included in association with each clinical question and are intended to offer short pieces of advice to the target user. Some of these good practice points may not have an evidence base but are viewed as good clinical practice by the expert panel. All good practice points were arrived at by consensus, based on the clinical experience of the guideline panel members.
Applicability/Implementability
Recommendations were formulated with the aim of being clear and actionable by clinicians within the user group, in accordance with best principles for guideline language and format.Citation29 Resource implications were considered for each recommendation and are implicitly stated within the values and preferences section.
The recommendations from this focused guideline update have been integrated with previous guidelinesCitation11,Citation12,Citation18 to create the CTS 2021 Asthma Guideline Update, which addresses all aspects of asthma care in a single document, to allow for easier implementation. The diagnosis and management of severe asthma has been kept as a separate position statementCitation2 as those individuals are typically managed in subspecialty clinics.
Review and approval process
In accordance with the CTS Guideline Production Methodology, before completion, the CTS independently invited formal review of the guideline by: 1) 1 external (non-CTS) international and 2 external (non-CTS) national content experts; and 2) 5 internal (CTS) reviewers. One of the internal reviewers performed an AGREE assessment of the guideline. The authors were blinded to the identities of the reviewers. Each reviewer then provided a detailed review and suggestions, and authors responded to these reviews in detail. These reviews and the AGREE II scoresheet were provided to CTS CRGC for review. Two members of the CRGC Executive then completed a review of the guideline and these documents, and provided further feedback for consideration by authors. Upon acceptance, the CRGC recommended approval of the guideline to the CTS Executive Committee. All reviews and author responses are posted on the CTS website.
Living guideline/future updates
The guideline will be formally reviewed every 3 years or sooner to determine the need for and nature of any updates, in accordance with the CTS Living Guideline Model. The CTS Asthma Assembly Steering Committee members will also use the continuously updated McMaster Plus database, whereby they will receive alerts when new articles pertaining to these PICO questions are published (starting from the last date of the literature search conducted for this guideline). This will serve to prompt members to consider timely guideline updates with evolving evidence and will facilitate formal literature reviews.
REVISIONS TO ASTHMA CONTROL CRITERIA AND ASSESSMENT OF EXACERBATION RISK
Asthma Control Criteria
Assessment of asthma control is a keystone to asthma management. Previous surveys of Canadians with asthma found that 93-97% consider their asthma controlled; however, 53-90% of individuals had 1 or more criteria for poorly-controlled asthma as per CTS criteria.Citation6,Citation7 This highlights the importance of performing structured assessments or using control questionnaires to assess asthma control, instead of asking general questions about the individual’s perception of their asthma control.
The evolution of the CTS Asthma Control Criteria
The CTS Asthma Control Criteria has undergone multiple changes since first introduced in 1989. The initial criteriaCitation30 included “minimal symptoms, ideally none” and inhaled beta-agonist needed “not more than twice daily and ideally none.” This was further quantified in the 1996 guideline as <3 days/week, allowing for 1 dose per day of SABA for prevention of exercise-induced symptoms (consensus).Citation31 In the 1999 update, this was increased to allow for <4 days/week of daytime symptoms and <4 doses/week of SABA, still allowing for 1 dose per day for prevention of exercise induced symptoms, acknowledging that “complete absence of respiratory symptoms and normal pulmonary function was difficult to achieve in individuals with asthma” and that “acceptable” control was the goal.Citation32 This was further revised in 2010 to count doses of FABA used to treat or prevent exercise-induced symptoms when evaluating FABA use (given that pre-exercise allowance of FABA was not evidence-based, and a concern that frequent use of FABA for exercise-induced symptoms indicated poorly-controlled asthma).Citation11 The term FABA replaced SABA in 2010 to recognize that PRN bud/form was approved to be used as a daily maintenance and reliever medication. In the last revision,Citation12 sputum eosinophils <2-3% were included for those with moderate to severe asthma.
In comparison, other national and international guidelines/strategies have used more stringent criteria for frequency of asthma symptoms and use of SABA. The National Heart, Lung, and Blood Institute (NHLBI)Citation21 and the GINACitation20 documents apply a cutoff of ≤2 days/week of symptoms or SABA use, and use different criteria for lung function (GINA no longer uses a specific criterion but highlights that a forced expiratory volume in 1 second (FEV1) <60% increases risk for future exacerbations; NHLBI uses an FEV1 > 80% predicted). A study comparing asthma control with and without spirometry criteria found that asthma control was overestimated if lung function parameters were not included, but there was no significant discrepancy in individuals considered poorly-controlled when comparing the 2010 GINA and CTS symptom control criteria.Citation33
Rationale for changes to the CTS Asthma Control Criteria
In this update, the frequency of daytime symptoms and need for reliever (SABA or PRN bud/form) defining well-controlled asthma were decreased from <4 days per week and <4 doses per week to ≤2 days per week and ≤2 doses per week, respectively. These changes were made for the following reasons:
RCTs reviewed for this update for the initiation of controller therapy most frequently used inclusion criteria of individuals with symptoms or use of SABA >2 days per week.Citation15,Citation34–38
Recommendations in previous guidelines for escalation of controller therapy, were based on RCTs that often used the cutoff of >2 days per week to define poorly-controlled asthma.Citation39–41
Future trials that use a “number of days/week with symptoms” as an inclusion criterion will likely continue to use the cutoff of >2 days per week given that RCTs often use GINA or NHLBI criteria to define controlCitation35,Citation36,Citation38,Citation42,Citation43 if they do not use a global score from a control questionnaire (e.g., Asthma Control Test, Asthma Control Questionnaire).
Aligning the Canadian control criteria with other national and international recommendations will allow future evidence to be generalizable in the Canadian context.
Aligning the criteria for initiation of controller treatment in preschoolers compared to older children and adults simplifies management guidance. The 2015 CTS/Canadian Pediatric Society (CPS) Preschool Position StatementCitation18 used a cutoff of symptoms ≥8 days per month (which roughly aligns with the cutoff of >2 days per week), and will be revised in the CTS 2021 Asthma Guideline Update to >8 days/month (thus approximating >2 days per week).
Previous control criteria were based on consensus opinion and it was not felt that a specific PICO question addressing this would yield evidence to support a cutoff of <4 days per week compared to ≤2 days per week.
There is also a discrepancy in the frequency of night symptoms, which is defined as <1/week in the CTS guideline and no night wakening in the prior 4 weeks in GINA, whereas NHLBI defines poor control as ≥1/month for children <11years of age and ≥2/month for children >12 years of age and adults. Given that there is no consistency across guidelines and that this criterion is not often used in inclusion criteria for RCTs, this criterion was not changed. However, we have specified that nocturnal symptoms should be considered mild.
For clarity, the term FABA has been replaced by SABA or bud/form as these are the medications approved for use as a reliever in Canada. Although there are no established control criteria when using bud/form as a reliever, the use of a reliever often indicates that an individual is having symptoms and is useful to track as it can be objectively assessed through prescription refills.
A mild exacerbation has been defined as an increase in asthma symptoms from baseline that does not require systemic steroids, an ED visit or a hospitalization. The frequency of mild exacerbations has not been specifically defined, as the frequency of mild exacerbations that leads to an impairment of quality of life differs for each patient or family. However, the frequency of mild exacerbations for well-controlled asthma has been qualified as a frequency which is not deemed by the patient or their family members to impair their quality of life.
Assessing Risk for Exacerbation
One of the goals of asthma treatment is to decrease the frequency and severity of asthma exacerbations, and many RCTs studying individuals with mild asthma have this as their primary outcome measure.Citation15,Citation34,Citation44,Citation45
An individual can have very mild or mild asthma as defined by the intensity of treatment required to maintain control and still be at risk for exacerbations and asthma-related death. Those with mild asthma represent 30-50% of individuals with acute exacerbations in the ED and 9-30% of those who died of asthma,Citation10,Citation46 although the definitions of mild asthma vary across studies and do not necessarily align with the definition used in this guideline. It is because of this morbidity and mortality that the updated GINA strategyCitation20 recommended that patients over the age 12 no longer receive PRN SABA as the only treatment for their asthma.
The guideline panel elected to keep PRN SABA as a treatment option in individuals with well-controlled asthma who are currently on PRN SABA; however, acknowledged that there were still individuals in this group at higher risk for asthma exacerbation who would benefit from the increased protection provided by a step-up in therapy (see PICO 1 for further explanation, p. 12). The panel reviewed other guidelinesCitation20–22 and the literature examining risk factors for asthma exacerbations and elected to adapt the tables used in the 2019 British Thoracic Society/Scottish Intercollegiate Guidelines Network guideline, as they clearly specified the odds ratio (OR) associated with each risk factor.Citation22 Factors that were included in the higher risk of exacerbation category were chosen based on having an OR > 1.5, certainty of the effect of the risk factorCitation22,Citation47 and ease of use in clinical practice. All chosen risk factors had consensus agreement.
Having a higher risk of exacerbation is defined by ANY of the following: 1) any history of a previous severe asthma exacerbation (requiring any of: systemic steroids, ED visit or hospitalization), 2) poorly-controlled asthma as per CTS criteria, 3) overuse of SABA (defined as use of more than 2 inhalers of SABA in 1 year)Citation48 or 4) current smoker. Patients without any of these features have a lower risk of exacerbation.
A more comprehensive table of risk factors is provided to facilitate discussions between clinicians and patients where different treatment options exist (). Some of the factors were altered slightly to provide a pragmatic definition for clinicians (Appendix 3). A table of risk factors associated with near-fatal or fatal asthma is also included to highlight those at highest risk. Patients with these risk factors require careful follow-up, and may benefit from a multi-disciplinary team, given that factors such as nonadherence, substance use and psychiatric illness increase their risk of death from asthma ().Citation22
Table 4. Risk factors associated with severe asthma exacerbations
(This table is adapted from SIGN 158 - British guideline on the management of asthma by kind permission of the Scottish Intercollegiate Guidelines Network).Citation22
Table 5. Risk factors associated with near-fatal or fatal asthmaCitation10,Citation51
(This table is adapted from SIGN 158 - British guideline on the management of asthma by kind permission of the Scottish Intercollegiate Guidelines Network).Citation22
SUMMARY OF RECOMMENDATIONS FROM THIS FOCUSED GUIDELINE UPDATE
Asthma is defined as well- or poorly-controlled as per CTS well-controlled asthma criteria table ().
RESULTS
PICO 1. In individuals on PRN SABA with well-controlled asthma is:
a) Daily ICS + PRN SABA safe and more effective than PRN SABA?
b) PRN bud/form safe and more effective than PRN SABA?
c) PRN bud/form safe and more effective than daily ICS + PRN SABA?
Recommendations
1.1 For individuals ≥12 years of age on PRN SABA with well-controlled asthma at lower risk for exacerbations, we recommend continuing PRN SABA or switching to either daily ICS + PRN SABA or PRN bud/form (based on patient preference). (Strong recommendation)
1.2 For individuals ≥12 years of age on PRN SABA with well-controlled asthma at higher risk for exacerbations, we recommend switching to either daily ICS + PRN SABA or PRN bud/form. In individuals with poor adherence to daily medication despite substantial asthma education and support, we recommend PRN bud/form over daily ICS + PRN SABA. (Strong recommendation)
1.3 For individuals <12 years of age with well-controlled asthma on PRN SABA at lower risk for exacerbations, we recommend continuing PRN SABA or switching to daily ICS + PRN SABA (based on patient preference). (Strong recommendation)
1.4 For individuals <12 years of age with well-controlled asthma on PRN SABA at higher risk for exacerbations, we recommend switching to daily ICS + PRN SABA. (Strong recommendation)
In this section, outcomes for safety and efficacy were prioritized by the panel resulted in ranking severe exacerbations, non-severe exacerbations, asthma control, safety/mortality, lung function and inflammation as important. All outcomes, except for lung function and inflammation, were considered critical for making a decision.
Clinical remarks
The choice between regimens recommended should be based on an assessment of patient preferences, ideally through a shared decision-making process. A choice to continue PRN SABA monotherapy in individuals at lower risk for exacerbations, rather than switching to daily ICS or PRN bud/form, would be for individuals who place a higher value on affordability and convenience of treatment regimen. They may place a relatively lower value on the possibility that a change in medication will decrease exacerbations, improve daily asthma control, lung function and inflammation.
A choice of daily ICS + PRN SABA over PRN bud/form would place a higher value on asthma control, lung function and inflammation and a relatively lower value on affordability and convenience of treatment regimen. Although we have provided a practical definition for higher versus lower risk of asthma exacerbation, there are varying risk levels within the lower risk category. The table of risk factors for asthma exacerbations () is provided to help practitioners discuss exacerbation risk individually with their patients, to reach a treatment decision. Particular attention should be paid to individuals who have behavioral or psychosocial issues that put them at high risk for near-fatal or fatal asthma ().
There are no data on the safety or efficacy of PRN bud/form in children under age 12 and bud/form is not approved for use in Canada for that age group. In individuals ≥ 12 years of age, bud/form 200/6 mcg 1 puff PRN is approved for use in Canada, to a maximum of 6 puffs in a single occasion and a maximum of 8 puffs per day.
Patient values and preferences
We placed a high value on affordability, convenience and acceptability of treatment. We placed a relatively lower value on exacerbations, asthma control and markers of airway inflammation given the high number needed to treat in this population.
Good practice points
Individuals frequently overestimate their asthma control; therefore, a structured assessment of individual elements of asthma control should be done at each visit. Pharmacy records to assess frequency of SABA inhaler refills should be used to provide an objective measure of SABA use, and if more than 2 inhalers have been filled in the last year, this should prompt discussion about SABA use.
Review of evidence by outcomes
Overall quality of evidence across all critical outcomes (severe exacerbations, non-severe exacerbations, asthma control, Safety/mortality):
Daily ICS + PRN SABA versus PRN SABA (all age groups): Moderate certainty
PRN bud/form versus PRN SABA:
≥ 18 years of age: Low certainty
12-17 years of age: Very low certainty
PRN bud/form versus Daily ICS + PRN SABA:
≥ 18 years of age: Low certainty
12-17 years of age: Very low certainty
No available meta-analysis of RCTs. Four trials were included in the comparison of daily ICS + PRN SABA (beclomethasoneEUR 800 mcg/day, budesonide 400 mcg/day, 200 mcg/day if <11 years of age) and PRN SABACitation44,Citation45,Citation52,Citation53 (Appendix 2). This included 1 blinded RCT in preschoolers (BEST pediatric),Citation53 1 blinded RCT in children and adults (START trial, 4-66 years of age)Citation45 and another unblinded RCT in adults (NovelSTART trial, 18-75 years of age).Citation44 The Lazarinis study was a small study (n = 66) that was only 6 weeks in duration and specifically looked at patients (≥12 years old although unclear if any adolescent patients were recruited) with exercise induced asthma confirmed with a ≥10% drop in FEV1 after exercise.Citation52 The other 3 trials included patients with a range of symptom frequency. BEST pediatric included preschoolers with 3 or more episodes of wheezing in 6 months but were excluded if they had required systemic steroids, START included patients with symptoms “at least once per week, but not as often as daily” and NovelSTART included patients with SABA use between 2 occasions in the last 4 weeks to less than 2 occasions per day. NovelSTART also included patients with a severe exacerbation in the last 12 months with no minimum requirement for symptom frequency (93% of the study population did not have an exacerbation in the last year). A post hoc analysis of the START trial did not show a difference in outcomes when comparing the group that had symptoms ≤2 times a week compared to the group that had symptoms >2 times a week at baseline.Citation45,Citation54 In both studies, there was an almost equal number of patients with symptoms or SABA use ≤2 times per week (58%Citation45, 54%Citation44) and >2 times per week.
There was only 1 unblinded RCT in adults (NovelSTART trial, 18-75 years of age) that compared PRN bud/form (200 mcg) to PRN SABA.Citation44 The same unblinded RCT compared PRN bud/form to Daily ICS + PRN SABA (budesonide 400 mcg/day), although this was not a pre-specified comparison.Citation44 The specific patient population included in NovelSTART is discussed in a previous section but included patients that had well- and poorly-controlled asthma on PRN SABA.
1. Severe exacerbations
Daily ICS + PRN SABA versus PRN SABA
In preschoolers, daily beclomethasone increased the time to first exacerbation requiring oral steroids compared to PRN SABA (p = 0.01).Citation53
In children and adults, 2 trials included data on severe exacerbations in patients on daily budesonide versus PRN SABA.Citation44,Citation45 The START trial found a reduced risk of a first severe asthma-related event in the daily budesonide versus PRN SABA group (HR 0.56, 95% Confidence Interval (CI) 0.45-0.71); whereas there was no difference found in number of severe exacerbations in NovelSTART (21 in daily budesonide versus 23 in PRN SABA). Overall, this led to a number of needed to treat (NNT) of 50 (95% CI 25-100) to prevent 1 severe exacerbation. There were many differences in the trials that could have accounted for the difference in findings including length of trial (3 years vs 1 year), study design (blinded vs unblinded), criteria for asthma diagnosis (self-report of physician diagnosis vs objective evidence of variable airflow limitation). Although adherence was based on self-report in the START trial, it was assessed using electronic monitors in NovelSTART and was only 56% for twice daily (bid) ICS, which may have led to decreased efficacy of daily ICS in that study. In a post hoc analysis of the START trial, the decrease in severe exacerbations was reduced regardless of baseline symptom frequency (Rate Ratio 0.48 (0.55 to 0.8) 0-1 symptom days per week, rate ratio 0.56 (0.44-0.71) <1 to ≤2 symptom days per week, rate ratio 0.66 (0.55-0.8) >2 symptom days per week, pinteraction = 0.11).
PRN bud/form versus PRN SABA
In adults, the NovelSTART trial, showed a decrease in severe exacerbations in the PRN bud/form group compared to the PRN SABA group (RR = 0.4, 95% CI 0.18-0.86). Citation44
PRN bud/form versus Daily ICS + PRN SABA
In adults, the NovelSTART trial, showed a decrease in severe exacerbations in favor of PRN bud/form over daily budesonide (RR = 0.44, 0.2-0.96)Citation44 although adherence to twice daily medication in this trial was only 56%.
2. Non-severe exacerbations
Daily ICS + PRN SABA versus PRN SABA
In preschoolers, there was an increase in time to any first exacerbation (severe and non-severe) in patients on daily beclomethasone (p = 0.03).Citation53
In adults, the NovelSTART trial showed a decrease in non-severe exacerbations in the daily budesonide group versus PRN SABA group with an annualized exacerbation rate of 0.175 in daily budesonide versus 0.4 in PRN SABA (relative rate 0.44, CI not provided as this was not a pre-specified comparison in this trial).Citation44
PRN bud/form versus PRN SABA
In adults, the NovelSTART trial showed a decrease in non-severe exacerbations in the PRN bud/form versus PRN SABA group (absolute rate/patient/year for either severe or non-severe exacerbations, RR 0.49 (0.33-0.72)) with a decreased risk of either severe or non-severe exacerbations in the time to first event analysis (HR 0.46, 95% CI 0.29-0.73).Citation44
PRN bud/form versus Daily ICS + PRN SABA
In adults, the NovelSTART trial showed that PRN bud/form led to a nonsignificant increase in severe or non-severe exacerbations compared to daily budesonide (absolute rate/patient/year for either severe or non-severe exacerbations, Relative rate 1.12 95% CI 0.7-1.79, p = 0.6) with a nonsignificant decrease in risk of either severe or non-severe exacerbations in the time to first event analysis (HR 0.93, 95% CI 0.55-1.57).Citation44
3. Asthma control
Daily ICS + PRN SABA versus PRN SABA
In preschoolers, there was a decrease in symptom free days in patients on daily beclomethasone (69.6 ± 21 vs 61 ± 24, p = 0.034).Citation53
In children and adults, the START trial showed an increase in symptom free days in the daily budesonide versus PRN SABA group (p < 0.0001),Citation45 with a post hoc analysis showing no significant difference in the magnitude of the effect when patients were stratified by symptom frequency (mean difference in symptom free days between daily ICS group compared to PRN SABA by baseline symptom frequency, 0-1 days with symptoms/week 3.11%, 2 days with symptoms/week 3.86%, >2 days with symptoms/week 4.71%).
In adults in the NovelSTART trial, there was improved asthma control in those receiving daily budesonide compared to PRN SABA, looking at Asthma Control Questionnaire (ACQ-5) scores (no statistics for comparison available).Citation44 In adults (≥12 years of age) with exercise-induced asthma in the Lazarinis trial, there was no difference in ACQ-5 at 6 weeks, between the daily ICS and PRN SABA groups.Citation52
PRN bud/form versus PRN SABA
In adults in the NovelSTART trial, there was a decrease in the ACQ-5 score in the PRN bud/form versus PRN SABA group (median difference -0.15, 95% CI (-0.24 to -0.06), but this did not meet the minimal clinically important difference (MCID) of 0.5.Citation44
In adults (≥12 years of age) with exercise induced asthma, the Lazarinis trial showed no difference in ACQ-5 at 6 weeks, between the PRN SABA and PRN bud/form groups.Citation52
PRN bud/form versus Daily ICS + PRN SABA
In adults in the NovelSTART trial, asthma control was worse as measured by the ACQ-5 in the PRN bud/form group compared to daily budesonide group (mean difference 0.14, 95% CI 0.05 to 0.23).Citation44
In adults (12 and over) with exercise induced asthma in the Lazarinis trial, there was no difference in the 6 week trial in ACQ-5 between the daily budesonide and PRN bud/form groups.Citation52
4. Safety/mortality
There were 13 deaths reported in all of the studies (4 daily ICS, 8 PRN SABA, 1 PRN bud/form) with 1 asthma related death in a patient receiving placebo.Citation45 There was no significant difference in serious adverse events (6 in the PRN SABA group, 7 in the daily ICS group, 13 in the PRN bud/form group), and most seemed unrelated to asthma.Citation44 Most common adverse events included upper respiratory tract infection, nasopharyngitis and asthma and did not differ between groups.Citation44
In the 1 trial (START trial) that included children,Citation45 there was a decrease in growth in children 5 to 15 years of age in the daily budesonide group (mean difference -0.43 centimeter (cm)/year, 95%CI -0.54 to -0.32, P < 0.0001) and this was seen in those <11 years of age receiving budesonide 200 mcg/day and those 12–15 years of age receiving budesonide 400 mcg/day. Other trials did not specifically examine this endpoint. The preschool trial did not report growth parameters and there was no difference in drug related adverse events or morning salivary cortisol in that trial.Citation53
FEV1
In children and adults in the START trial, there was a small but significant improvement in FEV1 at 1 year and 3 years in the group on daily ICS versus PRN SABA (FEV1 was higher in budesonide versus placebo, at year 1 by 2.24% pre-bronchodilator (BD) p < 0.0001, 1.48% post-BD, p < 0.0001, at year 3 by 1.71% pre-BD, p < 0.0001 and 0.88% post-BD, p = 0.0005).Citation45
In the adult NovelSTART study, there was no difference in FEV1 at the end of 1 year when comparing PRN bud/form, PRN SABA and daily ICS, although adherence to daily ICS in that trial was only 56%.Citation44
In the Lazarinis study looking at those with exercise-induced asthma,Citation52 there was a difference in the maximum post-exercise FEV1 fall after 6 weeks of treatment favoring daily ICS 6.6% smaller (95% CI -10.3 to -3) and PRN bud/form 5.4% smaller (95% CI -8.9 to -1.8)), compared to PRN SABA (1.5% greater (95% CI -2.1 to +5.1); p = 0.017 for bud/form versus PRN SABA, p = 0.026 daily ICS versus PRN SABA. In that analysis, PRN bud/form was noninferior to daily ICS based on a cutoff post-exercise FEV1 fall of <7.28%.
6. Inflammation
The Novel START study in adults found a trend of decrease in fraction of exhaled nitric oxide (FeNO) in the daily ICS versus PRN SABA group (budesonide FeNO Median interquartile range (IQR): 38 (20-76) visit 1, 25 (16-45) visit 7 versus PRN SABA FeNO Median (IQR): 40 (23-75) visit 1, 36 (22-66) visit 7).Citation44
That study also found a decrease in FeNO in the PRN bud/form group compared to PRN SABA (ratio of geometric means, 0.83; 95% CI, 0.75 to 0.91) and a higher FeNO in the bud/form group compared to daily ICS (ratio of geometric means 1.13; 95% CI, 1.02 to 1.25).Citation44
Expert panel discussion of additional considerations and clinical judgment of risk versus benefit
For recommendation 1.1 and 1.3, the decision to keep PRN SABA as a treatment option for individuals with well-controlled asthma and lower risk of exacerbation was based on the low (PRN bud/form vs SABA) to moderate (daily ICS + PRN SABA vs PRN SABA) level of evidence in this patient population for alternative regimens, the lower acceptability of more costly and inconvenient treatment regimens, and the implementation challenges of changing current standard of practice considering the aforementioned issues. The panel acknowledges that the criteria used to define individuals as lower risk for exacerbation have not been prospectively validated; however, given that the potential benefit from daily ICS + PRN SABA or PRN bud/form is largely a decrease in exacerbations, the panel felt that it was important to provide practitioners with a practical way to identify individuals at higher risk of exacerbation. The recommendations were worded to be implementable by practitioners, which necessitated combining different levels of evidence into 1 recommendation. It is acknowledged that the strength of evidence for benefit of daily ICS versus PRN SABA is moderate, warranting a strong recommendation; whereas the evidence of benefit for PRN bud-form versus PRN SABA is of low certainty and would typically warrant a weak recommendation.
Moderate quality evidence shows that daily ICS is superior to PRN SABACitation44,Citation45 in studies that included individuals with symptoms 0-2 times per week (NovelSTART and START trials) for outcomes including symptom control, exacerbations, lung function and inflammation. However, in deciding to keep PRN SABA as an option for such individuals, the panel considered the acceptability of daily treatment in individuals with infrequent symptoms and the lower risk of exacerbations with the NNT of 50 to prevent 1 severe exacerbation in all individuals. Given that the evidence shows that daily ICS prevents exacerbations in comparison to PRN SABA, it is recommended that individuals at higher risk of exacerbation start daily ICS instead of continuing PRN SABA.
Low quality evidence from 1 unblinded RCT (NovelSTART) in adults with no objective evidence of asthma diagnosis shows that PRN bud/form is superior to PRN SABACitation44 in individuals 18 years of age and older with symptoms as infrequent as 2 times per month but as often as daily, for outcomes including symptom control and exacerbations. Although the inclusion criteria in this trial called for patients with symptoms at least 2 times per month, actual SABA use in the 4 weeks prior to trial entry was 3.8 +/- 3.5 times per week, and 46% of patients used SABA > 2 times per week. No subgroup analysis in patients with symptoms ≤2 times per week was provided. The panel considered the cost of treatment, the level of evidence, and the implementation challenge given the level of evidence in deciding to keep PRN SABA as an option for individuals. This recommendation was also extended to children 12 years of age or older, after extrapolating from studies that included individuals 12-18 years old with more frequent symptoms.Citation37
Limited low quality evidence demonstrates that PRN bud/form is similar to daily ICS in reducing exacerbations in this patient population.Citation44 However, there is moderate evidence demonstrating the benefit of daily ICS for reducing exacerbations when compared to PRN SABA alone, and evidence for improved asthma control and inflammation with daily ICS compared to PRN bud/form.Citation44 These pros and cons were considered by the panel when recommending that either option could be considered (based on patient preferences).
There was only 1 asthma related death in all of the aforementioned trials, and it was a patient on placebo.Citation45 In children 5-15 years of age on daily budesonide (200 mcg daily if <11 years of age or 400 mcg daily if ≥11 years of age) there was a 0.43 cm/year decrease in height compared to the group on PRN SABA. Similar to other studies of ICS in children, this small difference in growth is not expected for each year on medication, as studies have shown that children on long term ICS either attain predicted adult heightCitation55 or have a 1.2 cm (95% CI (-1.9 to -0.5)) difference in adult height compared to placebo.Citation56 There were no data on the growth effect of the PRN bud/form regimen on children aged ≥12 years of age.
The panel cautions against the off-label use of PRN bud/form in children 6-11 years of age, given the lack of evidence in this age. Although the annual dose of ICS was lower in the bud/form group compared to the daily ICS group, the use was clustered around short bursts,Citation57 which may lead to safety issues in children. In addition, in comparison to the evidence-base in adults, the efficacy of ICS/LABA medication in preventing exacerbations in children is not as strong.Citation12
Future research questions
In individuals with well-controlled asthma on PRN SABA, are other formulations of ICS/formoterol used PRN as safe and effective as PRN bud/form, daily ICS + PRN SABA, or PRN SABA?
Is PRN bud/form safe and effective in children 6-11 years of age with well-controlled asthma on PRN SABA?
Do individuals with well-controlled asthma on PRN SABA with lower risk of exacerbation benefit from PRN bud/form or daily ICS compared to individuals with well-controlled asthma at higher risk of exacerbation?
In individuals with well-controlled asthma on PRN SABA, are LTRAs as safe and effective as PRN bud/form?
PICO 2. In individuals on PRN SABA with well-controlled asthma is ICS taken each time SABA is taken (PRN ICS-SABA) safe and more effective than PRN SABA?
Recommendation
2.1 We suggest that individuals on PRN SABA with well-controlled asthma who are at lower risk for exacerbations continue to take PRN SABA alone instead of taking an ICS each time PRN SABA is taken. (Weak recommendation)
As a harm reduction strategy, individuals ≥18 years of age at higher risk for exacerbations who are unable to take a daily ICS or PRN bud/form (as per recommendation 1.2) can be given the option of taking an ICS each time a SABA is taken. (Weak recommendation)
As per recommendation 1.4, individuals <12 years of age with well-controlled asthma at higher risk for exacerbations should take a daily ICS + PRN SABA instead of taking PRN SABA alone. Similarly, as per recommendation 1.2, individuals 12–18 years of age with well-controlled asthma at higher risk for exacerbations should take either daily ICS + PRN SABA or PRN bud/form.
Clinical remarks
In Canada, ICS are not currently approved by Health Canada to be used on a PRN basis. The RCT that evaluated this strategy in adults using 2 separate inhalersCitation58 used a regimen of beclomethasone 50 mcg 2 puffs each time salbutamol 100 mcg 2 puffs was used. If practitioners recommend this strategy (off-label), we suggest that the maximum approved daily ICS dose should not be exceeded (see ).
Patient values and preferences
For this recommendation we placed a high value on minimizing the potential for improper use of this medication regimen given the lack of a single inhaler containing an ICS and SABA and the possibility that this strategy may be interpreted as starting a short course of ICS with the onset of symptoms (discussed further in PICO 6). However, in individuals ≥18 years of age at higher risk for exacerbations, we placed a higher value on reducing exacerbations. We also considered the availability of other treatment options for this patient group.
Review of evidence by outcomes
Overall quality of evidence across all critical outcomes (severe exacerbations, non-severe exacerbations, asthma control, Safety/mortality): Very low certainty
There were no trials that looked at this question in individuals with well-controlled asthma on PRN SABA. There was 1 meta-analysis of intermittent ICS compared to placebo for mild persistent asthma,Citation59 which included trials using PRN ICS-SABA (1 preschool,Citation53 1 pediatricCitation36 and 1 adult trial.Citation38) However, because that review also included data from trials that used short courses of ICS, which may not have the same efficacy as PRN ICS-SABA, the meta-analysis could not be used. Data for this recommendation were derived from the 3 aforementioned trials: 1 that included children (1-4 years of age) with 3 or more episodes of wheezing requiring medical attention in the last 6 months (excluding those that needed systemic steroids);Citation53 1 in children (TREXA, 6-18 years of age) controlled on low dose ICS or with a history of 1-2 exacerbations in the previous year on no controller therapy;Citation36 and 1 in adults (BEST, ≥18 years of age) with symptoms more than once a week but less than once a day, nocturnal symptoms more than twice a month and exacerbations that may affect activity and sleep.Citation38 The preschool trial used a nebule that had a combination of beclomethasone and salbutamol (beclomethasoneEUR 800 mcg + salbutamol 1600 mcg), the pediatric trial used salbutamol 100 mcg and beclomethasone 50 mcg in 2 separate inhalers,Citation36 and the adult trial used an inhaler that had a combination of beclomethasone and salbutamol in a single inhaler (beclomethasoneEUR 250 mcg + salbutamol 100 mcg) (Appendix 2).
1. Severe exacerbations
In preschoolers, there was no difference in exacerbations requiring systemic steroids between the PRN beclomethasone-SABA (beclo-SABA) and PRN SABA groups (OR 0.48, 0.15-1.57).
There were no trials in children over 4 years of age or in adults that directly contributed to this outcome, as the exacerbation outcome reported in the pediatricCitation36 and adultCitation38 trials combined both severe and non-severe exacerbations (by our definition). The TREXA trial did report first exacerbation requiring prednisone; however, prednisone was given per protocol definition of exacerbation (any of the following: use if more than 12 puffs of SABA in 24 hours, peak expiratory flow <70% of reference value before SABA, symptoms leading to inability to sleep or do daily activities for 2 or more consecutive days, peak expiratory flow of less than 50% of reference value despite relief treatment, ED visit), which did not meet our definition for severe exacerbations.Citation36 The definition for severe exacerbation in the BEST trial did include use of oral corticosteroids but also considered a peak flow <30% below baseline for 2 days and use of more than 8 puffs of rescue inhaler for 3 days as “severe exacerbation.”Citation38
2. Non-severe exacerbations
There were no trials in any age group that directly contributed to this outcome, as none looked exclusively at non-severe exacerbations.
In preschoolers, there was no difference in the time to first exacerbation (including exacerbations of all severities) between the PRN beclo-SABA and PRN SABA groups (p = 0.88).Citation53
In children, the TREXA trial did not find a statistically significant difference in the probability of an exacerbation (including exacerbations of all severities) in the PRN beclo-SABA group versus the PRN SABA group (HR 0.62, 0.37–1.05, p = 0.073).Citation36
In adults, the BEST trial found a decreased percentage of patients with at least 1 exacerbation (including exacerbations of all severities) in the PRN beclo-SABA group compared to the PRN SABA group (4.92% vs 17.8%, p = 0.002).Citation38 There was no significant difference in the percentage of patients with at least 1 “severe” exacerbation in the PRN beclo-SABA group compared to the PRN SABA group (0% vs 3.4%, p = 0.057), although there were only 10 “severe” exacerbations in 242 patients.
3. Asthma control
In preschoolers, there was an increase in symptom-free days only when looking at weeks 9 to12 (PRN beclo-SABA 77.4 vs PRN SABA 69.5, p = 0.033), but not when looking at weeks 1 to 12 (PRN beclo-SABA 64.9 vs PRN SABA 61, p = 0.248).Citation53
In children the TREXA trial showed no significant difference in the number of days with well-controlled asthma between the PRN beclo-SABA group and the PRN SABA group, with both groups having 80-90% of days with well-controlled asthma.Citation36
In adults, the BEST trial found no difference in most measures of asthma control, except for a decrease in the nocturnal awakening score with PRN beclo-SABA compared to PRN SABA (0.1 vs 0.21, difference -0.1, p = 0.03).Citation38 There was no difference in the daytime asthma symptom score (difference -0.28, p = 0.11), rescue medication use/day (difference -0.16, p = 0.11) or symptom free days (difference 5.69, p = 0.13).
4. FEV1
In children in the TREXA trial, there was no difference in FEV1 between groups, or in methacholine challenge (PC20) results at week 24.Citation36
In the adult BEST trial, there was a 3.89% difference in the improvement in FEV1% predicted in the PRN beclo-SABA group versus the PRN SABA group (p = 0.005).Citation38
5. Inflammation
The pediatric trial measured FeNO at baseline and then every 8 weeks starting at week 8 and did not report a difference between the PRN beclo-SABA versus PRN SABA groups, although both groups had an elevated FeNO throughout the study compared to the groups that were on daily beclomethasone.Citation36
6. Safety/mortality
There was no difference in severe adverse events in any of the trials.Citation36,Citation38,Citation53 In the preschool trial there was no difference in morning salivary cortisol between the 2 groups,Citation53 and in the pediatric trial no difference in growth between the PRN beclo-SABA versus SABA group.Citation36 There was 1 serious adverse event in the adult trial, which was hemoptysis of undetermined cause in a patient on PRN beclo-SABA,Citation38 and 1 severe adverse event in the preschool trial in the PRN SABA group (details of event not reported).
Expert panel discussion of additional considerations and clinical judgment of risk versus benefit
Evidence for this recommendation was extrapolated from studies that included individuals with more severe asthma. The panel felt that the use of PRN ICS-SABA compared to PRN SABA was more relevant for individuals with less severe asthma, as the current standard of care for this group would be PRN SABA.
In individuals with more frequent symptoms, there is evidence from 1 RCT in those 18 years of age and older that taking an ICS each time SABA is taken reduces “severe” asthma exacerbations, improves some aspects of asthma control and improves lung function compared to PRN SABA.Citation38 This adult trial included a broader definition of severe exacerbations, and therefore, there is not strong evidence for the prevention of severe exacerbations when comparing these 2 regimens. Also, this trialCitation38 used a single inhaler containing SABA (100 mcg salbutamol) and beclomethasoneEur (250 mcg). These data cannot be generalized to our setting, as there is no single inhaler containing SABA and ICS approved for use in Canada, and none of the ICS medications are approved to be used as needed. However, given that this strategy has been shown to decrease asthma exacerbations, it could be considered as a harm reduction strategy in those 18 of years and older at higher risk for exacerbations, and who cannot take daily ICS + PRN SABA or PRN bud/form.
There is limited evidence of benefit in individuals <18 years of age. For children 6-18 years of age with more frequent symptoms or a history of severe exacerbation, there was a nonsignificant trend that an ICS taken each time a SABA was taken decreases exacerbations compared to PRN SABA, with no difference in safety outcomes (of note in that trial, daily ICS significantly decreased exacerbations compared to PRN SABA).Citation36 In children 1-4 years of age, there was no difference in exacerbations but an improvement in some measures of asthma control, with no difference in safety outcomes.Citation53 However, that trial used nebulized medication, which is not the preferred modality for delivery of asthma medication in Canada (it also used a nebule that contained ICS and SABA, which is not available in Canada). Given the possibility of overuse of ICS in this patient group, the current level of evidence, and the lack of a combined ICS-SABA inhaler on the Canadian market, the panel does not recommend this strategy for individuals under 18 years of age.
Future research question
In individuals with well-controlled asthma on PRN SABA, particularly those at higher risk for exacerbation, is an ICS taken whenever a SABA is taken safe and effective at preventing exacerbations compared to PRN SABA?
PICO 3. In individuals on PRN SABA with poorly-controlled asthma is:
a) Daily ICS + PRN SABA safe and more effective than PRN SABA?
b) PRN bud/form safe and more effective than daily ICS + PRN SABA?
Recommendations
3.1 We recommend that all individuals on PRN SABA with poorly-controlled asthma take a daily ICS + PRN SABA instead of PRN SABA or PRN bud/form. (Strong recommendation)
3.2 In individuals ≥12 years of age with poor adherence to daily medication despite substantial asthma education and support, we recommend PRN bud/form instead of daily ICS + PRN SABA. (Strong recommendation)
Clinical remarks
Before escalating therapy, any individual with poorly-controlled asthma should be assessed to determine what can be done to improve asthma control, including an assessment of inhaler technique, adherence to medication, trigger avoidance, and treatable comorbidities.
There are no data on the safety or efficacy of PRN bud/form in children under age 12 and bud/form is not approved for use in Canada for that age group. In individuals ≥12 years of age, bud/form 200/6 mcg 1 puff PRN is approved for use in Canada, to a maximum of 6 puffs in a single occasion and a maximum of 8 puffs per day.
Patient values and preferences
We placed a high value on daily asthma control and prevention of exacerbations. We placed relatively low value on affordability and convenience of treatment.
Review of evidence by outcomes
Overall quality of evidence across all critical outcomes (severe exacerbations, non-severe exacerbations, asthma control, Safety/mortality):
Daily ICS versus PRN SABA: High certainty
PRN bud/form versus Daily ICS + PRN SABA: Moderate certainty
No available meta-analysis of RCTs. Six RCTs were included in the comparison of daily ICS + PRN SABA and PRN SABA (Appendix 2).Citation36–38,Citation44,Citation45,Citation53 This included 1 blinded RCT in preschoolers 1–4 years of age (BEST pediatric, n = 166),Citation53 1 blinded RCT in children 6-18 years of age (TREXA, n = 146),Citation36 1 blinded RCT in children and adults (4–66 years of age) (START, n = 7165),Citation45 2 blinded RCTs in adults (SYGMA1, ≥12 years of age, n = 2570,Citation37 BEST, 18-65 years of age, n = 224),Citation38 and an unblinded RCT in adults (NovelSTART, 18-75 years of age, n = 448).Citation44 The START and NovelSTART trials included an almost equal number of patients with symptoms ≤2/week and >2/week (Appendix 2).
Four RCTs compared the effectiveness of bud/form PRN to daily budesonide + PRN SABA; however, 2 of these studies were unblinded.Citation15,Citation34,Citation37,Citation44 The 2 blinded RCTs included patients 12-18 years old (SYGMA1, total n = 2569)Citation37 (SYGMA2, total n = 4176),Citation34 and the unblinded trials included patients 18-75 with a patient report of physician diagnosed asthma which did not require objective confirmation (NovelSTART, n = 445,Citation44 PRACTICAL, n = 885Citation15) (Appendix 2).
1. Severe exacerbations
Daily ICS + PRN SABA versus PRN SABA
In preschoolers, daily beclomethasone increased the time to first exacerbation requiring oral steroids compared to PRN SABA (p = 0.01).Citation53
In older patients, the data for severe exacerbations for daily ICS + PRN SABA compared to PRN SABA is from 3 studies (2 blinded RCTs and 1 unblinded RCT) looking at the effectiveness of daily ICS (budesonide 200 mcg daily for those under age 12 and otherwise 400 mcg daily) compared to PRN SABA.Citation37,Citation44,Citation45
In children and adults, daily budesonide use was associated with a significant decrease in the annualized severe exacerbation rate and reduced the risk of a first severe asthma-related event over 36 months by 44% (HR 0.56, 95% CI 0.45-0.71).Citation37,Citation45 Daily budesonide also prolonged the time to first severe exacerbation (p < 0.0001).Citation45 Combining data from these studies led to a NNT of 33 (95% CI 17-100) to prevent a patient from having a severe exacerbation.Citation37,Citation44,Citation45
SYGMA1 found in children (≥12 years of age) and adults, that the proportion of patients with a severe exacerbation requiring systemic prednisone for ≥3 days was 5.8% in the budesonide group and 11% in the terbutaline PRN group.Citation37 In the NovelSTART study, the number of severe exacerbations was similar between daily budesonide and PRN SABA, but there was a trend that the annualized exacerbation rate was lower in the budesonide group (0.175 vs 0.4, no statistics available).Citation44
PRN bud/form versus Daily ICS + PRN SABA
In children ≥12 years of age and adults involved in the 2 blinded RCTs (SYGMA-1 and 2),Citation34,Citation37 there was no significant difference in the annual rate of exacerbations [(Rate ratio 0.83, 95% CI 0.59-1.16) and (Rate ratio 0.97, 1-sided CI 1.16)] or time to first exacerbation [(HR 0.9, 95% CI 0.65-1.24) and (HR 0.97, 95% CI 0.78-1.17)] between the bud/form arm and the daily budesonide arm.
In the 2 unblinded RCTs, involving adults ≥18 years of age, there was a relative risk reduction for severe exacerbations with bud/form compared to daily budesonide in the NovelSTART study (RR 0.44, 95% CI 0.2-0.96) and a trend towards benefit in the PRACTICAL study.Citation15,Citation44 Furthermore, the time to first severe exacerbation was longer in the bud/form arm versus daily budesonide (HR 0.60, 95% CI 0.40-0.91) in the PRACTICAL study.Citation15 Adherence in the daily budesonide arm varied from 56 to 78% in all studies but was lowest in the NovelSTART study, which showed the largest benefit of PRN bud/form over daily budesonide.Citation44
2. Non-severe exacerbations
Daily ICS + PRN SABA versus PRN SABA
Although no studies looked exclusively at non-severe exacerbations, 5 RCTs (including 1 unblinded RCT) reported all exacerbations (including moderate to severe), comparing budesonide 200 mg bid or beclomethasoneEUR 250 mcg bid (beclomethasone 50 mcg bid for ages 6-18 yr, beclomethasoneEUR 400 mcg bid nebules for age 1-4 yr) (as the ICS) to terbutaline or salbutamol (as the SABA).Citation36–38,Citation44
In adults, all studies showed a reduction in the rate of moderate to severe exacerbations in the ICS arm compared to SABA (annualized exacerbation rate ratio varied from 0.42 to 0.44).Citation37,Citation44 The number of patients with at least 1 exacerbation was significantly lower in the beclomethasone arm compared to SABA (5.66% vs 17.8%, p = 0.005).Citation38
In children 6-18 years of age, daily beclomethasone was associated with a significantly lower frequency of exacerbations (28% vs 49%, p = 0.03) vs SABA PRN.Citation36
In preschoolers, there was an increase in time to any first exacerbation (severe and non-severe) in patients on daily beclomethasone (p = 0.03).Citation53
PRN bud/form versus Daily ICS + PRN SABA
Three studies, 2 including children 12 to 18 years of age, compared the effectiveness of PRN bud/form compared to daily budesonide + PRN SABA in reducing severe and non-severe exacerbations; the results were variable.Citation15,Citation37,Citation44 SYGMA-1 (children ≥12 years of age and adults) was the only blinded study and there was no significant difference in the annualized rate of exacerbations between the PRN bud/form and daily budesonide + PRN SABA group.Citation37 Results in the unblinded RCTs (NovelSTART and PRACTICAL) in adults ≥18 years of age diverged. The PRACTICAL trial showed a reduced relative rate of exacerbations in the bud/form arm (RR 0.70, 95%CI 0.51-0.95) and the NovelSTART trial found no significant difference between daily budesonide and PRN bud-form (RR 1.12, 95% CI 0.70-1.79). The time to event analysis showed similar results.Citation15,Citation44
3. Asthma control
Daily ICS + PRN SABA versus PRN SABA
There were 5 blinded RCTs and 1 unblinded RCT that examined the outcome of asthma control, comparing daily ICS to PRN SABA. Three studies involving children and adults used budesonide 400 mcg per day (200 mcg once daily if <11 years of age),Citation37,Citation44,Citation45 1 study in preschoolers used beclomethasoneEUR 400 mcg bid (nebulized),Citation53 and the others used beclomethasone250EUR mcg bid for >18 years of age,Citation38 and beclomethasone 50 mcg bid for children 6-18 years of age.Citation36 All of the trials, except TREXA,Citation36 showed a significant increase in either symptom free days, or well-controlled asthma weeks in favor of the daily ICS group.Citation37,Citation44,Citation45,Citation53
PRN bud/form versus Daily ICS + PRN SABA
Four studies compared asthma control between PRN bud/form and daily budesonide + SABA PRN, 2 of which were blinded RCTs and 2 unblinded RCTs. The outcomes measured were ACQ-5 and/or weeks with well-controlled asthma. Three of the studies showed better asthma control with daily budesonide compared to PRN bud/form, in terms of change in ACQ-5 score or mean weeks of well-controlled asthma.Citation15,Citation34,Citation37 Although these results were statistically significant, most of the differences in ACQ-5 score did not meet the minimal clinically important difference for this measure. Only the NovelSTART trial showed no difference in ACQ-5 scores between the 2 groups.Citation44
4. FEV1
Daily ICS + PRN SABA versus PRN SABA
Four blinded RCTs and 1 unblinded RCT examined the difference in FEV1 between daily ICS (budesonide 200-400 mcg total daily dose or beclomethasone 100-500 mcg total daily dose) + PRN SABA and PRN SABA alone. In children ≥12 years of age and adults, SYGMA1 and START trialsCitation37,Citation45 showed a difference in pre-BD FEV1 between the groups in favor of the daily budesonide arm, although for SYGMA1 no statistical analysis was presented for these data as this was not a pre-specified comparison (mean change from baseline FEV1 119.3 mL in budesonide vs 11.2 mL in SABA arm).Citation37 In the START study, small but statistically significant improvements were seen in both pre and post-BD FEV1 in the daily budesonide group compared to PRN SABA (difference between daily budesonide versus PRN SABA in pre-BD FEV1 2.24% (SE 0.31), p < 0.0001, post-BD FEV1 1.48% (SE 0.22), p < 0.0001) at 1 year and this difference was still seen at 3 years (difference between daily budesonide vs PRN SABA in pre-BD FEV1 1.71% (SE 0.32), p < 0.0001, post-BD FEV1 0.88% (SE 0.25), p < 0.0001).Citation45
PRN bud/form versus Daily ICS + PRN SABA
Both SYGMA 1 and 2 looked at change in baseline pre-BD and post-BD FEV1 in children ≥12 years of age and adults, comparing PRN bud/form to daily budesonide + PRN SABA. In both studies, there was a small but significant improvement in pre-BD FEV1 in the daily budesonide group compared to PRN bud/form group (54.3 mL and 32.6 mL improvements in SYGMA 1 and 2, respectively).Citation34,Citation37
In adults, both the NovelSTART and PRACTICAL unblinded RCTs showed no significant difference in mean FEV1 between daily budesonide and PRN bud/form.Citation15,Citation44
5. Inflammation
Daily ICS + PRN SABA versus PRN SABA
Only 2 studies looked at inflammatory markers, primarily the FeNO. One trial was a blinded RCT and the other an unblinded RCT. Both studies, which involved children and adults, showed a significantly lower FeNO in the daily ICS group compared to PRN SABA (p < 0.0001).Citation36,Citation44
PRN bud/form versus Daily ICS + PRN SABA
FeNO levels were also compared between daily budesonide and PRN bud/form in 2 open-label RCTs involving adults ≥18 years of age. The median FeNO was not different between the 2 arms of the studies but the geometric mean of FeNO was higher with PRN bud/form compared to daily budesonide in both studies, albeit the difference was small (1.13, 95% CI 1.07-1.21 and 1.13, 95% CI 1.02-1.25 NovelSTART and PRACTICAL trials, respectively).Citation15,Citation44
6. Safety/mortality
Daily ICS + PRN SABA versus PRN SABA
In terms of mortality, there was no significant difference between the groups; however, there was 1 asthma related death in the SABA group in the START trial.Citation45 The SYGMA trial showed slightly more increased adverse events and discontinuation due to adverse events in the SABA groups compared to daily ICS (42.7% vs 39.9% adverse events, 2.9% vs 1.2% treatment discontinuation due to adverse events).Citation37
Among the trials which included children (<18 years of age) there was a significant decline in linear growth in the daily ICS groups compared to groups with SABA PRN monotherapy.Citation36,Citation45 In the TREXA trial, which included only children, there was a 1.1 cm difference in linear growth between children on beclomethasone compared to PRN SABA.Citation36 The preschool trial did not report growth parameters and there was no difference in drug related adverse events or morning salivary cortisol in that trial.Citation53
PRN bud/form versus Daily ICS + PRN SABA
Overall, there were no significant differences in mortality between the daily ICS and PRN bud/form groups. There was 1 death in the SYGMA2 trial in the daily budesonide group, related to asthma.Citation34 In terms of others adverse outcomes, they were not significantly different between daily budesonide and PRN bud/form.Citation15,Citation34,Citation37,Citation44 The most common adverse events were viral upper respiratory tract infection (URTI) and nasopharyngitis.
Expert panel discussion of additional considerations and clinical judgment of risk versus benefit
As discussed in the expert panel discussion for PICO 1, there is no evidence to date that those with symptoms 0-2 times per week as opposed to >2 times per week have a differential response to daily ICS. This recommendation for ICS use pertains to individuals with poorly-controlled asthma, as defined by symptoms >2 times per week (among other poor control criteria) and in this group there is additional evidence (SYGMA1, TREXA) to increase the strength of evidence for daily ICS compared to PRN SABA. In addition, the acceptability of daily treatment in a group with more frequent symptoms or a history of exacerbations requiring oral steroids (which is associated with a greatly increased risk for future exacerbations) was felt to be higher than in the patient group with well-controlled asthma and a lower risk of exacerbation. Although the evidence presented for daily ICS + PRN SABA versus PRN SABA included only 1 trial with children <6 years old, the additional evidence in favor of daily ICS from 2 systematic reviewsCitation60,Citation61 was discussed in the 2015 CTS/CPS Diagnosis and management of asthma in preschoolers position statement.Citation18
Although there is evidence that PRN bud/form is similar to daily ICS in reducing exacerbations (NovelSTART, PRACTICAL, SYGMA1/2), the prior extensive evidence demonstrating the benefit and safety of daily ICS for reducing exacerbations when compared to PRN SABA alone,Citation62–65 and improved asthma control, lung function and inflammation with daily ICS compared to PRN bud/form in some trials, was considered by the panel when recommending daily ICS over PRN bud/form.
Future research questions
In individuals with poorly-controlled asthma on PRN SABA, are other formulations of ICS/formoterol used PRN as safe and effective as PRN bud/form, daily ICS + PRN SABA, or PRN SABA?
In individuals with poorly-controlled asthma on PRN SABA, aged 6-11 years old, is PRN bud/form safe and effective compared to daily ICS + PRN SABA, or PRN SABA?
In individuals with poorly-controlled asthma on PRN SABA, aged 12-18 years old, does PRN bud/form cause a decrease in linear growth or other adverse events (i.e., adrenal suppression) assessed for more commonly in the pediatric population?
PICO 4. In individuals on PRN SABA with poorly-controlled asthma is ICS taken each time SABA is taken (PRN ICS-SABA) safe and more effective than daily ICS + PRN SABA?
Recommendations
4.1 We recommend that individuals on PRN SABA with poorly-controlled asthma take daily ICS instead of taking an ICS each time a SABA is taken.
As a harm mitigation strategy, we recommend that individuals ≥18 years of age at higher risk for exacerbations who are unable to take a daily ICS + PRN SABA or PRN bud/form (as per recommendations 3.1 and 3.2), can be given the option of taking an ICS each time a SABA is taken. (Strong recommendation)
Clinical remarks
Before escalating therapy, any individual with poorly-controlled asthma should be assessed to determine what can be done to improve asthma control, including an assessment of inhaler technique, adherence to medication, trigger avoidance, and treatable comorbidities.
In Canada, ICS are not currently approved by Health Canada to be used on a PRN basis. The clinical trials that evaluated this strategy in adults using 2 separate inhalersCitation58 used a regimen of beclomethasone 50 mcg 2 puffs each time salbutamol 100 mcg 2 puffs was used. If practitioners recommend this strategy (off-label), we suggest that the maximum approved daily ICS dose should not be exceeded (see ).
Patient values and preferences
For this recommendation, we placed a high value on minimizing the potential for improper use of this medication regimen given the lack of a single inhaler containing an ICS and SABA. We placed a moderate value on decreasing the risk of exacerbation in individuals at risk for exacerbation and a relatively low value on affordability of medication.
Review of evidence by outcomes
Overall quality of evidence across all critical outcomes (severe exacerbations, non-severe exacerbations, asthma control, safety/mortality):
≥12 years of age, 6-11 years of age: Low to moderate certainty with no discrete data for severe exacerbations
1-5 years of age: Low certainty
No available meta-analysis of RCTs. There were 5 blinded RCTs that compared daily ICS + PRN SABA to PRN ICS-SABA (Appendix 2). Two trials were in preschool-aged children (INFANT, BEST pediatric, n = 680),Citation35,Citation53 1 in children 6-18 years of age (TREXA, n = 143),Citation36 and 2 in adults ≥ 18 years of age (BEST, BASALT n = 457).Citation38,Citation58 The design of all of the trials was similar except for the INFANT trial, which was a triple cross-over trial examining differential response to medication (fluticasone propionate 50 mcg 2 puffs bid + PRN SABA, fluticasone propionate 50 mcg 2 puffs each time salbutamol 100 mcg 2 puffs given, daily montelukast + PRN SABA) and the BASALT trial, which compared symptom-based management (beclomethasone 50 mcg 2 puffs each time salbutamol 100 mcg 2 puffs was used) to physician-based care (beclomethasone 50 mcg 2 puffs bid + PRN SABA adjusted every 2-6 weeks by physician as per NHLBI guidelines) to FeNO-based care (beclomethasone 50 mcg 2 puffs bid + PRN SABA adjusted based on FeNO).Citation58
1. Severe exacerbations
There were 2 studies in preschoolers that looked at severe exacerbations. One compared beclomethasoneEUR 800 mcg/day to beclomethasoneEUR 800 mcg + salbutamol 1600 mcg/vial PRN via nebulizer,Citation53 and the other compared fluticasone 200 mcg/day to fluticasone 100 mcg taken with salbutamol 200 mcg PRN via MDI + spacer.Citation35 One study found no difference in time to first severe exacerbation (p > 0.1)Citation53 and the other found decreased exacerbations in patients on daily ICS compared to the group that received PRN ICS-SABACitation35 (daily ICS n = 47 vs PRN ICS-SABA n = 69, p = 0.027), although no difference in hospitalizations was shown (note that there was only 1 hospitalization in the trial).
There were no trials in children over 4 years of age or adults that contributed to this outcome as the definition of exacerbations used in TREXA, BEST, BASALT Citation36,Citation38,Citation58 included both severe and non-severe exacerbations according to our definition. TREXA did report first exacerbation requiring prednisone, however, prednisone was given per protocol definition of exacerbation (any of: use of more than 12 puffs of SABA in 24 hours, peak expiratory flow <70% of reference value before SABA, symptoms leading to inability to sleep or do daily activities for 2 or more consecutive days, peak expiratory flow of less than 50% of reference value despite relief treatment, ED visit), which did not meet our definition for severe exacerbations.Citation36 The definition for severe exacerbation in BEST did include use of oral corticosteroids but also considered a peak flow <30% below baseline for 2 days and use of more than 8 puffs of rescue inhaler for 3 days as “severe exacerbation.”Citation38 BASALT included use of oral steroids, but also increased ICSs or additional medications for asthma in the definition of “severe exacerbation.”Citation58
2. Non-severe exacerbations
In preschoolers, the time to first non-severe or severe exacerbation was longer in the daily beclomethasone versus PRN beclo-SABA group (p = 0.03).Citation53
In children, the TREXA study comparing beclomethasone 100 mcg to PRN beclomethasone 100 mcg each time salbutamol 200 mcg was used did not statistically compare exacerbations between these 2 arms. However, compared with the PRN SABA group, there was a significantly decreased risk of any asthma exacerbations in the daily beclomethasone group (HR 0.49, 95% CI 0.28-0.85, p = 0.033) but the difference was not statistically significant in the PRN beclo-SABA group (HR 0.62, 95% CI 0.37-1.05, p = 0.073).Citation36
In adults, the 2 trials that provided data for this outcome had slightly different study designs but found similar results. The BEST trial compared symptom-driven use of PRN beclomethasoneEUR 250 mcg/day + salbutamol 100 mcg (in single inhaler) with a fixed dose of beclomethasoneEUR 500 mcg/day.Citation38 It found that the mean number of non-severe exacerbations per patient per year was not different in the daily beclomethasone versus PRN beclo-SABA groups (0.71 vs 0.74, p = 0.099), and both of these groups had decreased number of non-severe exacerbations/patient/year compared to the PRN SABA group (1.63, P < 0.001).Citation38 The BASALT trial compared symptom-driven use of PRN beclomethasone 100 mcg each time SABA taken with a physician adjusted dose of beclomethasone every 2 to 6 weeks.Citation58 The exacerbation rate was not statistically different in the physician adjusted beclomethasone group (0.23) compared to the PRN beclomethasone SABA group (0.12), with a HR of 2 (97.5% CI 0.8-5.4).Citation58
3. Asthma control
In preschoolers, there were more days with well-controlled asthma in patients on daily fluticasone (94%) versus PRN fluticasone-SABA (88.4%, p = 0.001) in 1 studyCitation35 and no difference in symptom-free days in the daily beclomethasone group (64.9%) versus the PRN beclo-SABA group in another (64.9%, p = 0.293).Citation53
In children, there was no difference in the proportion of asthma control days in the daily beclomethasone versus PRN beclo-SABA groups (80-90% in both groups). They also reported no difference in asthma control tests and frequency of SABA use.
In adults, there was no difference in daytime asthma symptom scores, nocturnal awakenings, rescue medication use or symptom-free days in the daily beclomethasone versus PRN beclo-SABA groups in 1 studyCitation38 and no difference in mean ACQ scores in another study (difference in mean ACQ in PRN beclo-SABA compared with daily beclomethasone 0.01 (95% CI -0.19-0.18).
4. FEV1
In children, TREXA found no significant differences in FEV1 between the PRN beclo-SABA group and the daily beclomethasone group. However, the FEV1 did decrease significantly from baseline in the PRN beclo-SABA group (-4.1% SD 1.8, p = 0.024) and not in the daily beclomethasone group (specific data not provided in manuscript or appendix). There was no difference in the methacholine challenge PC20 between these 2 groups at 24 weeks.Citation36
In adults, there was no difference in FEV1 between the daily beclomethasone and PRN beclo-SABA groups.Citation38,Citation58 BEST found that at 6 months there was no difference in the FEV1% predicted between the daily beclomethasone (90.32+/-1.25%) and the PRN beclo-SABA (92.23+/-1.05%, difference 2.07, 95% CI -0.71-4.79) groups.Citation38 BASALT found that the difference in pre-BD FEV1% predicted in the PRN beclo-SABA versus the daily beclomethasone group was 0.01 (95% CI -2.17–2.18).Citation58
5. Inflammation
In children, there was no difference in FeNO between study groups at the randomization visit. Increases in FeNO, beginning at week 8, were seen in individuals in the PRN beclo-SABA and PRN SABA groups, whereas individuals in the daily beclomethasone group (and the group receiving daily beclomethasone + PRN beclo-SABA) had significantly lower FeNO compared to the PRN beclo-SABA and PRN SABA groups (p < 0.0001).Citation36
In adults, there was no difference in the natural log FeNO between the daily ICS group and PRN beclo-SABA groups (difference in natural log PRN ICS-SABA vs daily ICS, 0.13, 95% CI -0.08-0.34, p = 0.15).Citation58 There was also no difference in sputum eosinophils (%) in the 2 groups (difference PRN beclo-SABA vs daily beclomethasone 0.25, 05% CI 0.2-1.4, p = 0.11).Citation58
6. Safety/mortality
In preschoolers, there was no significant difference in drug related adverse events and no serious adverse events were reported in either trial. Morning salivary cortisol reported in 1 trialCitation53 was no different than baseline in either group, and height velocity was not different between the 2 groups over 16 weeks (difference in height velocity with PRN fluticasone-SABA vs daily fluticasone 0.20, standard deviation (SD 0.2097, p = 0.34).Citation35
In the pediatric trial, TREXA found 1 individual with a severe adverse event in these 2 groups (viral meningitis in a subject on daily beclomethasone). Children in the daily beclomethasone group grew 1.1 cm (SD 0.3) less than the children in the PRN SABA group (p < 0.0001),Citation36 but there was no significant difference in linear growth in the PRN beclomethasone-SABA groupCitation36 (0.3 cm, SD 0.2, p = 0.26) compared to the PRN SABA group.
In adults, there was no difference in severe adverse events in the PRN beclo-SABA compared to daily beclomethasone groups.Citation38,Citation58 Serious adverse events were reported in only 2 patients: 1 patient receiving PRN ICS-SABA had hemoptysis of undetermined cause; and 1 patient receiving daily ICS had myocardial ischemia.Citation38,Citation58
Expert panel discussion of additional considerations and clinical judgment of risk versus benefit
In children 1-4 years of age, there was a decrease in the number of severe exacerbations with daily ICS versus PRN ICS-SABA in 1Citation35 of 2 trials, with patients having an elevated serum eosinophil count (>300 cells/µL) and aeroallergen sensitization predicting the best response to daily ICS in that trial. There was an improvement in asthma control in 1 of 2 trials, with no difference in safety outcomes including growth velocity.Citation35,Citation53
For children 6-18 years of age, daily ICS but not PRN ICS-SABA was shown to decrease the time to first exacerbation compared to placebo. There was 1.1 cm (SD 0.3 cm) less linear growth in the daily ICS group compared to placebo, with no difference in linear growth when comparing the PRN ICS-SABA group with placebo.Citation36
In individuals 18 years of age and older, there was no significant decrease in non-severe exacerbations or differences in asthma control between daily ICS and PRN ICS-SABA;Citation38,Citation58 however, one of these studiesCitation38 used a single inhaler containing ICS-SABA, making it difficult to apply those results to a Canadian population that would be using separate ICS and SABA inhalers.
Given the lack of data for severe exacerbations, the difficulty in implementing this strategy, and the other therapeutic options with stronger evidence available for this population, ICS-SABA is not recommended except as a harm reduction strategy in individuals ≥18 years of age who are unable to take daily ICS + PRN SABA or PRN bud/form.
Given the benefit of daily ICS, the possibility of overuse of ICS when used PRN with SABA PRN particularly in children (in whom growth and adrenal suppression are concerns), the current level of evidence, and the lack of a combined ICS-SABA inhaler on the Canadian market, the panel does not recommend this strategy for individuals under 18 years of age.
Future research questions
In children <6 years of age with poorly-controlled asthma on PRN SABA, do elevated serum eosinophils and aeroallergen sensitization consistently predict benefit from daily ICS?
What is the safety of PRN ICS-SABA compared to daily ICS in pragmatic, longer-term trials?
Are PRN ICS-SABA and daily ICS equivalent in preventing the decline in lung function with exacerbations?
PICO 5. In individuals on PRN SABA with poorly-controlled asthma or individuals on daily ICS with well-controlled asthma, is the use of a daily SABA inhalation immediately prior to daily ICS inhalation + PRN SABA safer or more effective than daily ICS + PRN SABA?
Recommendation
5.1 We recommend that individuals on PRN SABA with poorly-controlled asthma or individuals on daily low dose ICS with well-controlled asthma, take daily ICS + PRN SABA instead of daily ICS with a daily SABA inhalation immediately prior to ICS inhalation + PRN SABA. (Strong recommendation)
Clinical remarks
Although not recommended in any previous guidelines, the practice of using a SABA inhalation daily before ICS to “open the airways” and putatively enable the ICS to work more effectively is still encountered in clinical practice.
Individuals who currently use SABA prior to ICS, either habitually or following previous instruction, should be advised to discontinue this practice as it increases the risk of exacerbation and normalizes the daily use of SABA, which should be discouraged. Providers should be clear in their instructions to patients with a newly prescribed management plan of daily ICS + PRN SABA that SABA should be used on an as-needed basis only.
Patient values and preferences
For this recommendation we placed a high value on minimizing exacerbations.
Review of evidence by outcomes
Overall quality of evidence across all critical outcomes (severe exacerbations, non-severe exacerbations, asthma control, safety/mortality):
≥18 years of age: Low to Moderate certainty
<18 years of age: Very low certainty
No available meta-analysis of RCTs. There was 1 blinded RCT in adults (18-65 years of age), the BEST trial, that included patients with symptoms more than once a week but less than once a day, nocturnal symptoms more than twice a month and exacerbations that may affect activity and sleep that compared taking a SABA prior to daily ICS + PRN SABA to daily ICS + PRN SABACitation38(Appendix 2).
1. Severe exacerbations
There were no trials that used the same definition of severe exacerbations as our guideline had pre-defined. The definition for severe exacerbation in BEST did include use of oral corticosteroids but also included either a peak flow <30% below baseline for 2 days or use of more than 8 puffs of rescue inhaler for 3 days.Citation38
2. Non-severe exacerbations
In the BEST study, regular use of a SABA with an ICS was associated with almost double the risk of exacerbation (severe and non-severe) when compared to ICS alone with as needed SABA although given the small number of exacerbations in these 2 groups, this was not significant. This study found that 5.66% of patients taking daily beclomethasoneEUR 500 mcg/day + PRN SABA experienced at least 1 exacerbation in the study period, compared to 10.09% of patients taking daily beclomethasoneEUR + SABA (in 1 inhaler) + PRN SABA although this was not significant. There was a significant difference in the exacerbations per patient per year, with 0.71 exacerbations/patient/year in the daily beclomethasone + PRN SABA group and 1.76 exacerbations/patient/year in the daily beclomethasone + SABA (in 1 inhaler) + PRN SABA group (p < 0.001).Citation38
3. Asthma control
Despite an increase in exacerbations with use of SABA prior to daily ICS, there was no difference in asthma control or change from baseline compared to regular ICS + PRN SABA. Both daytime and nocturnal symptoms were patient-rated on a scale of 0 (no symptoms) to 4 (symptoms most of the day/did not allow sleep). Daytime symptom score was 0.87+/-1.53 in the beclomethasone group and 0.83+/- 0.63 in the beclomethasone with salbutamol group. Nocturnal symptom scores were also comparable, with 1.04+/- 0.17 in the beclomethasone group and 0.84+/-0.13 in the beclomethasone with salbutamol group. There was no difference in nocturnal waking (0.13+/-0.04 nights and 0.14+/-0.03 nights, respectively). Lastly, reliever use was similar, with 0.44+/-0.77 puffs per day in the beclomethasone group and 0.51+/-0.08 puffs per day in the beclomethasone with albuterol group.Citation38
4. FEV1Citation38
Lung function was similar between groups and demonstrated no significant change from baseline values. Patients in the daily ICS group demonstrated an FEV1 of 90.32+/-1.25% while those in the daily ICS + SABA (in 1 inhaler) + PRN SABA group had an FEV1 of 89.49+/-1.21%. This was similar to their baseline FEV1 of 88.8+/-11.1% and 87.2+/-10.7%, respectively (no statistical analysis provided for this secondary outcome as these 2 groups were not the main comparison groups).
5. Inflammation
This outcome was not examined in the clinical trialCitation38 that compared these interventions.
6. Safety/mortality
The number of adverse events was similar in all arms of the study,Citation38 with no increased safety risk based on treatment regime. Two patients on daily beclomethasone had oral candidiasis and 1 suffered myocardial ischemia. One patient on daily beclomethasone + SABA (in 1 inhaler) + PRN SABA withdrew from the study early due to acute tonsillitis.
Expert panel discussion of additional considerations and clinical judgment of risk versus benefit
One study included a comparison of daily ICS + PRN SABA and daily ICS + SABA (in 1 inhaler) + PRN SABA.Citation38 It found an almost doubled risk of exacerbations/patient/year (severe and non-severe) in the daily ICS + SABA (in 1 inhaler) + PRN SABA group compared to daily ICS + PRN SABA group. There was no benefit in asthma control or FEV1 in the daily ICS + SABA (in 1 inhaler) + PRN SABA group.Citation38
Although there is limited evidence for this recommendation and the trial only included patients over 18 years of age, given that this is not currently the standard of care, the harms of normalizing regular SABA use, biological plausibility that tachyphylaxis from daily SABA use could lead to increased exacerbations, and lack of evidence of benefit along with evidence of harm, taking a SABA before daily ICS is not recommended for any age group.
Future research question
What is the mechanism by which daily ICS + SABA and PRN SABA leads to increased exacerbations, whereas PRN ICS-SABA does not?
PICO 6. In individuals on PRN SABA with well- or poorly-controlled asthma is a short course of very high dose ICS taken with acute loss of asthma control safe and more effective than PRN SABA?
Recommendation
6.1 We do not suggest that individuals on PRN SABA with well- or poorly-controlled asthma take a very high-dose short course of ICS with acute loss of asthma control. (Weak recommendation)
As per recommendations 1.1 and 1.3, we recommend that: individuals on PRN SABA with well-controlled asthma at lower risk of asthma exacerbation have the option of taking PRN SABA monotherapy or daily ICS + PRN SABA, with those ≥12 years of age being provided with the additional option of PRN bud/form. As per recommendations 1.2 and 1.4, individuals on PRN SABA with well-controlled asthma at higher risk of exacerbations should take daily ICS (all ages), with those ≥12 years of age being provided with the additional option of PRN bud/form.
As per recommendation 3.1, individuals on PRN SABA with poorly-controlled asthma should take a daily ICS with PRN SABA.
Clinical remarks
The recommendation related to PICO 6 is specific to individuals on PRN SABA. Individuals already on maintenance ICS + PRN SABA are addressed in the aforementioned recommendations. In the face of an acute loss of asthma control, those individuals should intensify therapy in accordance with a self-management asthma action plan. These recommendations are unchanged from the CTS 2012 guidelineCitation12 for acute loss of control:
Adults with a history of severe exacerbations in the past year requiring systemic steroids should undertake a trial of increasing the ICS maintenance dose by 4- or 5-fold for 7 to 14 days
Children and adults on maintenance ICS monotherapy should not routinely double the dose of their ICS
Children on maintenance ICS monotherapy should not routinely increase the dose of their ICS by four-fold or more.Citation66
Patient values and preferences
For this recommendation, we placed a high value on minimizing the potential for side effects, given the high doses of ICS used in clinical trials of ICS for acute loss of asthma control (daily doses: budesonide 1600 mcg-2000 mcg, fluticasone propionate 1500 mcg, beclomethasoneEUR 2250 mcg). A lower value was placed on exacerbation prevention, symptom scores and convenience of regimen.
Review of evidence by outcomes
Overall quality of evidence across all critical outcomes (severe exacerbations, non-severe exacerbations, asthma control, Safety/mortality): Low to Moderate certainty
There was 1 meta-analysis in patients of all ages,Citation59 1 in preschoolersCitation61 and 5 trialsCitation67–71 all in children under 6 years of age included in this evidence review. The systematic review in all ages looked at the use of intermittent ICS versus placebo for persistent asthma, however, combined data from trials using PRN ICS-SABACitation36,Citation38,Citation53 with trials using short course ICS for symptomatic worsening.Citation67,Citation69,Citation70 Given that there may be a difference in outcomes for trials using PRN ICS-SABA compared to trials using short course ICS with symptoms, the data from that meta-analysis was not included below. The evidence for this recommendation, therefore, comes from 1 meta-analysis (including 3 conventional RCTs)Citation61 and 2 crossover RCTsCitation68,Citation71 in preschoolers (total n = 431) using short courses of very high doses of ICS with onset of symptoms, compared to PRN SABA.Citation67–71 Doses of ICS used in these trials included budesonide 1 mg nebulized bid,Citation67 fluticasone MDI 750 mcg bid,Citation69 budesonide MDI 1600 mcg to 3200 mcg per day,Citation68,Citation70 or beclomethasoneEUR MDI 2250 mcg per day,Citation71 for durations ranging from 5 to 10 days (Appendix 2).
1. Severe exacerbations
The meta-analysis found that short courses of very high doses of ICS reduced the risk of severe exacerbations (RR 0.68, 95% CI 0.53-0.86).Citation61 The 2 crossover trials did not find a difference in the number of exacerbations requiring systemic steroids, although they were small and not powered for this outcome.Citation68,Citation71
2. Non-severe exacerbations
Non-severe exacerbations were not assessed in the RCTs.
3. Asthma control
The meta-analysis did not find a difference in asthma free days.Citation61 Some measures of asthma control were improved in the very high dose short course ICS group compared to the PRN SABA group in some trials. Improvement was seen in scores of daytime and night symptomsCitation71 and daytime and nighttime wheeze, but no differences were seen in daytime and nighttime cough, usage of SABA, duration of asthma symptoms,Citation68 or wheezing or breathlessness scores.Citation70
4. FEV1
This outcome was not assessed in the RCTs.
5. Inflammation
This outcome was not assessed in the RCTs.
6. Safety/mortality
The meta-analysis did not report adverse events given the lack of systematic documentation in RCTs. Two trials specifically reported safety data.Citation67,Citation69 There were 8 serious adverse events in the very high-dose short course ICS group compared to 13 in the PRN SABA group, however, the 9 adverse events in the PRN SABA group reported in 1 studyCitation69 were judged not to be related to the study.
In the study comparing fluticasone 1500 mcg/day (to a maximum of 10 days) to PRN SABA (n = 129), there were statistically significant differences in gains from baseline in measures of both height and weight between the study groups, with the fluticasone group increasing less than the control group (difference in height increase from baseline, -0.61cm (95% CI -1.31-0.09), difference in height z-score from baseline -0.24 (-0.4 to -0.08), difference in weight from baseline -0.71 kilogram (kg) (95% CI -1.19 to -0.24) and difference in weight z-score from baseline -0.26 (95% CI -0.41 to -0.09).Citation69 There was a correlation between cumulative ICS dose and height (r= -0.21, p = 0.02), but not weight (r = -0.11, p = 0.21). In terms of bone mineral density, basal cortisol level and adverse events, there were no differences between the groups. The same study noted an average of 8 URTIs per patient, with 89-93% of URTI episodes associated with asthma symptoms. The other trial that compared budesonide 2 mg/day nebulized (for 7 days) with PRN SABA, did not find a difference in the increase in height between groups (budesonide 7.8 cm, 95% CI 7.4-8.1 cm; PRN SABA 7.5 cm, 95% CI 7.0-8.1 cm).Citation67
Expert panel discussion of additional considerations and clinical judgment of risk versus benefit
Intermittent use of ICSs at the start of symptoms until symptoms resolve is a common practice in all age groups but there is no evidence that short courses of low- to high-dose ICS prevents progression from an acute loss of asthma control to a full-blown exacerbation, compared to PRN SABA. The only trials to compare these interventions are in children under 6 years of age.
Although there is moderate certainty evidence that short courses of very high-dose ICS decrease exacerbations and improve some measures of asthma control in children under 6 years of age, the studies that showed these benefits used very high doses of ICS that are not approved for use in children: budesonide 2 mg/day (nebule); budesonide 1600 mcg to 3200 mcg/day (MDI with spacer); beclomethasoneEUR (MDI 2250 mcg/day). The 1 trial that showed a decrease in severe exacerbations noted a decrease in growth, with a correlation between cumulative ICS dose and decrease in height even when courses were limited to 10 days. This is likely due to the frequency of URTIs accompanied by asthma symptoms in children 1-6 years of age (7.4 URTIs with asthma symptoms over the study duration, which was a median of 40 weeks).Citation69 Although this effect on growth was not seen in the other clinical trial involving preschoolers, this may be due to decreased absorption of nebulized medication and the decreased dose used (budesonide 2 mg/day nebulized). Given that nebulized medication is not recommended as first-line management for asthma, even in preschoolers, the use of budesonide 2 mg/day nebulized would not be a recommended approach.
The wide use of this approach outside of a well-controlled RCT setting carries a potential for young children to be exposed to very high cumulative doses of ICS, given the frequency of viral infections, and although the current trials do not show serious adverse events aside from growth, the panel was not convinced of the safety of this approach given the small numbers included in trials. From an implementation perspective, it is likely that in practice, individuals would be prescribed short courses of low- to high doses of ICS; a strategy for which there is no evidence base. There are also other treatment options, given the high quality evidence for daily ICS + PRN SABA in preventing exacerbations even in preschoolers.Citation61 Accordingly, the panel did not feel that this approach should be suggested in children <6 years of age.
Although the studies using PRN ICS-SABA and PRN bud/form also use intermittent ICS, the panel did not feel that these data could be extrapolated to assess the efficacy and safety of intermittent courses of ICS with acute loss of asthma control, given the differences in timing and dose of ICS, and potential differences between SABAs and fast acting LABAs.
Future research questions
Are low to medium doses of ICS + PRN SABA, taken at the onset of an acute loss of asthma control and stopped when symptoms resolve, more effective and as safe as SABA PRN in children under 6 years of age?
Is any dose of ICS + PRN SABA, taken at the onset of an acute loss of asthma control and stopped when symptoms resolve, more effective and as safe as PRN SABA in children 6 years of age or older and adults?
Is the seasonal use (started 2-4 weeks prior to the trigger season until the end of the season) of daily ICS + PRN SABA more effective than and as safe as PRN SABA in individuals of all ages?
DISSEMINATION AND IMPLEMENTATION
Our guideline will be disseminated via traditional channels including this publication, through the CTS website at https://cts-sct.ca/guideline-library/, the CTS Journal Canadian Journal of Respiratory, Critical Care, and Sleep Medicine at www.tandfonline.com, social media channels, through an accompanying slide deck which will be used to present our findings at key meetings such as the Canadian Respiratory Conference. An online educational module will be developed and posted on the CTS website at https://cts-sct.ca/education-and-accreditation/cts-e-learning-modules/.
Our goal is to monitor the impact of these actionable recommendations through their ability to correct knowledge gaps and improve actual behaviors within the target user groups. The CTS Asthma Clinical Assembly welcomes the opportunity to partner with other organizations and stakeholders in the development of educational tools and resources that support the implementation of the key messages described herein, with various targeted groups. For messages targeting patients and their families, we will seek to tailor messages and produce corresponding educational content, in collaboration with key stakeholders such as provincial lung associations, and the Respiratory Training and Educator Course (RESPTREC®). In addition, we will work with The Pan-Canadian Respiratory Standards Initiative for Electronic Health Records Initiative (PRESTINE)Citation72 working group to update asthma elements that prompt and enable adherence with this new guideline.
Factors that would facilitate implementation of this guideline into practice include:
Access to province-wide pharmacy records showing prescription fills of asthma medication, particularly SABA and oral steroids, to provide clinicians with objective evidence of asthma control, risk of exacerbation, and adherence to medication. Ideally this would be integrated into office electronic medical records (EMRs)
Access to province-wide data sharing platforms showing acute care visits (e.g., walk in clinic), ED visits, and hospitalizations for asthma, to provide clinicians with objective evidence of disease activity and risk of future exacerbation. Ideally this would be integrated into office EMRs
EMRs that prompt clinicians to ask about all components of asthma control and risk of exacerbation and then provide management algorithms
Individuals having access to providers that provide asthma education to remediate factors that lead to poorly-controlled asthma, including advice on proper device use, smoking cessation counselling and addressing misconceptions around the definition of well-controlled asthma and risk factors for exacerbation
Providing education to physicians and individuals that poorly-controlled asthma is only 1 risk factor for asthma exacerbations and that those with very mild or mild asthma can still be at risk for asthma- related morbidity and mortality
Improving access to spirometry for diagnosis as well as assessment of ongoing asthma control and risk stratification
Access to multidisciplinary teams to address larger psychosocial and behavioral issues that increase risk for near-fatal and fatal asthma
Anticipated barriers to implementation into practice:
Poor access to timely spirometry
Some provincial or private medication plans may not allow individuals to fill a prescription for bud/form without failing other steps of management (i.e., daily ICS)
Cost of asthma controller medications for individuals without provincial or private medication plans
Fragmented asthma care in which acute exacerbations are treated in the ED or after-hours clinic, where prevention is less likely to be discussed. Individuals may lack primary care providers and/or providers may not be aware of all of the severe exacerbations that an individual has had.
Lack of knowledge of control criteria among both patients and providers
Complexity and lack of familiarity with escalation and de-escalation recommendations among providers
Poor adherence to medication due to: cost, convenience, concern about side effects or not wanting to take “too much'' medication, lack of perceived efficacy of ICS, perceived lack of seriousness of disease
Advice and/or tools to put recommendations into practice
EMRs that integrate patient-reported asthma control criteria, medication records from pharmacy and acute care visits for asthma and provide management algorithms
Decision-making tools to facilitate discussions between clinicians and patients, particularly when there are multiple options for therapy
Educational material for individuals that highlight what well-controlled asthma is, what puts them at a higher risk for exacerbation, and the different management options, to facilitate discussion between clinicians and patients
Integrating recommendations into 1 asthma guideline that incorporates diagnosis and management across the spectrum of asthma severities
Monitoring and auditing adherence to this guideline
Individuals on PRN SABA or no medication with poorly-controlled asthma who have treatment escalated (numerator) among individuals on PRN SABA or no medication with poorly-controlled asthma (denominator)
Individuals on PRN SABA or no medication who had a severe exacerbation that have controller therapy escalated (numerator) among individuals on PRN SABA or no medication who had a severe exacerbation (denominator)
^ Time between severe exacerbation and treatment escalation beyond PRN SABA
Individuals on PRN SABA or no medication with well-controlled asthma at higher risk of exacerbation who have treatment escalated (numerator) among individuals on PRN SABA or no medication with well-controlled asthma at higher risk of exacerbation
^ Individuals on PRN bud/form with poorly-controlled asthma who have treatment escalated (numerator) among individuals on PRN bud/form with poorly-controlled asthma (denominator)
^ Individuals on PRN ICS-SABA with poorly-controlled asthma who have treatment escalated (numerator) among individuals on PRN ICS-SABA with poorly-controlled asthma (denominator)
^ Number of clinic visits for asthma where asthma control and risk for exacerbation are assessed (numerator) among total clinic visits for asthma (denominator)
^ Number of individuals with poorly-controlled asthma provided with asthma education (numerator) among individuals with poorly-controlled asthma (denominator)
^ Number of individuals with asthma referred for spirometry as part of asthma control assessment (numerator) out of total number of individuals with asthma (denominator)
^ Individuals <12 years old prescribed PRN bud/form as monotherapy (numerator) among all individuals with asthma <12 years old (denominator) (practice should not occur)
^ Individuals <18 years old prescribed ICS-SABA (numerator) among all individuals with asthma <18 years old (denominator) (practice should not occur)
^ Individuals prescribed SABA to be taken regularly before daily ICS (numerator) among individuals prescribed daily ICS (practice should not occur)
^ Individuals prescribed short courses of ICS (numerator) among individuals with asthma (denominator) (practice should not occur)
FUTURE RESEARCH
Future research questions related to each PICO section are included within the guideline.
Very mild and mild asthma are thought to affect approximately 28 to 41% of the Canadian population, however, more accurate estimates of their prevalence as defined in this guideline are required. Epidemiologic studies are needed to determine both the prevalence of very mild and mild asthma and the degree of risk they pose for severe exacerbations and asthma-related death. It would be particularly informative to understand the morbidity and mortality experienced by individuals on PRN SABA with well-controlled asthma at low risk for exacerbations, as this guideline suggests that these individuals can be given the option of remaining on PRN SABA. Such data would inform future guideline updates and provide information for an economic analysis, which should be performed to determine the cost-effectiveness of the recommendations in this guideline.
The definition used for higher risk of exacerbation in this guideline should be validated. In particular, it should be determined in the Canadian context if a history of a severe asthma exacerbation poses a lifetime increased risk of asthma exacerbation or if the risk of future exacerbations, particularly in preschoolers and children, significantly decreases over time.
Poor adherence to daily ICS decreases the efficacy of this treatment; further studies are needed to determine how to improve adherence to daily medication in individuals with asthma.
CONCLUSION
There continues to be evidence that daily ICS decreases exacerbations and improves asthma control compared to PRN SABA in individuals with very mild and mild asthma. There is new evidence in children ≥12 years of age and adults that PRN bud/form decreases exacerbations in comparison to PRN SABA, with more robust evidence in those with mild asthma compared to very mild asthma. Individuals with very mild asthma at higher risk of exacerbation should be switched from PRN SABA to daily ICS (all ages) or PRN bud/form (only an option in those ≥12 years of age). Individuals with very mild asthma at lower risk of exacerbation should be given the option of switching to daily ICS (all ages) or PRN bud/form (only an option in those ≥12 years of age). In individuals with mild asthma, daily ICS are still recommended as first line; however, in individuals ≥12 years of age with poor adherence despite substantial asthma education and support, PRN bud/form is a reasonable alternate. Using an ICS each time a SABA is taken (PRN ICS-SABA) is not recommended except as a harm reduction measure in individuals ≥ 18 years of age who are unable to take PRN bud/form or daily ICS + PRN SABA. Intermittent use of ICS with acute loss of asthma control is not suggested, given the lack of evidence for benefit when using approved ICS doses and potential for harm, particularly in preschoolers.
EDITORIAL INDEPENDENCE
The CTS Asthma guideline panel is accountable to the CTS Canadian Respiratory Guidelines Committee and the CTS Board of Directors. The CTS Asthma guideline panel is functionally and editorially independent from any funding sources of the CTS and does not receive any direct funding from external sources. The CTS receives unrestricted grants which are combined into a central operating account to facilitate the knowledge translation activities of the CTS Assemblies and its guideline panels. No funders played a role in the collection, review, analysis or interpretation of the scientific literature or in any decisions regarding the key messages presented in this document.
DISCLOSURES
Members of the CTS Asthma Guideline Panel declared potential conflicts of interest at the time of appointment and these were updated throughout the process in accordance with the CTS Conflict of Interest Disclosure Policy. Individual member conflict of interest statements are posted on the CTS website.
| ABBREVIATIONS | ||
| ACQ | = | Asthma Control Questionnaire |
| BD | = | bronchodilator |
| beclo | = | beclomethasone |
| bud/form | = | budesonide/formoterol in single inhaler |
| CI | = | confidence interval |
| CPS | = | Canadian Pediatric Society |
| CRE | = | certified respiratory educator |
| CRGC | = | Canadian Respiratory Guidelines Committee |
| CTS | = | Canadian Thoracic Society |
| DART | = | Documentation and Appraisal |
| ED | = | Emergency Department |
| EMR | = | electronic medical records |
| FABA | = | fast-acting beta-agonist |
| FeNO | = | fraction of exhaled nitric oxide |
| FEV1 | = | forced expiratory volume in 1 second |
| GINA | = | Global Initiative for Asthma |
| HR | = | hazard ratio |
| ICS | = | inhaled corticosteroids |
| LABA | = | long-acting beta-agonist |
| LTRA | = | leukotriene receptor antagonists |
| MCID | = | minimal clinically important difference |
| MDI | = | metered-dose inhaler |
| NHLBI | = | National Heart, Lung, and Blood Institute |
| NNT | = | number needed to treat |
| OR | = | odds ratio |
| PC20 | = | provocative concentration to cause a 20% decrease in FEV1 |
| PEF | = | peak expiratory flow |
| PICO | = | Patient/problem, Intervention, Comparison, Outcome |
| PRN | = | pro re nata, use as needed |
| PRN ICS- SABA | = | as needed use of an inhaled corticosteroid each time a short-acting beta-agonist is taken |
| RCT | = | randomized control trial |
| RR | = | relative risk |
| SABA | = | short-acting beta-agonist |
| SD | = | standard deviation |
| URTI | = | upper respiratory tract infection |
ACKNOWLEDGMENTS
The authors would like to recognize and thank CTS staff (Anne Van Dam), CTS member (Om Kurmi), CTS Executive members (Dina Brooks, Paul Hernandez, Richard Leigh, Mohit Bhutani, and John Granton), CTS CRGC Executive members (Samir Gupta and Christopher Licskai), and CTS Asthma Assembly Steering Committee members (Diane Lougheed, Francine Ducharme, Dhenuka Radhakrishnan, Andréanne Côté, Tania Samanta) for their input and guidance. We would also like to acknowledge with sincere appreciation our expert reviewers who made valuable contributions to the manuscript: Andrew Menzies-Gow, Director of the Lung Division, Deputy Medical Director, Royal Brompton & Harefield National Health Service Foundation Trust, London, England; Robert Newton, Professor and Head, Department of Physiology & Pharmacology, Airways Inflammation Research Group, Snyder Institute for Chronic Diseases, Cumming School of Medicine, University of Calgary, Calgary, Alberta; Emilie Zannis, Clinical Editor, Canadian Pharmacists Association, Ottawa, Ontario; Yue Chen, Professor, School of Epidemiology and Public Health, Faculty of Medicine, University of Ottawa, Ottawa, Ontario.
REFERENCES
- Government of Canada. Asthma and Chronic Obstructive Pulmonary Disease (COPD) in Canada, 2018. Report from the Canadian Chronic Disease Surveillance System. Available from: https://www.canada.ca/en/public-health/services/publications/diseases-conditions/asthma-chronic-obstructive-pulmonary-disease-canada-2018.html#a1.1. Accessed on: September 18, 2020.
- FitzGerald JM, Lemiere C, Lougheed MD, et al. Recognition and management of severe asthma: A Canadian Thoracic Society position statement. Can J Respir Crit Care Sleep Med. 2017;1(4):199–221. doi:https://doi.org/10.1080/24745332.2017.1395250.
- Firoozi F, Lemière C, Beauchesne MF, et al. Development and validation of database indexes of asthma severity and control. Thorax. 2007;62(7):581–587. doi:https://doi.org/10.1136/thx.2006.061572.
- Ungar WJ, Chapman KR, Santos MT. Assessment of a Medication-Based Asthma Index for Population Research. Am J Respir Crit Care Med. 2002;165(2):190–194. doi:https://doi.org/10.1164/ajrccm.165.2.2102012.
- Bahadori K, Doyle-Waters MM, Marra C, et al. Economic burden of asthma: a systematic review. BMC Pulmonary Medicine. 2009;9(1):24. doi:https://doi.org/10.1186/1471-2466-9-24.
- Canadian Lung Association, Asthma Control in Canada™ Survey, 2016. Available from: https://www.lung.ca/news/advocacy-tools/our-publications. Accessed on September 18, 2020.
- FitzGerald JM, Boulet LP, McIvor RA, et al. Asthma control in Canada remains suboptimal: the Reality of Asthma Control (TRAC) study. Can Respir J. 2006;13(5):253–259. doi:https://doi.org/10.1155/2006/753083.
- Ismaila AS, Sayani AP, Marin M, et al. Clinical, economic, and humanistic burden of asthma in Canada: a systematic review. BMC Pulm Med. 2013;13(1):70. doi:https://doi.org/10.1186/1471-2466-13-70.
- To T, Simatovic J, Zhu J, et al. Asthma Deaths in a Large Provincial Health System. A 10-Year Population-Based Study. Ann Am Thorac Soc. 2014;11(8):1210–1217. doi:https://doi.org/10.1513/AnnalsATS.201404-138OC.
- Royal College of Physicians. Why asthma still kills: the National Review of Asthma Deaths (NRAD) Confidential Enquiry report. 2014. Available from: https://www.rcplondon.ac.uk/projects/outputs/why-asthma-still-kills. Accessed on September 18, 2020.
- Lougheed MD, Lemière C, Dell SD, et al. Canadian Thoracic Society Asthma Management Continuum–2010 Consensus Summary for children six years of age and over, and adults. Can Respir J. 2010;17(1):15–24. doi:https://doi.org/10.1155/2010/827281.
- Lougheed MD, Lemiere C, Ducharme FM, et al. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Can Respir J. 2012;19(2):127–164. doi:https://doi.org/10.1155/2012/635624.
- Bateman ED, Harrison TW, Quirce S, et al. Overall asthma control achieved with budesonide/formoterol maintenance and reliever therapy for patients on different treatment steps. Respir Res. 2011;12(1):38. doi:https://doi.org/10.1186/1465-9921-12-38.
- Bender BG, Pedan A, Varasteh LT. Adherence and persistence with fluticasone propionate/salmeterol combination therapy. J Allergy Clin Immunol. 2006;118(4):899–904. doi:https://doi.org/10.1016/j.jaci.2006.07.002.
- Hardy J, Baggott C, Fingleton J, et al. Budesonide-formoterol reliever therapy versus maintenance budesonide plus terbutaline reliever therapy in adults with mild to moderate asthma (PRACTICAL): a 52-week, open-label, multicentre, superiority, randomised controlled trial. Lancet. 2019;394(10202):919–928. doi:https://doi.org/10.1016/S0140-6736(19)31948-8.
- Bibi HS, Feigenbaum D, Hessen M, et al. Do current treatment protocols adequately prevent airway remodeling in children with mild intermittent asthma? Respir Med. 2006;100(3):458–462. doi:https://doi.org/10.1016/j.rmed.2005.06.011.
- Lange P, Scharling H, Ulrik CS, et al. Inhaled corticosteroids and decline of lung function in community residents with asthma. Thorax. 2006;61(2):100–104. doi:https://doi.org/10.1136/thx.2004.037978.
- Ducharme FM, Dell SD, Radhakrishnan D, et al. Diagnosis and Management of Asthma in Preschoolers: A Canadian Thoracic Society and Canadian Paediatric Society Position Paper. Can Respir J. 2015;22:101572.
- Brouwers MC, Kho ME, Browman GP, et al. AGREE II: advancing guideline development, reporting and evaluation in health care. CMAJ. 2010;182(18):E839–842. doi:https://doi.org/10.1503/cmaj.090449.
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2020. Available from: www.ginasthma.org. Accessed on September 18, 2020.
- National Heart, Lung, and Blood Institute. Advisory Council Asthma Expert Working Group. Guidelines for the Diagnosis and Management of Asthma (EPR-3). 2007. Available from: https://www.nhlbi.nih.gov/health-topics/guidelines-for-diagnosis-management-of-asthma. Accessed on September 18, 2020.
- British Thoracic Society/Scottish Intercollegiate Guidelines Network. British guideline on the management of asthma. 2019. Available from: https://www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/. Accessed on September 18, 2020.
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (Clinical research ed.). 2011;343:d5928. doi:https://doi.org/10.1136/bmj.d5928.
- Diekemper R, Ireland B, Merz L. Development of the Documentation and Appraisal Review Tool (Dart) For Systematic Reviews. BMJ Quality & Safety. 2013;22(Suppl 1):61–62. doi:https://doi.org/10.1136/bmjqs-2013-002293.184.
- Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi:https://doi.org/10.1016/j.jclinepi.2010.04.026.
- Alonso-Coello P, Schünemann HJ, Moberg J, et al. GRADE Evidence to Decision (EtD) frameworks: a systematic and transparent approach to making well informed healthcare choices. 1: Introduction. BMJ (Clinical research ed.). 2016;353:i2016.
- Atkins D, Best D, Briss PA, et al. Grading quality of evidence and strength of recommendations. BMJ (Clinical research ed.). 2004;328(7454):1490.
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ (Clinical research ed.). 2008;336(7650):924–926.
- Gupta S, Rai N, Bhattacharrya O, et al. Optimizing the language and format of guidelines to improve guideline uptake. CMAJ. 2016;188(14):E362–e368. doi:https://doi.org/10.1503/cmaj.151102.
- Hargreave FE, Dolovich J, Newhouse MT. The assessment and treatment of asthma: a conference report. J Allergy Clin Immunol. 1990;85(6):1098–1111. doi:https://doi.org/10.1016/0091-6749(90)90056-a.
- FitzGerald JM, Spier S, Ernst P. Evidence-Based Asthma Guidelines. CHEST. 1996;110(6):1382–1383. doi:https://doi.org/10.1378/chest.110.6.1382.
- Boulet LP, Becker A, Bérubé D, et al. Canadian asthma consensus report, 1999. CMAJ. 1999;161(11 suppl 1):S1–S5.
- Dostaler SM, Olajos-Clow JG, Sands TW, et al. Comparison of Asthma Control Criteria: Importance of Spirometry. J Asthma. 2011;48(10):1069–1075. doi:https://doi.org/10.3109/02770903.2011.631243.
- Bateman ED, Reddel HK, O’Byrne PM, et al. As-Needed Budesonide–Formoterol versus Maintenance Budesonide in Mild Asthma. NEJM. 2018;378(20):1877–1887. doi:https://doi.org/10.1056/NEJMoa1715275.
- Fitzpatrick AM, Jackson DJ, Mauger DT, et al. Individualized therapy for persistent asthma in young children. J Allergy Clin Immunol. 2016;138(6):1608–1618.e1612.
- Martinez FD, Chinchilli VM, Morgan WJ, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377(9766):650–657. doi:https://doi.org/10.1016/S0140-6736(10)62145-9.
- O’Byrne PM, FitzGerald JM, Bateman ED, et al. Inhaled Combined Budesonide–Formoterol as Needed in Mild Asthma. NEJM. 2018;378(20):1865–1876. doi:https://doi.org/10.1056/NEJMoa1715274.
- Papi A, Canonica GW, Maestrelli P, et al. Rescue Use of Beclomethasone and Albuterol in a Single Inhaler for Mild Asthma. NEJM. 2007;356(20):2040–2052. doi:https://doi.org/10.1056/NEJMoa063861.
- Bateman ED, Boushey HA, Bousquet J, et al. Can Guideline-defined Asthma Control Be Achieved? Am J Respir Crit Care Med. 2004;170(8):836–844. doi:https://doi.org/10.1164/rccm.200401-033OC.
- Lanier B, Bridges T, Kulus M, et al. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124(6):1210–1216. doi:https://doi.org/10.1016/j.jaci.2009.09.021.
- Lemanske RF, Mauger DT, Sorkness CA, et al. Step-up Therapy for Children with Uncontrolled Asthma Receiving Inhaled Corticosteroids. NEJM. 2010;362(11):975–985. doi:https://doi.org/10.1056/NEJMoa1001278.
- Busse WW, Shah SR, Somerville L, et al. Comparison of adjustable- and fixed-dose budesonide/formoterol pressurized metered-dose inhaler and fixed-dose fluticasone propionate/salmeterol dry powder inhaler in asthma patients. J Allergy Clin Immunol. 2008;121(6):1407–1414, e1401-1406.
- Lazarus SC, Krishnan JA, King TS, et al. Mometasone or Tiotropium in Mild Asthma with a Low Sputum Eosinophil Level. NEJM. 2019;380(21):2009–2019. doi:https://doi.org/10.1056/NEJMoa1814917.
- Beasley R, Holliday M, Reddel HK, et al. Controlled Trial of Budesonide–Formoterol as Needed for Mild Asthma. NEJM. 2019;380(21):2020–2030. doi:https://doi.org/10.1056/NEJMoa1901963.
- Pauwels RA, Pedersen S, Busse WW, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361(9363):1071–1076. doi:https://doi.org/10.1016/S0140-6736(03)12891-7.
- Dusser D, Montani D, Chanez P, et al. Mild asthma: an expert review on epidemiology, clinical characteristics and treatment recommendations. Allergy. 2007;62(6):591–604. doi:https://doi.org/10.1111/j.1398-9995.2007.01394.x.
- Buelo A, McLean S, Julious S, et al. At-risk children with asthma (ARC): a systematic review. Thorax. 2018;73(9):813. doi:https://doi.org/10.1136/thoraxjnl-2017-210939.
- Nwaru BI, Ekström M, Hasvold P, et al. Overuse of short-acting β2-agonists in asthma is associated with increased risk of exacerbation and mortality: a nationwide cohort study of the global SABINA programme. Eur Respir J. 2020;55(4):1901872. doi:https://doi.org/10.1183/13993003.01872-2019.
- Bayly JE, Bernat D, Porter L, et al. Secondhand Exposure to Aerosols From Electronic Nicotine Delivery Systems and Asthma Exacerbations Among Youth With Asthma. Chest. 2019;155(1):88–93. doi:https://doi.org/10.1016/j.chest.2018.10.005.
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Population Health and Public Health Practice; Committee on the Review of the Health Effects of Electronic Nicotine Delivery Systems. Public Health Consequences of E-Cigarettes. Eaton DL, Kwan LY, Stratton K, editors. Washington (DC): National Academies Press (US). 2018.
- To T, Zhu J, Williams DP, et al. Frequency of health service use in the year prior to asthma death. J Asthma. 2016;53(5):505–509. doi:https://doi.org/10.3109/02770903.2015.1064949.
- Lazarinis N, Jørgensen L, Ekström T, et al. Combination of budesonide/formoterol on demand improves asthma control by reducing exercise-induced bronchoconstriction. Thorax. 2014;69(2):130–136. doi:https://doi.org/10.1136/thoraxjnl-2013-203557.
- Papi A, Nicolini G, Baraldi E, et al. Regular vs prn nebulized treatment in wheeze preschool children. Allergy. 2009;64(10):1463–1471. doi:https://doi.org/10.1111/j.1398-9995.2009.02134.x.
- Reddel HK, Busse WW, Pedersen S, et al. Should recommendations about starting inhaled corticosteroid treatment for mild asthma be based on symptom frequency: a post-hoc efficacy analysis of the START study. Lancet. 2017;389(10065):157–166. doi:https://doi.org/10.1016/S0140-6736(16)31399-X.
- Agertoft L, Pedersen S. Effect of Long-Term Treatment with Inhaled Budesonide on Adult Height in Children with Asthma. NEJM. 2000;343(15):1064–1069. doi:https://doi.org/10.1056/NEJM200010123431502.
- Kelly HW, Sternberg AL, Lescher R, et al. Effect of Inhaled Glucocorticoids in Childhood on Adult Height. NEJM. 2012;367(10):904–912. doi:https://doi.org/10.1056/NEJMoa1203229.
- Baggott C, Hardy J, Sparks J, et al. Self-titration of inhaled corticosteroid and β(2)-agonist in response to symptoms in mild asthma: a pre-specified analysis from the PRACTICAL randomised controlled trial. Eur Respir J. 2020;56(4). doi:https://doi.org/10.1183/13993003.00170-2020.
- Calhoun WJ, Ameredes BT, King TS, et al. Comparison of physician-, biomarker-, and symptom-based strategies for adjustment of inhaled corticosteroid therapy in adults with asthma: the BASALT randomized controlled trial. JAMA. 2012;308(10):987–997. doi:https://doi.org/10.1001/2012.jama.10893.
- Chong J, Haran C, Chauhan BF, et al. Intermittent inhaled corticosteroid therapy versus placebo for persistent asthma in children and adults. Cochrane Database Syst Rev. 2015(7):Cd011032.
- Castro-Rodriguez JA, Rodrigo GJ. Efficacy of inhaled corticosteroids in infants and preschoolers with recurrent wheezing and asthma: a systematic review with meta-analysis. Pediatrics. 2009;123(3):e519-525. doi:https://doi.org/10.1542/peds.2008-2867.
- Ducharme FM, Tse SM, Chauhan B. Diagnosis, management, and prognosis of preschool wheeze. Lancet. 2014;383(9928):1593–1604. doi:https://doi.org/10.1016/S0140-6736(14)60615-2.
- Adams NP, Bestall JC, Jones P. Budesonide versus placebo for chronic asthma in children and adults. Cochrane Database Syst Rev. 1999(4): 1465–1858.
- Adams NP, Bestall JC, Lasserson TJ, Jones P, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database Syst Rev. 2008;(4):CD003135.
- Adams NP, Bestall JC, Malouf R, Lasserson TJ, Jones P. Beclomethasone versus placebo for chronic asthma. Cochrane Database Syst Rev. 2005;(1):CD002738.
- Manning P, Gibson PG, Lasserson TJ. Ciclesonide versus placebo for chronic asthma in adults and children. Cochrane Database Syst Rev. 2008;2008(2):CD006217.
- Jackson DJ, Bacharier LB, Mauger DT, et al. Quintupling Inhaled Glucocorticoids to Prevent Childhood Asthma Exacerbations. NEJM. 2018;378(10):891–901. doi:https://doi.org/10.1056/NEJMoa1710988.
- Bacharier LB, Phillips BR, Zeiger RS, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol. 2008;122(6):1127–1135.e1128.
- Connett G, Lenney W. Prevention of viral induced asthma attacks using inhaled budesonide. Arch Dis Child. 1993;68(1):85–87. doi:https://doi.org/10.1136/adc.68.1.85.
- Ducharme FM, Lemire C, Noya FJ, et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. NEJM. 2009;360(4):339–353. doi:https://doi.org/10.1056/NEJMoa0808907.
- Svedmyr J, Nyberg E, Thunqvist P, et al. Prophylactic intermittent treatment with inhaled corticosteroids of asthma exacerbations due to airway infections in toddlers. Acta Paediatr. 1999;88(1):42–47. doi:https://doi.org/10.1111/j.1651-2227.1999.tb01266.x.
- Wilson NM, Silverman M. Treatment of acute, episodic asthma in preschool children using intermittent high dose inhaled steroids at home. Arch Dis Child 1990;(65):407–410. doi:https://doi.org/10.1136/adc.65.4.407.
- Lougheed MD, Taite A, ten Hove J, et al. Pan-Canadian asthma and COPD standards for electronic health records: A Canadian Thoracic Society Expert Working Group Report. Can J Respir Crit Care Sleep Med. 2018;2(4):244–250. doi:https://doi.org/10.1080/24745332.2018.1517623.
- Fuhlbrigge AL, Kitch BT, Paltiel AD, et al. FEV1 is associated with risk of asthma attacks in a pediatric population. J Allergy Clin Immunol. 2001;107(1):61–67. doi:https://doi.org/10.1067/mai.2001.111590.
- Schatz M, Cook EF, Joshua A, et al. Risk factors for asthma hospitalizations in a managed care organization: development of a clinical prediction rule. Am J Manag Care. 2003;9(8):538–547.
- Tanaka A, Uno T, Sato H, et al. Predicting future risk of exacerbations in Japanese patients with adult asthma: A prospective 1-year follow up study. Allergol Int. 2017;66(4):568–573. doi:https://doi.org/10.1016/j.alit.2017.02.013.
- Stanford RH, Shah MB, D'Souza AO, et al. Short-acting β-agonist use and its ability to predict future asthma-related outcomes. Ann Allergy Asthma Immunol. 2012;109(6):403–407. doi:https://doi.org/10.1016/j.anai.2012.08.014.
- Suissa S, Ernst P, Boivin JF, et al. A cohort analysis of excess mortality in asthma and the use of inhaled beta-agonists. Am J Respir Crit Care Med. 1994;149(3 Pt 1):604–610. doi:https://doi.org/10.1164/ajrccm.149.3.8118625.
- Bloom CI, Nissen F, Douglas IJ, et al. Exacerbation risk and characterisation of the UK’s asthma population from infants to old age. Thorax. 2018;73(4):313–320. doi:https://doi.org/10.1136/thoraxjnl-2017-210650.
APPENDIX 1
Search Strategy
PRISMA Diagram
Articles selected for inclusion in the systematic review of the evidence reported data on individuals well-controlled as per CTS criteria on SABA PRN for PICO 1 and data on individuals where the CTS recommends ICS maintenance therapy (not well-controlled on PRN SABA or well-controlled on daily low dose ICS) for PICO 2. Therefore, we excluded studies in PICO 1 if the individuals with asthma were not well-controlled on SABA PRN. In PICO 2 we excluded studies if individuals were well-controlled on PRN SABA based on CTS criteria for asthma control.
After reviewing the papers, the panel focused the scope of the guideline and included the comparisons between the current standards of care PRN SABA (PICO 1) and daily ICS + PRN SABA (PICO 2) versus PRN ICS-SABA, PRN ICS-FABA, Short courses of ICS, daily ICS + daily SABA + PRN SABA, as well as the comparison between PRN SABA and daily ICS + PRN SABA for PICO 1. This resulted in 9 comparisons.
PICO 1. In individuals where the CTS currently recommend SABA PRN monotherapy (well-controlled as per CTS criteria) what are the best options for management?
PICO 2. In individuals where the CTS currently recommends ICS maintenance therapy + PRN SABA (not well-controlled on PRN SABA or well-controlled on daily low dose ICS + PRN SABA) what are the preferred treatment options for management?
APPENDIX 2
APPENDIX 3
List of changes to original SIGN 158 - British guideline on the management of asthma tableCitation45 or from the definitions used in the original papers
Persistent asthma symptoms was changed to poorly-controlled asthma to provide criteria for assessing frequency of symptoms.
Suboptimal drug regimen (ICS:SABA refill ratio <0.5) was changed to excessive SABA use (>2 inhalers/year) as it was felt that ICS:SABA refill ratio was too difficult to use in clinical practice and to align with adult criteria.
>2 inhalers/year was added to provide an additional objective way of assessing SABA use in addition to the traditional method of assessing frequency of SABA use in a week by self-report. In 1 study, individuals with >2 SABA inhaler refills (assuming an average of 150 inhalations per inhaler) in a year were found to have more asthma exacerbations, asthma-related hospitalizations and outpatient hospital visits compared to individuals filling ≤2 inhalers/year.Citation48 In that study, the pattern of SABA overuse was stable in patients over a 3- year period. There was a dose dependent increase in exacerbations and asthma-related deaths with increasing number of SABA inhalers filled. This is consistent with previous studies showing that filling ≥3 inhalers of SABA/year is associated with an increased risk of ED visits,Citation76 and filling 12 or more inhalers or SABA is associated with a high risk of death.Citation77 The 3 relievers in Canada contain 200 inhalations/inhaler (salbutamol), 100 inhalations/inhaler (terbutaline), and 120 inhalations/inhaler (bud/form) however for ease of use, the cutoff chosen was >2 inhalers of any type. Given that typically 2 inhalations of salbutamol, 1 inhalation of terbutaline and 1 inhalation of bud/form are used for relief, the approximation of >2 inhalers across all reliever types was felt to be reasonable.
Smoking was divided into current smoker and previous smokerCitation78 to illustrate the different OR in these 2 groups. Smoking in the original paper referred to tobacco smoke, however with the increased prevalence of vaping and marijuana smoking, these should also be considered when assessing an individual’s risk of exacerbation.