The risk of healthcare-associated transmission of Mycobacterium tuberculosis (M. tuberculosis) varies with the type of healthcare setting, health care worker occupational group, patient care activity, patient/resident/client/health care worker tuberculosis risk factors and the effectiveness of tuberculosis infection prevention and control measures.
The most important contributor to healthcare-associated transmission of M. tuberculosis is the presence of individuals with unrecognized respiratory tuberculosis that results in the failure to apply appropriate infection prevention and control measures. Hence, the most important elements in preventing the transmission of tuberculosis are engineering and administrative controls that reduce transmission risk even when tuberculosis is not suspected; recognition of individuals at risk for respiratory tuberculosis; prompt application of airborne precautions; rapid diagnosis; and initiation of effective antimicrobial therapy.
Remote and isolated healthcare settings in which populations at risk for respiratory tuberculosis are cared for should have access to infection prevention and control and occupational health, safety and wellness expertise to facilitate implementation of the recommended engineering, administrative and personal protective equipment controls.
All healthcare settings should have a tuberculosis infection prevention and control program that involves a hierarchical approach to infection prevention and control measures and includes a policy and procedure for contact tracing in the event that a patient/resident/client/health care worker is diagnosed with respiratory tuberculosis.
Airborne precautions should be initiated immediately for everyone admitted to a healthcare facility with, or being evaluated for, respiratory tuberculosis.
A medical mask should be used by individuals with, or being evaluated for, respiratory tuberculosis when in the healthcare setting and outside an airborne infection isolation room.
Baseline tuberculin skin testing is recommended for all health care workers in all healthcare settings. Recommendations for periodic and serial (repeated) tuberculin skin testing for health care workers vary with the setting, but periodic (repeated) testing is no longer routinely recommended for all health care workers.
KEY POINTS
1. Introduction and general principles
While the overall incidence of tuberculosis (TB) in Canada is low, it is higher in certain population groups and geographic regions and exposure to people with unsuspected respiratory TB, whether patients, visitors or other health care workers (HCWs), followed by its transmission, does occur in healthcare settings. As detailed in Chapter 1: Epidemiology of Tuberculosis in Canada, populations at higher risk of active TB include Indigenous Peoples, those born or previously residing in high-TB-incidence countries (especially low- and high-human immunodeficiency virus (HIV) prevalence regions in Africa, and the Western Pacific and Southeast Asia regions), HIV-infected persons and people with a history of active TB disease. Staff and residents of homeless shelters and injection drug users may have a higher risk of TB than the general population.Citation1,Citation2
While some studies have indicated that HCWs are at increased risk of occupationally acquired TB,Citation3 more recent literature from a number of low-TB-incidence countries has found that the rate of latent and active TB infection in HCWs is similar to that in the general population, particularly after adjusting for country of birth.Citation4–10 However, occupationally acquired TB does occur.Citation11–15 Occupational risk factors appear to be providing direct care to those with respiratory TB and participation in aerosol-generating medical procedures on individuals with infectious TB.Citation16–18
In hospitals and other settings where people congregate and share indoor air (in the same room or via the building ventilation system), the risk of M. tuberculosis transmission can be increased if ventilation and other infection prevention and control (IPC) measures are inadequate.Citation11,Citation12,Citation16,Citation19 A number of studies have identified that TB exposures within healthcare facilities are most often due to failure to suspect or diagnose active TB and implement appropriate IPC measures.Citation12,Citation16,Citation17,Citation20–24 As a result, recommendations for the prevention of healthcare-associated transmission of M. tuberculosis to HCWs, patients/residents and visitors have been developed.Citation25–27 Despite limited high-quality evidence on preventing TB transmission, implementation of the recommended hierarchy of IPC measures in hospitals in high-income countries was followed by a reduction in its transmission.Citation28
This chapter reviews factors that determine or affect transmission of M. tuberculosis within hospitals and other healthcare settings, while focusing on measures to prevent transmission.
Recommendations provided are based, as much as possible, on published evidence. Evidence from randomized controlled trials, generally considered the strongest level of evidence, is limited, as this is generally not feasible when analyzing risk factors or situations involving natural exposure (e.g., TB outbreaks). As a result, all available evidence comes from observational studies, such as cohort studies and outbreak investigations. This chapter cites the evidence base from these primary studies, published literature reviews and a grey literature search of relevant international guidelines.Citation25,Citation27,Citation29–32
1.1. Determinants of transmission in healthcare settings
Aerosolization of infectious M. tuberculosis bacteria occurs when individuals with respiratory TB cough, sneeze, sing, or speak. Aerosol-generating medical procedures (e.g., bronchoscopy, intubation, sputum induction), and some patient-care activities (e.g., irrigating a mycobacterial-containing wound), laboratory and autopsy procedures can also cause aerosolization of mycobacteria. Once infectious M. tuberculosis bacteria are aerosolized, they may be carried throughout a room or building by air currents and inhaled by another individual, with the possibility of TB infection. Although the risk of transmitting M. tuberculosis is highly variable, the presence of certain factors (discussed in the following sections) predicts an increased transmission risk. In general, the more of these factors present, the greater the risk of M. tuberculosis transmission (see Chapter 2: Transmission and Pathogenesis of Tuberculosis).
1.2. Factors associated with increased risk of health care-associated M. tuberculosis
1.2.1. Delayed diagnosis
Many outbreak investigations, as well as a root-cause analysis exploring factors contributing to TB exposures in a tertiary-care hospital in Canada, identify delay in making the diagnosis of TB as the most common reason for the exposures (see Appendix 1 and Appendix 2, ).Citation11,Citation12,Citation16–18,Citation20–23,Citation33–41 The root-cause analysis noted failures to consider TB as a possible diagnosis and failure to obtain or correctly interpret imaging findings as common errors, with 80% of the errors being preventable.Citation20 A Canadian study examining IPC failures contributing to bronchoscopy-associated exposures found a failure to obtain a pre-procedure sputum or determine whether the patient was known to have a positive sputum smear prior to the procedure.Citation42 Even when TB has been initially considered in the diagnosis, precautions may be inappropriately discontinued if HCWs have not been systematic in determining whether the diagnosis has been accurately excluded. The importance of considering a diagnosis of TB, and not prematurely excluding it, in an individual presenting with sub-acute or chronic respiratory symptoms, even when an alternate diagnosis is plausible, cannot be over-emphasized.
1.2.2. Number of patients with respiratory TB
It seems intuitive that a larger number of hospitalized patients with respiratory TB is an important determinant of higher institutional transmission risk. However, results from a study involving 17 acute care hospitals in Canada showed that institutional risk of M. tuberculosis transmission was better correlated with delayed diagnosis and treatment which in turn was associated with having a small number of admissions with respiratory TB disease.Citation43 The study results suggest that healthcare facilities with more experience managing patients with TB are, not surprisingly, less likely to miss a diagnosis of respiratory TB and that facilities seeing fewer patients with TB need ongoing reeducation on recognizing a patient who might have respiratory TB.
1.2.3. Inadequate ventilation
The exchange of indoor air with outdoor air reduces the risk of infection transmission by diluting the concentration of viable airborne M. tuberculosis bacteria present. Several studies report inadequate ventilation as a risk factor that contributes to transmission.Citation11,Citation22,Citation23,Citation44
1.2.4. Duration of exposure and proximity to infectious patient
The risk of TB infection varies with duration of exposure, form of tuberculous disease, and type of patient care activity. Even when the relative risk of infection is low, close proximity and repeated exposure can lead to a higher cumulative risk.Citation24,Citation34
1.3. Risk classification
1.3.1. Healthcare settings
The risk of healthcare-associated transmission of M. tuberculosis to HCWs, patients/residents and visitors varies with the type of setting, occupational group, effectiveness of TB IPC measures and patient/resident population. Previous Canadian guidance provided thresholds for annual numbers of patients admitted with respiratory TB that could be used by facilities to determine their risk for healthcare-associated transmission of TB and guide their IPC interventions. Given the annual number of TB cases alone may not accurately reflect risk and that other factors such as effectiveness of IPC measures must be taken into account,Citation43 this type of risk classification may no longer be suitable. A review of the community profile of TB disease and the number of TB admissions in the course of a year, independent of a risk-classification scheme, should inform determination of a healthcare facility and unit risk category as a framework to predict whether and where their HCWs are at increased risk of TB exposure and guide their IPC strategies.
1.3.2. Health care worker activities
Patient-care activities are associated with varying degrees of exposure risk and subsequent infection with M. tuberculosis. Performing high-risk procedures and activities are contributing factors to transmission of M. tuberculosis.Citation16 The risk of transmission increases with the duration of exposure and higher amounts of airborne mycobacteria. As a result, it is recommended that HCWs perform a risk assessment prior to interactions with all patients, including people with confirmed TB or who have symptoms of TB.Citation45 This risk assessment involves evaluating the likelihood of exposure to M. tuberculosis for a specific patient-care activity, with a specific patient, in a specific environment and under particular conditions. This is referred to as a point-of-care risk assessment and is described in the Public Health Agency of Canada’s (PHAC’s) Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings.Citation45 The point-of-care risk assessment informs HCWs’ decisions regarding the IPC measures needed to minimize the risk of exposure for themselves, other HCWs, patients and visitors.
1.3.2.1. High-risk activities
Aerosol-generating medical procedures (such as sputum induction, intubation, bronchoscopy)
High-pressure irrigation of M. tuberculosis infected wounds
Autopsy
Morbid/pathologic anatomy
Mycobacteriology laboratory procedures, especially handling cultures of M. tuberculosis
1.3.2.2. Intermediate-risk activities
Work requiring regular, direct patient contact on units (including emergency departments as well as inpatient pediatric, neonatal, adult respiratory, medicine and thoracic surgery wards) where patients with respiratory TB may be present (this includes work done by all HCWs in these units).
1.3.2.3. Low-risk activities
Work requiring minimal patient contact (such as clerical, reception and administration).
Work on units where patients with respiratory TB disease are unlikely to be present.
Classification of such units as low risk may be inaccurate if the population they are serving has a high incidence of TB (e.g., patients born or previously residing in countries with a high-TB incidence or other at-risk populations). Some of the longest delays in diagnosis may occur in such settings.Citation14,Citation22
Risk can be mitigated by use of engineering and administrative controls and appropriate personal protective equipment (PPE).
2. Prevention and control of transmission of M. tuberculosis in healthcare settings
Recommendations for the prevention of healthcare-associated transmission of M. tuberculosis involve application of a tiered framework of measures that enables healthcare organizations to comprehensively evaluate the risk of HCW exposure to workplace hazards, including M. tuberculosis, and the effectiveness of the organization’s mitigation responses.Citation45 This involves collaboration between IPC, Occupational Health Safety and Wellness and building engineers.
The ideal approach to containing a hazard is to implement a hierarchy of controls: 1) elimination; 2) substitution; 3) engineering controls; 4) administrative controls; and 5) PPE.Citation46 Elimination of TB from the healthcare setting is not always possible, but effective treatment of respiratory TB is equivalent. Substitution is not a relevant approach to preventing transmission of TB.
3. Engineering controls
These are measures built into healthcare facility design to reduce the likelihood of HCW, patient/resident and visitor exposure to viable airborne M. tuberculosis. They reduce the number of infectious particles in the air by ventilation, high-efficiency particulate air (HEPA) filtration and/or disinfection using ultraviolet germicidal irradiation (UVGI). It is important that engineering controls be regularly checked to ensure that they meet recommendations.Citation27
3.1. Ventilation
The exchange of indoor air with outdoor air reduces the risk of infection by diluting the concentration of airborne pathogens. Theoretically, the risk of transmission should decrease with increasing fresh-air ventilation.
To achieve a balanced ventilation system, the amount of supply air (air mechanically pushed into a room) and the amount of exhaust air (air mechanically pulled from a room) must be set to ensure room conditions are stable. Factors such as infiltration (e.g., space around doors, windows and curtains), doors and conditions of an adjacent room need to be considered when balancing a ventilation system.
Ventilation recommendations for airborne infection isolation rooms and other select areas are of critical importance because of their positive impact on reducing the risk for healthcare-associated transmission of M. tuberculosis. The supply and exhaust air system need to be properly designed to achieve effective air changes within a space. The location of the supply and exhaust air diffusers, the speed of the air, furniture in the room and other items that affect air flow patterns will affect the effectiveness of the air changes per hour (ACH). Increasing the number of ACH from 1 to 6 will result in more rapid clearing of infectious airborne microorganisms from the room air. However, further increases above 6 ACH will have progressively less effect, and increases above 12 ACH may provide minimal additional benefit.Citation47,Citation48 In general, as ACH rates are increased, there are increased costs for building and maintaining the ventilation system. Pressurization, which prevents particulates from leaving the room through infiltration, is equally, if not more, important than ACH. Pressurization is also critical for when doors are opened and closed, to ensure that air keeps flowing into the room and not out through the door. Opening the window may cause reversal of the direction of air flow, depending upon the prevailing wind direction and outdoor temperature.
A number of organizations and agencies, such as American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE), Canadian Standards Association (CSA) and Centers for Disease Control and Prevention (CDC) have published recommendations regarding ventilation levels to reduce the risk of healthcare-associated transmission of airborne pathogens, such as M. tuberculosis.Citation49–51 Differences among their recommendations () are not based on differing evidence but, rather, on the risk-benefit assessment of each organization. In deciding which recommendations to implement, hospital administrators may take into account factors such as facility design, latest scientific evidence, facility risk assessment, financial resources, environmental conditions and, at a minimum, provincial and territorial building regulations.
Table 1. Ventilation recommendations for selected areas in healthcare facilities.
3.1.1. General hospital areas
Adequate ventilation in general areas such as inpatient, examination and treatment rooms is important because people with unsuspected respiratory TB may be placed in them, posing a risk of transmission to other patients and HCWs.Citation44,Citation53 Ventilation in these areas may be poor and further disrupted by opening and closing doors, installation of ceiling fans post-design and use of items such as ground circulation fans and electric heaters.
3.1.2. Airborne infection isolation rooms
Measures to ensure that adequate ventilation is in place for airborne infection isolation rooms are outlined in the following sections and discussed in more detail in other guidelines.Citation45,Citation54
Regulation
Air should be exhausted to the outdoors through a dedicated exhaust system, ideally exiting from the roof of the building. It is important that the exhausted air does not re-enter the building or an adjacent occupied building.
Good practice statements
In the absence of provincial/territorial building standards for healthcare facilities, healthcare organizations should follow either the Canadian Standards Association, American Society of Heating, Refrigerating and Air-Conditioning Engineers, or the Centers for Disease Control and Prevention ventilation recommendations for healthcare facilities.
With the exception of rooms in which operative procedures are performed, the direction of air flow should be inward from the hall into the room (negative pressure), and then the air should be exhausted outdoors, or high-efficiency particulate air filtered.
If an anteroom is used, the air from both the anteroom and patient room should be exhausted outdoors; if an airborne infection isolation room does not have an anteroom, there shall be an adequate pressure differential between it and the corridor and adjacent rooms.
For positive pressure rooms in which sterile operative procedures are performed, there should be an anteroom for which the direction of air flow should be inward from the corridor and also from the operating room into the anteroom. In this way, clean air enters the operating room, but air does not go from the operating room into the corridor or beyond. The air should be exhausted outdoors from the anteroom.
Windows and doors should be kept closed, both during and after aerosol-generating medical procedures (long enough for air clearance in the room), and in the state that the heating, ventilation, and air conditioning system was designed to function.
The rate of air changes and pressurization should be verified at least every 6 months when the room is not being used as an airborne infection isolation room; rooms that are balanced with a Building Automation System should be under constant verification and have integrated alarms that notify maintenance staff of operational issues. Older buildings may require alternative air flow indicator devices.
When the airborne infection isolation room is in use, the direction of air flow should be verified daily and recorded, and the facility’s infection prevention and control team should review these ventilation system monitoring results.
Airborne infection isolation rooms, as well as other areas routinely used for care of patients with respiratory tuberculosis, should have at least 12 air changes per hour.
The number of airborne infection isolation rooms in hospitals should be based on the number of patients admitted each year with suspected respiratory tuberculosis; in organizations with very few admissions for tuberculosis, the number of such rooms should be decided by the organizational authorities according to an analysis of airborne infection isolation room utilization in the previous 2-to-3 years. One or two more rooms than needed in the past at peak times could be considered as the optimal number, recognizing that they may be used for airborne infections other than respiratory tuberculosis. Appropriate resources should be made available to hospitals that will have such rooms and therefore receive patients with respiratory tuberculosis from other healthcare facilities.
3.1.3. Sputum induction and administration of aerosolized pentamidine
The smaller the room where these procedures are performed, the easier and more practical it is to achieve required ventilation levels. Sputum collection booths, which are a type of enclosed local exhaust ventilation device, are commercially available for these purposes.
Good practice statements
Acute care hospitals should have an airborne infection isolation room or sputum collection booth for sputum induction; a sputum collection booth is preferred.
A sputum collection booth should meet the ventilation requirements of an airborne infection isolation room, be properly functioning, used and maintained according to manufacturer’s instructions, and adequate time allowed for air clearance between users.
If a room is used for sputum induction, it should meet the ventilation requirements of an airborne infection isolation room.
3.1.4. Bronchoscopy and autopsy
Areas where these procedures are performed tend to be much larger than inpatient rooms, making it difficult to achieve consistently high levels of ventilation with an inward direction of air flow.
Good practice statement
All bronchoscopies should occur in an airborne infection isolation room unless tuberculosis has been excluded as a diagnosis; if tuberculosis is confirmed, the necessity of bronchoscopy for optimal patient management should be carefully considered, in view of the considerable risk of transmission.
3.1.5. Entering a room after generation of infectious aerosols has ended or patient with respiratory TB has been discharged
provides guidance on when it is safe to enter a room previously occupied by a patient with respiratory TB without needing to wear a respirator or when a procedure room can be used for another patient after generation of infectious aerosols has ceased. The time required to remove airborne particles from an enclosed space depends on the number of ACH, which is a function of the volume (cubic feet of air) in the room or booth; the rate at which air is exiting the room or booth; the location of the ventilation inlet and outlet; and the configuration of the room or booth.Citation49 The minutes required to allow at least 99% removal of airborne microorganisms is considered sufficient to allow room entry.
Table 2. Time needed (by number of air changes per hour) to remove airborne microorganisms after generation of infectious droplet nuclei has ceased.Table Footnote a
3.2. Filtration
HEPA filters have a minimum removal efficiency of 99.97% for all particles.Citation30,Citation49 Air can be recirculated through HEPA filters in areas in which no general ventilation system is present, an existing system is incapable of providing sufficient ACH or air-cleaning (particulate removal) without affecting the fresh air supply or negative-pressure system is desired.Citation30,Citation49
Small HEPA units, either fixed or portable, may be used to filter recirculated air in a room. Portable industrial grade HEPA units, sometimes referred to as Room Air Purifiers with a HEPA filter, are available that can filter air at a range of airflows. The quality of these units must be carefully analyzed by the hospital engineering department prior to purchase and use. Placement of the unit within the room must be specified by this department to ensure laminar airflow distribution and maximize air changes throughout the room. HEPA filters must be installed to ensure that air flowing through the unit cannot bypass the filter. Refer to the CDC guideline for additional details.Citation49
HEPA filters typically last 5-10 years if a proper pre-filter to protect it from common environmental particles is used and maintained. HEPA filters require regular monitoring to ensure that the filter has not clogged, which will result in decreased filtering efficiency. For further information on HEPA filtration and details on safety issues when handling spent filters, refer to the CDC guidelines.Citation49
Good practice statements
If the air from the room in which bronchoscopy is performed will be recirculated, or if the exhausted air could re-enter a building, it should be passed through a high-efficiency particulate air filter before being exhausted.
The use, maintenance and monitoring of high-efficiency particulate air filters should be according to manufacturer’s instructions where provided and under the direction of the hospital engineering department. The filters should be serviced at a minimum once per year or whenever a major event has occurred that could have influenced the functionality of the filter and airflow.
3.3. Ultraviolet germicidal irradiation
Ultraviolet germicidal irradiation (UVGI) is effective at inactivating airborne bacteria and in reducing the risk of M. tuberculosis transmission.Citation55–57 Upper-room UVGI directs UV-C energy to the upper portions of a room to create a disinfection zone so that pathogens in the air that pass through this disinfection zone are inactivated.Citation58 The effectiveness of upper-room UVGI depends on multiple factors, including UVGI dose, air circulation, ventilation, temperature, humidity, room configuration and proper UVGI system installation and maintenance.
The interest in UVGI in IPC is growing, and safety and application standards are in development. However, safety data, real-world effectiveness and accepted industry standards remain limited. For further information on UVGI, refer to the CDC environmental guidance document.Citation49
3.4. Equipment cleaning and disinfection
As transmission of M. tuberculosis in healthcare settings occurs almost exclusively by the airborne route, preventive measures target airflow controls and ventilation systems. However, there have been rare instances of transmission via improperly reprocessed bronchoscopes.Citation59,Citation60
Good practice statement
Healthcare settings should have policies in place regarding requirements for cleaning, disinfection and sterilization of medical equipment based on its intended use.
4. Administrative controls
These are institutional policies or measures that 1) aim to reduce the time between the arrival of an individual with undiagnosed respiratory TB at a healthcare facility and making a presumptive diagnosis of their condition, placing the patient on airborne precautions, establishing the diagnosis and starting antimicrobial treatment; 2) provide the HCW with respiratory protection; and 3) evaluate the effectiveness of its IPC strategies and interventions.
4.1. TB infection prevention and control program
The goal of a TB IPC Program is to prevent M. tuberculosis transmission to HCWs, patients/residents/clients and visitors.
Good practice statements
All healthcare facilities/organizations, including emergency medical services, should have a TB Infection Prevention and Control (IPC) Program supported at the highest administrative level and with the components detailed in the following section. This Program may be facilitated through existing IPC and Occupational Health Safety and Wellness programs; the components of the Program should be adapted to the facility/organization needs based on a facility/organization risk assessment.
The hospital IPC Committee (or other appropriate committee) should be given responsibility for oversight of the TB IPC Program. Committee members should include people with day-to-day responsibility for IPC and Occupational Health Safety and Wellness, as well as representation from public health, senior administration, the microbiology laboratory, nursing, medicine, facility management and other groups as needed (e.g., respiratory technology, housekeeping, pharmacy).
The IPC Program should include policies and procedures that clearly delineate administrative responsibility for developing, implementing, reviewing and evaluating various program components. The evaluation should include quality control and audits for all components of engineering, administrative and personal protective equipment controls. Personnel with responsibility for the Program should be designated.
IPC policies and procedures should be in place for rapid diagnosis, isolation and treatment of patients with respiratory TB; reduction of healthcare-associated transmission through engineering and administrative controls, including contact tracing in the event that a patient/resident/client/health care worker is diagnosed with respiratory TB; and protection of staff through appropriate use of personal protective equipment, education and screening for active and latent TB infection.
Hospital administrators, in collaboration with appropriate jurisdictional authorities, should coordinate efforts to ensure availability of adequate numbers of hospitals with resources to receive patients with, or being evaluated for, respiratory TB from facilities/organizations without the required engineering, administrative or personal protective equipment controls, with minimum delay.
In facilities/organizations without an airborne infection isolation room, the following should be in place as components of the TB IPC Program:
Pre-arrangement to transfer patients/residents with, or being evaluated for, respiratory TB to a center with appropriate engineering controls; and at least one separate, well-ventilated area or single room with the door closed, away from immune-compromised patients/residents, where such patients/residents can be cared for until transfer.
4.1.1. Risk assessment
The first step of an effective TB IPC Program in every healthcare setting should be to perform an organizational risk assessment in order to understand what measures are required to decrease the risk of patient/resident/client, visitor and HCW exposure to M. tuberculosis. The exposure risk for HCWs engaged in different activities should be evaluated during this assessment. For further information on an organizational risk assessment, refer to PHAC’s Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings.Citation45
Good practice statements
All healthcare facilities should perform an organizational risk assessment that includes evaluation of exposure risk for health care workers (HCWs) and also includes:
an annual review of the indicators of healthcare-associated TB transmission, including (i) tuberculin skin test conversion rates among HCWs; (ii) the total number of people with respiratory TB admitted annually; (iii) the number of occupational exposure episodes (i.e., admitted individuals with respiratory TB who were not placed under airborne precautions such that contact tracing was required; and (iv) the number of previously admitted patients whose TB was diagnosed only at autopsy;
an annual summary of the clinical, epidemiologic and microbiologic features of inpatients newly diagnosed with respiratory TB to be made available to HCWs caring for these patients as a tool to increase awareness of which patients in the population served are at risk of respiratory TB and their clinical manifestations; and
a root-cause analysis to identify and mitigate factors that contributed to healthcare-associated TB exposures.
4.1.2. Respiratory protection program
Essential components of a respiratory protection program are selecting appropriate respirators (N95 or equivalent) for HCWs and HCW education regarding the occupational risk of TB, the role of respiratory protection in reducing that risk and the correct use of respirators, including performing a seal check.Citation61 For cost-efficiency purposes, it is important to provide respirator models with inherently good fit characteristics that will fit the majority of HCWs.
4.1.2.1. Respirators
Respiratory protection of HCWs involves the use of a Health Canada-approved respirator with a filter class equivalent to or higher than an N95, to prevent inhalation of aerosols containing infectious microorganisms.Citation45 These respirators are certified to filter 95% of particles of diameter 0.3 microns (µm) or larger with less than a 10% leak, thus protecting wearers against airborne infectious microorganisms such as M. tuberculosis.Citation25 Medical masks are not designed for respiratory protection of HCWs against M. tuberculosis.
4.1.2.2. Fit testing
Fit testing is used to determine whether a particular size and model of respirator fits a given person, by assessing leakage around the face-respirator seal. Each time the HCW puts on a respirator, a user seal check (according to manufacturer’s instructions) is required to determine whether the respirator is properly sealed to the face. Most Canadian jurisdictions require fit testing for HCWs to determine their ability to obtain a satisfactory seal during respirator use.Citation62 HCWs are referred to jurisdictional requirements regarding the processes and frequency of fit testing. In the absence of requirements, consult provincial/territorial public health authorities.
Regulations
Health care workers should be fit-tested for an N95 or equivalent respirator, and monitored for proper wearing, seal checking and removal of their assigned size and type of N95 or equivalent respirators according to workplace health and safety policies.
Health Canada-approved respirators (N95 or equivalent) should be used by all health care workers providing care to, involved in transport of or otherwise in direct contact with patients with, or being evaluated for, respiratory TB.
Individuals performing maintenance and replacing filters on any ventilation system that is potentially contaminated with Mycobacterium tuberculosis should wear a respirator.
Good practice statements
The respiratory protection program should be committed to developing, implementing, maintaining and evaluating the program.
The healthcare organization should ensure that sufficient numbers of the appropriate respirators are available for use by health care workers and others who are in contact with patients/residents/clients with or being evaluated for respiratory TB.
Health care workers participating in diagnostic bronchoscopies should wear a respirator (N95 or equivalent).
4.1.3. Identifying individuals with respiratory TB in the healthcare setting
Healthcare-associated transmission of TB to other patients and HCWs is uncommon; when it occurs, however, it is most often due to a failure to consider respiratory TB in the diagnosis and to place the patient/resident on airborne precautions.Citation11,Citation14,Citation20,Citation24 One factor leading to delayed diagnosis is not realizing that the patient (or family member) is at risk for TB.Citation22 This may be especially relevant in the patient with minimal respiratory symptoms who is presenting for unrelated reasons, including labour and delivery.Citation63,Citation64,104 For young children (less than five years old) with respiratory TB, it should be noted that they likely recently acquired their infection from an adult family member with active respiratory TB, who may pose a risk to HCWs and other patients while visiting the child in hospital.Citation63,Citation65
Even when the diagnosis of respiratory TB is considered, it may be discounted in the patient for whom another diagnosis seems plausible, such as a non-mycobacterial, community-acquired pneumonia, lung abscess or malignancy, and airborne precautions prematurely discontinued (see Appendix 2, ). Guidelines that direct HCWs on when to consider the diagnosis of respiratory TBCitation66–68 and place the patient on airborne precautions, and under what circumstances airborne precautions can be discontinued, as well as regular HCW education on those guidelines, are important administrative controls.
The diagnosis of TB rests on detection of M. tuberculosis from a respiratory tract specimen.Citation69,Citation70 Three sputum specimens (spontaneous, induced or post-bronchoscopy) from adolescents and adults can be collected on the same day, a minimum of one hour apart. Three gastric washes can be collected from young children in the same morning. A single negative smear from bronchial alveolar lavage does not definitively exclude respiratory TB disease but may be used in certain situations where it is not possible to collect sputum (see Chapter 3: Diagnosis of Tuberculosis Disease and Drug-resistant Tuberculosis).
Good practice statements
Healthcare facilities should provide clinical guidelines for assisting health care workers in making a prompt diagnosis of respiratory TB.
For pediatric patients with, or being investigated for, respiratory TB, family members should be screened by symptoms and radiography for active TB and wear a medical mask as source control during visits (when not in the airborne infection isolation room) until respiratory TB is excluded.
4.1.4. Airborne precautions
Airborne precautions refer to multiple measures applied to prevent airborne exposure to M. tuberculosis in the healthcare setting (see ). They include source-control measures, patient accommodation, limiting patient movement and use of respirators, all to reduce the risk of patient-to-patient and patient-to-HCW transmission.
Figure 1. Isolation of patients with, or being evaluated for, respiratory tuberculosis (TB) in healthcare settings.ASee Section 4.1.3; BSee Section 4.1.4; CSee Chapter 3: Diagnosis of Tuberculosis Disease and Drug-resistant Tuberculosis; DSee Chapter 5: Treatment of Tuberculosis Disease. Abbreviations: AIIR, airborne infection isolation room; IPC, infection prevention and control; PHA, public health authority.
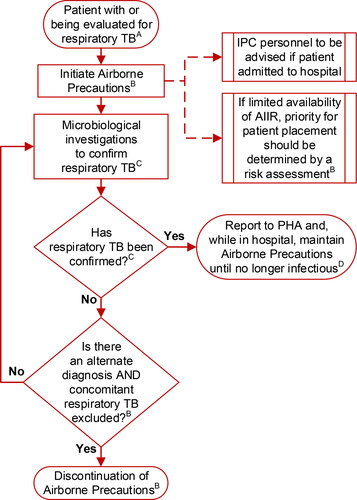
Medical masks include procedure masks and surgical masks. Either type of medical mask, when worn by patients with respiratory TB, serves as a source-control measure to trap infectious respiratory secretions and is not intended as a form of PPE for the wearer.Citation71 Although there is concern that because masks are loose fitting they may allow the escape of aerosols (particularly during coughing), tight-fitting respirators may be uncomfortable for patients (particularly those with limited respiratory reserve) and are therefore not recommended for source control.
Airborne precautions for a patient with symptoms of respiratory TB can be discontinued once suspicion of TB is appropriately excluded, based on results of microbiological investigation and establishment of an alternate diagnosis.
Although the degree and duration of infectiousness of patients after initiation of effective therapy remains unclear, it is known that effective therapy will rapidly reduce cough and the number of viable mycobacteria in the sputum. In patients who are no longer able to spontaneously produce a sputum specimen, sputum induction is useful and appropriate. A poor response to therapy should raise the possibility of drug resistance, even before susceptibility results are available, and inform the decision on discontinuing precautions.
Most people with respiratory TB can be managed in the outpatient setting. If hospitalization is needed, patients with TB are not necessarily required to remain in hospital until no longer infectious. While smear-positive patients are still potentially infectious, their household contacts have already been exposed and are often receiving therapy for latent TB infection when discharge from hospital is being considered. The risk of transmission to these contacts should be balanced by the social, mental and physical health benefits of the patient’s return home. Patients with TB should be discharged as soon as there is no further medical indication to continue hospitalization and criteria for home isolation are met (see Appendix B: De-isolation Review and Recommendations).
On the one hand, there is no evidence of TB being transmitted from persons who have received at least 2 weeks of effective anti-TB therapy. On the other hand, the evidence of no transmission is of poor quality. Given the uncertainty that arises from poor evidence and the potential exposure of highly susceptible contacts (e.g., very young children or highly immune-compromised patients), especially within the acute care hospital setting, the decision to discontinue airborne precautions is individualized. As such, airborne precautions may be continued for a longer period of time in hospital, where many patients are immune-compromised, than is the case in community settings.
Recommendations
We strongly recommend initiating airborne precautions immediately for all those with, or being evaluated for, respiratory TB, both in the healthcare setting and in a long-term care home (good evidence).
For hospitalized patients being evaluated for respiratory TB, we strongly recommend that criteria for discontinuing airborne precautions in hospitalized adolescents and adults include three consecutive smear-negative sputum samples and an alternative diagnosis that explains the patient’s condition and eliminates suspicion of TB; discontinuation of airborne precautions in hospitalized children cannot be based on negative gastric aspirate smears alone (good evidence).
Good practice statements
When availability of airborne infection isolation rooms is limited, priorities for placement of patients should be determined by a risk assessment.
Patients with, or being evaluated for, respiratory TB should have priority over most other indications for an airborne infection isolation room and should not share rooms with each other.
In the absence of an airborne infection isolation room, the patient/resident with, or being evaluated for, respiratory TB should be placed in a single room (with the door closed and a portable high-efficiency particulate air filtration unit used if available) and instructed to wear a medical mask until and during transfer to a facility or unit where an airborne infection isolation room is available.
In the absence of an airborne infection isolation room and when outdoor temperature permits, use natural ventilation to assist in reducing the risk of transmission of airborne pathogens.
Airborne precautions include the use of respirators by health care workers in direct contact with patients/residents with, or being evaluated for, active respiratory TB.
Institutional policies should designate Infection Prevention and Control personnel with the authority to discontinue airborne precautions, as well as to manage both breaches of and adherence to airborne precautions.
The patient should remain in the airborne infection isolation room until airborne precautions are discontinued by designated personnel; patients on airborne precautions may leave the airborne infection isolation room for medical reasons provided they wear a medical mask.
Patients should be discharged home as soon as there is no further medical indication to continue hospitalization and criteria for home isolation are met.
Institutional policies should specify the criteria for discontinuing airborne precautions for patients who need continuing hospital care:
Hospitalized patient with confirmed respiratory TB
Smear-negative, rifampin-susceptible: Airborne precautions can be discontinued once there is clinical evidence of improvement and a minimum of two weeks of effective therapy has been completed.
Smear-positive, rifampin-susceptible: Airborne precautions can be discontinued once there is clinical evidence of improvement, a minimum of 2 weeks of effective therapy has been completed and there are 3 consecutive negative acid-fast bacilli sputum smears.
Persistent smear-positive, rifampin-susceptible: Discontinuing airborne precautions may be considered once there is clinical evidence of improvement and a minimum of 4 weeks of effective therapy has been completed.
Confirmed or suspect rifampin-resistant: Discontinuing airborne precautions may be considered once there is clinical improvement, second-line drug susceptibility results are available, a minimum of 4 weeks of effective therapy has been completed and, for those initially smear-positive, three consecutive sputum smears are negative.
Discharge to home of any patient presumed to be still infectious should be co-ordinated with the patient’s TB physician and local public health authorities to ensure that:
follow-up of household members and home measures to protect any vulnerable individuals are in place, and
patients are not being discharged to a setting where they would expose previously unexposed individuals or a large number of people (e.g., congregate settings).
4.1.5. Transport of patients with, or being evaluated for, respiratory TB
There is a potential for exposure to, and transmission of, TB during patient transport that can be prevented by applying appropriate IPC measures.
Good practice statements
Prior to transport, health care workers involved in patient transport, transport personnel and the receiving healthcare facility should be advised of the need for airborne precautions.
If transport between facilities is required, or for infectious patients traveling to outpatient appointments, patients should not use public transportation (e.g., buses, ride share services, commercial flights).
Patients should be transported in well-ventilated vehicles (ie, with the windows open when possible).
Where air transport is required (e.g., from remote settings), transport personnel should refer to their organization’s policies on medical transport of patients with respiratory TB.
4.1.6. Education of health care workers
An important component of any TB IPC Program is HCW education on how to recognize and protect themselves from exposure to M. tuberculosis. This includes information on epidemiologic and medical risk factors for TB, signs and symptoms of TB (respiratory and non-respiratory), mechanisms of transmission and principles of control.Citation72
Recommendations
We conditionally recommend that all health care workers receive education on TB that is relevant to their work activity, both at the time of hiring and periodically thereafter (poor evidence).
We conditionally recommend that all health care workers be educated on the principles of engineering, administrative (including signage) and personal protective equipment controls in the prevention of transmission of TB and how to apply them (poor evidence).
4.1.7. Health care worker testing and treatment for TB infection
The importance of conducting a baseline HCW assessment for the presence of latent TB infection cannot be overemphasized. At the time of employment, there may be HCWs with latent TB infection because of prior exposure, particularly the situation for HCWs born or previously living in high-TB-incidence countries (see Appendix 2, ).Citation4,Citation6,Citation37,Citation73 Foreign-born HCWs represent an increasing proportion of the workforce in Canadian hospitals and long-term care homes.Citation74 HCWs with reactivation of a latent TB infection can be a source of TB transmission in the healthcare setting where they work.Citation6,Citation75 As per the evidence summary in Appendix 1, , 7 out of 16 identified exposure events were due to an index HCW case. Testing and treating for TB infection is expected to reduce this source of exposures.
Historically, the tuberculin skin test (TST) has been the standard for making a diagnosis of latent TB infection. More recently, interferon-gamma release assays (IGRAs) have been introduced as another diagnostic test. However, the use of IGRA for serial (repeated) testing of HCWs is not recommended because serial-testing studies have shown high rates of conversions and reversions, unrelated to exposure or treatment (see Chapter 4: Diagnosis of Tuberculosis Infection).
Prior exposure to M. tuberculosis or Bacille Calmette-Guérin (BCG) vaccination can result in a boosting phenomenon due to immune recall to a mycobacterial antigen, which may be misdiagnosed as a TST conversion. Interpreting a positive TST performed as part of contact tracing in response to a potential TB exposure in an individual with preexisting latent TB infection or BCG vaccination and for whom an employment test result is unknown can incorrectly over-count TST conversions in relation to a healthcare exposure event. Therefore, a 2-step TST is recommended to establish baseline (see Chapter 4: Diagnosis of Tuberculosis Infection).Citation4,Citation76
Studies from the United States and the United Kingdom indicate that HCWs in low-incidence countries are at no higher risk for TB than the general population, when adjusted for country of origin (see Appendix 2, ).Citation4,Citation6,Citation77 As such, there is no indication for routine organization-wide periodic TST of all HCWs.Citation29,Citation76 Periodic screening (e.g., annual testing) of HCWs at higher risk for occupationally acquired TB, based on the organization risk assessment, may be warranted. Examples of such situations might be HCWs working in bronchoscopy suites or on units identified as having exposure episodes.
Any HCW identified as having had unprotected exposure (termed an exposure episode) to a patient/resident/client or coworker confirmed to have respiratory TB disease should be assessed for TB infection (see See section 9 in this chapter and Chapter 11: Tuberculosis Contact Investigation and Outbreak Management).
Recommendations
We strongly recommend that all health care workers should have a baseline TB screening, including:
an individual risk assessment that identifies risks for TB (temporary or permanent residence in a high-incidence country, prior TB, current or planned immune suppression or close contact with someone who has had infectious TB since the last tuberculin skin test);
a symptom evaluation; and
a tuberculin skin test for those without documented prior TB disease or latent TB infection (good evidence).
We strongly recommend against routine periodic TB testing of all health care workers with negative baseline tuberculin skin test (good evidence).
Good practice statements
The tuberculin skin test is the preferred diagnostic test for pre-employment and periodic testing (if indicated) for TB infection among health care workers.
While volunteers should be screened for risk factors for latent TB infection, consideration could be given to performing a tuberculin skin test only in those who expect to volunteer at least one-half day/week or who have risk factors for latent TB infection.
A baseline 2-step tuberculin skin test should be done unless there is documentation of a prior negative 2-step test, in which case a single-step test should be done, and all results entered into the health care worker’s health record.
All health care workers with a positive tuberculin skin test should be assessed for active TB disease, including a chest x-ray and a medical evaluation, including consideration for treatment of TB infection by a physician experienced in management of TB and latent TB infection; they should also be educated on the signs and symptoms of TB.
A tuberculin skin test should not be performed on a health care worker who was previously TST-positive or has prior documented TB disease.
Health care organizations can consider whether periodic screening for selected health care workers is warranted based on their organizational risk assessment.
Symptom evaluation for all health care workers should be performed by Occupational Health Safety and Wellness when an exposure is recognized and referral for medical assessment be made as required.
The health care worker with a baseline negative tuberculin skin test should have another such test 8 weeks after exposure.
Treatment of health care workers with latent TB infection is encouraged in the absence of contraindications to the recommended medications.
5. Personal protective equipment
The PPE tier refers to the availability and appropriate use of protective equipment that a susceptible host may wear to provide a physical barrier between them and an infectious agent/infected source. The use of PPE is dependent on HCW adherence and competence and, as a result, is the control that is most easily compromised.Citation45 Use of respirators is addressed in section 4.1.2.
6. Prevention of transmission of M. tuberculosis in specific units and populations within hospitals
6.1. Specific units
In certain units (e.g., chemotherapy, HIV and dialysis units), there may be a greater number of patients at higher risk for TB or at greater risk for acquiring TB if exposed (see Chapter 4: Diagnosis of Tuberculosis Infection and Chapter 10: Treatment of Active Tuberculosis in Special Populations). Special consideration is required to prevent the transmission of TB to HCWs, other patients and visitors in such units.
Good practice statement
A unit that treats or cares for patients at risk for TB should have a plan in place for how it will manage a patient with respiratory TB.
6.2. Special populations
There are certain individuals whose immunocompromising conditions or immunosuppressive therapy places them at higher risk of progression from TB infection to disease. They include HIV-infected individuals, transplant patients and people undergoing anti-tumor necrosis factor therapy. While TB incidence rates in patients with end-stage kidney disease are higher than those in the general population and symptoms of respiratory TB may be atypical, case numbers are low.Citation78 Providing care for immune-compromised patients requires a higher index of suspicion for TB and increased vigilance to prevent its transmission before diagnosis. Screening for latent TB infection is recommended for selected high-risk populations (see Chapter 4: Diagnosis of Tuberculosis Infection and Chapter 10: Treatment of Active Tuberculosis in Special Populations).
7. Prevention of transmission of M. tuberculosis within other healthcare settings
Although the principles of TB IPC found in Sections 2 through 6 inclusive are the same across the continuum of healthcare, there is variation in transmission risk and availability of control measures associated with different settings. Modification of control measures applied, and alternative strategies may be required. This section identifies additional specific considerations for non-hospital settings.
7.1. Long-term care homes
Residents of long-term care (LTC) homes are considered to be at the same risk for having latent TB infection as other populations in the community, and have the same risk of developing active TB as persons of the same age in the general population, with the exception of those belonging to identified at-risk groups (see Chapter 6: Tuberculosis Preventive Treatment in Adults, ). However, because of the concern for transmission of TB in LTC homes and the anticipated need for contact tracing should there be an exposure, many guidelines recommend screening newly admitted residents.
Across the Canadian provinces and territories, practices around assessment of new admissions for TB vary. In some provinces/territories, pre-admission and admission screening for symptoms and/or risk of active TB are recommended or mandated. In many provinces, screening for latent TB infection is recommended with a TST and/or chest x-ray, but the criteria for testing vary from all new residents to those who are at increased risk for TB. Some jurisdictions stipulate that the decision for screening should be based on a facility risk assessment and local epidemiology. For discussion and recommendations regarding testing for TB infection in this population, see Chapter 4: Diagnosis of Tuberculosis Infection.
Good practice statements
An assessment of likelihood of respiratory TB should be done on or before admission to a long-term care home.
A symptom screen to rule out active TB should be done, preferably prior to, and on admission to a long-term care home.
A posteroanterior and lateral chest x-ray should be performed if a resident is symptomatic and the resident should be referred for medical assessment if indicated.
Routine tuberculin skin testing on (or prior to) admission and periodic tuberculin skin tests (such as annually) are not recommended for residents.
If a resident has had exposure to respiratory TB, the need for testing should be individualized as part of contact tracing.
7.2. Ambulatory care/outpatient clinics
Ambulatory care settings include locations where health services are provided to patients who are not admitted to inpatient hospital units. This includes, but is not limited to, outpatient diagnostic and treatment facilities (e.g., diagnostic imaging, phlebotomy sites, pulmonary function laboratories, TB treatment facilities), community health centers or clinics, physician offices and offices of allied health professionals (e.g., physiotherapists).Citation25,Citation45
Good practice statements
If possible, non-urgent assessments of people with, or being evaluated for, respiratory TB should be postponed until no longer infectious.
If a visit cannot be postponed, it should be scheduled at the end of the day to minimize exposure to others and, when possible, staff should be alerted of these visits to allow for prompt use of airborne precautions.
The patient should be provided with a medical mask before arrival or immediately upon arrival to be worn until an airborne infection isolation room becomes available. If an airborne infection isolation room is unavailable, the patient should be temporarily assessed or treated in a single room with the door closed, away from vulnerable patients, and transferred as soon as medically feasible to a facility with airborne infection isolation rooms if admission is required.
7.3. Remote and isolated healthcare settings
In remote and isolated communities there are many challenges to TB IPC. Resource limitations may result in difficulties with access to adequate diagnostic facilities for bacteriologic examinations and chest x-ray. A high index of suspicion for a patient having respiratory TB is required. If a chest x-ray is difficult to organize because patients must fly out of the community, then sending sputum samples for TB smear and culture, or nucleic acid amplification test, may be a more rapid way to make a diagnosis of respiratory TB, with less risk of transmission to others. Cohorting may need to be considered as a strategy to accommodate inpatients with respiratory TB if there are insufficient numbers of AII or private rooms.
Recommendations
We conditionally recommend that, if patients with respiratory TB need admission for medical attention and cannot be transferred to a facility with an airborne infection isolation room, cohorting patients with smear-positive TB may be considered, provided they are receiving treatment and there is no suspicion of drug resistance (and/or the prevalence of drug-resistance is known to be very low) (poor evidence).
We conditionally recommend establishing effective community-based treatment programs (in homes) to complete treatment started in the hospital (poor evidence).
Good practice statements
Healthcare facilities that care for populations at-risk for TB should have access to Infection Prevention and Control and Occupational Health Safety and Wellness expertise that will facilitate implementation of engineering, administrative and personal protective equipment controls.
Outpatient visits from people with, or being evaluated for, respiratory TB should be scheduled at the end of the day or after regular hours.
7.4. Home care settings
Home care is delivered to patients who reside in their home or a community care residence. M. tuberculosis transmission to HCWs who work in home-based healthcare settings has been documented, with recommendations developed to prevent transmission.Citation25
Good practice statements
Home care agencies, in consultation with public health authorities, should develop a system for screening at-risk clients for signs of respiratory TB before and during visits, thus facilitating earlier diagnosis and use of appropriate infection prevention and control measures.
The room in the home where the health care worker sees the patient should be well ventilated.
Health care workers should not perform aerosol-generating medical procedures in the home on clients with, or being evaluated for, respiratory TB.
8. Prevention of transmission of M. tuberculosis within congregate settings
In some congregate settings, such as correctional facilities, homeless shelters or hospices there may be a higher proportion of persons with risk factors that put them at greater risk of developing TB, with subsequent transmission to others. The principles and recommendations for preventing transmission of TB in healthcare settings can inform TB IPC policies and procedures for congregate settings.
9. Contact tracing in healthcare settings
While there are many publications regarding outbreak investigations and the outcome of contact tracing efforts arising from TB exposures in the healthcare setting, there is much variation in the exposure criteria used, the extent and duration of the investigation and the diagnostic tests used (see Appendix 1). Most contact investigations following healthcare-associated exposures find few secondary cases of either active or latent TB.Citation10 Despite that, there is the expectation that health care organizations will undertake appropriate contact tracing and a need for relevant contact-tracing guidance in this setting.
Contact tracing following identification of a patient/resident with TB who has not been on airborne precautions or a HCW with TB who worked while infectious must be undertaken in an organized, systematic fashion and in close collaboration with local public health authorities.
Contact tracing principles and steps are described in Chapter 11: Tuberculosis Contact Investigation and Outbreak Management, including determination of infectiousness of the index case, likely period of infectiousness, degree of exposure and prioritization of contacts for screening and evaluation. Although the principles are the same in healthcare settings, there are some specific considerations.
Assessment of exposure: Exposure of HCWs or other patients is to be considered if they shared space with a patient with respiratory TB who was not on airborne precautions for any period of time during the infectious period. Exposure can also occur when an aerosol-generating medical procedure (e.g., high-pressure wound irrigation, procedure using power tools or cautery) is performed at or near the site of extra-pulmonary TB without airborne precautions.
Contact priority: In a typical contact investigation approach, contacts are classified as high-, medium- and low-risk priority, based on level of exposure and risk of progression to active disease.
In healthcare settings, the following are considered high-priority:
Contacts equivalent to household members (e.g., roommate(s) in hospital or LTC home).
Contacts anticipated to be at high risk of progression from latent TB infection to disease (e.g., aged less than 5 years, HIV infected, on dialysis, immune-compromised).
Contacts exposed during aerosol-generating medical procedures without appropriate respiratory protection.
Initial investigation of contacts: In healthcare settings, it is typical to use somewhat lower thresholds for the initial contact investigation compared to the community, because of potential vulnerability of the patient population and risk for further transmission in the healthcare setting. However, it is still important to have a risk-based approach and not to include patients/residents and HCWs with minimal or no exposure whose risk of infection is negligible, and in whom screening could cause more harm than benefit.
The exact duration of exposure that defines a significant risk for acquiring TB is not defined. There is general agreement that any duration of exposure to an aerosol-generating medical procedure warrants contact tracing. Individual facility policies for contact tracing in healthcare settings have used exposure durations in non-aerosol-generating medical procedure situations that vary between two and 48 hours, modulated by the index case’s level of infectiousness, facility ventilation and the contact’s risk of developing active TB. In hospital settings, it may be useful to measure air change rates in the exposure areas, to help prioritize contacts. It is important to remember the need to expand an investigation to include a shorter cumulative duration of exposure if transmissions have been identified during the initial investigation. Outcomes for the investigation across all settings (healthcare and community) should be pooled to guide decisions around expanding the investigation. provides a framework for establishing exposure thresholds for contact investigations in acute care settings, based on a range of exposure durations used by different jurisdictions. No evidence is available to recommend the use of a specific threshold.
Table 3. Suggested framework for TB transmission risk algorithm and contact follow-up.Table Footnote a
Amanda Graham and Chatura Prematunge for background work, literature screening, evidence summary tables, data extraction and text summaries
Joe Tanelli and David M. Barnes for engineering expert review and input
Hayley Watt for background work and literature screening
Ella Westhaver for literature search
The following members of the Public Health Agency of Canada’s National Advisory Committee on Infection Prevention and Control for review of the chapter:
Molly Blake, RN, BN, MHS, CIC
Program Director, Infection Prevention & Control | WRHA (Winnipeg Regional Health Authority)
Acting Program Director, Medical Device Reprocessing | WRHA
Winnipeg, MB
Nan Cleator, RN
National Practice Consultant (formerly)
Practice Quality & Risk Team
Victorian Order of Nurses (VON) Canada
Bracebridge, ON
Joanne Embree, MD, MSc
Pediatric Infectious Disease Specialist, Shared Health
Professor, University of Manitoba
Winnipeg, MB
Jennifer Happe, BSc, MSc
Infection Control Professional
Alberta Health Services
Director, Infection Prevention and Control Canada
Red Deer, AB
Susy Hota, MD, MSc, FRCPC
Medical Director, Infection Prevention and Control
University Health Network
Associate Professor, Department of Medicine, Division of Infectious Diseases
University of Toronto
Toronto, ON
Jennie Johnstone, MD, PhD, FRCPC
Medical Director IPAC, Sinai Health
Toronto, ON
Anne Masters-Boyne, RN, MN
Employee Health Services at Horizon Health Network
Fredericton, NB
Matthew Muller, MD, PhD, FRCPC
Assistant Professor, University of Toronto
Medical Director, Infection Prevention & Control, Unity Health Toronto
Toronto, ON
Patsy Rawding, RN, BScN, CIC
Health Services Manager, Infection Prevention & Control
Western Zone, NSHA Lead Manager in LTC
Middleton, NS
Suzanne Rhodenizer, RN, BScN, MHS, CIC
Director, Clinical Planning
IPAC Consultant QEII New Generation Project
Nova Scotia Health
Halifax, NS
Brian Sagar
Senior Director, Communicable Disease
British Columbia Ministry of Health
Victoria, BC
Patrice Savard, MD, MSc, FRCPC
Clinical Associate Professor, University of Montreal
Clinical Microbiologist and Infectious Diseases Specialist, CHUM
Medical Director, Nosocomial Infection Prevention and Control Unit, CHUM
Montréal, QC
Nisha Thampi, MD, MSc, FRCPC
Medical Director, Infection Prevention and Control Program, Division of Infectious Diseases
Children’s Hospital of Eastern Ontario
Associate Professor, Department of Pediatrics, University of Ottawa
Ottawa, ON
Acknowledgments
Employees of the Public Health Agency of Canada:
Disclosure statement
The CTS TB Standards editors and authors declared potential conflicts of interest at the time of appointment and these were updated throughout the process in accordance with the CTS Conflict of Interest Disclosure Policy. Individual member conflict of interest statements are posted on the CTS website.
Funding
The 8th edition Canadian Tuberculosis Standards are jointly funded by the Canadian Thoracic Society (CTS) and the Public Health Agency of Canada, edited by the CTS and published by the CTS in collaboration with AMMI Canada. However, it is important to note that the clinical recommendations in the Standards are those of the CTS. The CTS TB Standards editors and authors are accountable to the CTS CRGC and the CTS Board of Directors. The CTS TB Standards editors and authors are functionally and editorially independent from any funding sources and did not receive any direct funding from external sources. The CTS receives unrestricted grants which are combined into a central operating account to facilitate the knowledge translation activities of the CTS Assemblies and its guideline and standards panels. No corporate funders played any role in the collection, review, analysis or interpretation of the scientific literature or in any decisions regarding the recommendations presented in this document.
References
- Khan K, Rea E, McDermaid C, et al. Active tuberculosis among homeless persons, Toronto, Ontario, Canada, 1998-2007. Emerg Infect Dis. 2011;17(3):357–365. doi:10.3201/eid1703.100833.
- Isler MA, Rivest P, Mason J, Brassard P. Screening employees of services for homeless individuals in Montréal for tuberculosis infection. J Infect Public Health. 2013;6(3):209–215. doi:10.1016/j.jiph.2012.11.010.
- Uden L, Barber E, Ford N, Cooke GS. Risk of Tuberculosis Infection and Disease for Health Care Workers: An Updated Meta-Analysis. Open Forum Infect Dis. 2017;4(3):ofx137.
- Davidson JA, Lalor MK, Anderson LF, et al. TB in healthcare workers in the UK: A cohort analysis 2009-2013. Thorax. 2017;72(7):654–659. doi:10.1136/thoraxjnl-2015-208026.
- Gisondi P, Pezzolo E, Lo Cascio G, Girolomoni G. Latent tuberculosis infection in patients with chronic plaque psoriasis who are candidates for biological therapy. Br J Dermatol. 2014;171(4):884–890. doi:10.1111/bjd.13130.
- Lambert LA, Pratt RH, Armstrong LR, Haddad MB. Tuberculosis among healthcare workers, United States, 1995-2007. Infect Control Hosp Epidemiol. 2012;33(11):1126–1131. doi:10.1086/668016.
- Mongkolrattanothai T, Lambert LA, Winston CA. Tuberculosis among healthcare personnel, United States, 2010-2016. Infect Control Hosp Epidemiol. 2019;40(6):701–704. doi:10.1017/ice.2019.76.
- Youakim S. The occupational risk of tuberculosis in a low-prevalence population. Occup Med (Lond)). 2016;66(6):466–470. doi:10.1093/occmed/kqw040.
- Gehanno JF, Abiteboul D, Rollin L. Incidence of tuberculosis among nurses and healthcare assistants in France. Occup Med (Lond)). 2017;67(1):58–60. doi:10.1093/occmed/kqw138.
- Schepisi MS, Sotgiu G, Contini S, Puro V, Ippolito G, Girardi E. Tuberculosis transmission from healthcare workers to patients and co-workers: a systematic literature review and meta-analysis. PLoS One. 2015;10(4):e0121639. doi:10.1371/journal.pone.0121639.
- Khalil NJ, Kryzanowski JA, Mercer NJ, Ellis E, Jamieson F. Tuberculosis outbreak in a long-term care facility. Can J Public Health. 2013;104(1):e28–e32. doi:10.1007/BF03405650.
- Medrano BA, Salinas G, Sanchez C, et al. A missed tuberculosis diagnosis resulting in hospital transmission. Infect Control Hosp Epidemiol. 2014;35(5):534–537. doi:10.1086/675833.
- Hazard R, Enfield KB, Low DJ, et al. Hidden Reservoir: An Outbreak of Tuberculosis in Hospital Employees with No Patient Contact. Infect Control Hosp Epidemiol. 2016;37(9):1111–1113. doi:10.1017/ice.2016.126.
- Grisaru-Soen G, Savyon M, Sadot E, et al. Congenital tuberculosis and management of exposure in neonatal and pediatric intensive care units. Int J Tuberc Lung Dis. 2014;18(9):1062–1065. doi:10.5588/ijtld.14.0160.
- Balmelli C, Zysset F, Pagnamenta A, et al. Contact tracing investigation after professional exposure to tuberculosis in a Swiss hospital using both tuberculin skin test and IGRA. Swiss Med Wkly. 2014;144:w13988. doi:10.4414/smw.2014.13988.
- de Vries G, van Hunen R, Meerstadt-Rombach FS, et al. Analysing Tuberculosis Cases Among Healthcare Workers to Inform Infection Control Policy and Practices. Infect Control Hosp Epidemiol. 2017;38(8):976–982. doi:10.1017/ice.2017.100.
- Bucher JN, Schoenberg MB, Freytag I, et al. Donor-derived tuberculosis after solid organ transplantation in two patients and a staff member. Infection. 2016;44(3):365–370. doi:10.1007/s15010-015-0853-z.
- Holden KL, Bradley CW, Curran ET, et al. Unmasking leading to a healthcare worker Mycobacterium tuberculosis transmission. J Hosp Infect. 2018;100(4):e226–e232. doi:10.1016/j.jhin.2018.05.003.
- Menzies D, Fanning A, Yuan L, Fitzgerald M. Tuberculosis among health care workers. N Engl J Med. 1995;332(2):92–98. doi:10.1056/NEJM199501123320206.
- Uppal N, Batt J, Seemangal J, et al. Nosocomial tuberculosis exposures at a tertiary care hospital: a root cause analysis. Am J Infect Control. 2014;42(5):511–515. doi:10.1016/j.ajic.2013.12.010.
- Muzzi A, Seminari E, Feletti T, et al. Post-exposure rate of tuberculosis infection among health care workers measured with tuberculin skin test conversion after unprotected exposure to patients with pulmonary tuberculosis: 6-year experience in an Italian teaching hospital. BMC Infect Dis. 2014;14(1):324. doi:10.1186/1471-2334-14-324.
- Harris TG, Sullivan Meissner J, Proops D. Delay in diagnosis leading to nosocomial transmission of tuberculosis at a New York City health care facility. Am J Infect Control. 2013;41(2):155–160. doi:10.1016/j.ajic.2012.02.015.
- de Perio MA, Niemeier RT. Evaluation of exposure to tuberculosis among employees at a medical center. J Occup Environ Hyg. 2014;11(6):D63–68. doi:10.1080/15459624.2014.888075.
- Jonsson J, Kan B, Berggren I, Bruchfeld J. Extensive nosocomial transmission of tuberculosis in a low-incidence country. J Hosp Infect. 2013;83(4):321–326. doi:10.1016/j.jhin.2012.11.028.
- Centers for Disease Control and Prevention (CDC). Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings. MMWR. 2005;54 (RR-17):1–140.
- Health Canada. Guidelines for preventing the transmission of tuberculosis in Canadian health care facilities and other institutional settings. Communicable Disease Report; No. 22S1, 1996. Available at: https://publications.gc.ca/site/eng/9.507730/publication.html. Accessed on July 19, 2021.
- World Health Organization (WHO). WHO Guidelines on Tuberculosis Infection Prevention and Control, 2019 Update. Geneva, Switzerland: World Health Organization 2019.
- Menzies D, Joshi R, Pai M. Risk of tuberculosis infection and disease associated with work in health care settings. Int J Tuberc Lung Dis. 2007;11(6):593–605.
- Centers for Disease Control and Prevention (CDC), National Tuberculosis Controllers Association. Tuberculosis screening, testing, and treatment of U.S. health care personnel. MMWR. 2019;68(19):439–443.
- Coulter C, The National Tuberculosis Advisory Committee. Infection control guidelines for the management of patients with suspected or confirmed pulmonary tuberculosis in healthcare settings. Commun Dis Intell Q Rep. 2016;40(3):E360–E366.
- New Zealand Ministry of Health. Guidelines for Tuberculosis Control in New Zealand, 2019. Wellington, New Zealand: Ministry of Health. 2019.
- Waring J, Waring J, National Tuberculosis Advisory Committee National Tuberculosis Advisory Committee Guideline: Management of Tuberculosis Risk in Healthcare Workers in Australia. Commun Dis Intell Q Rep. 2017;41(3):E199–E203.
- Kelly AM, D’Agostino JF, Andrada LV, Liu J, Larson E. Delayed tuberculosis diagnosis and costs of contact investigations for hospital exposure: New York City, 2010-2014. Am J Infect Control. 2017;45(5):483–486. doi:10.1016/j.ajic.2016.12.017.
- Khatami A, Outhred AC, Maldigri PI, Isaacs D, Marais B, Kesson AM. Nosocomial transmission from an adolescent with sputum smear-negative pulmonary tuberculosis. Pediatr Infect Dis J. 2017;36(8):814–816. doi:10.1097/INF.0000000000001554.
- Orenstein P, Desmarais A, Maalouf L. TB in a healthcare worker - Exposure management of patients, families and personnel. Paper Presented at: 40th Annual APIC Conference; June 8-10, 2013: Ft Lauderdale, FL.; 2013.
- Romagnoli C, Riccardi R, Purcaro V, Villani A, et al. Neonatal tuberculosis: an experience that teaches. J Matern Fetal Neonatal Med. 2012;25:38–41. doi:10.3109/14767058.2012.714984
- Mor Z, Nuss N, Savion M, et al. Tuberculosis outbreak in a nursing home involving undocumented migrants and Israeli citizens. Isr J Health Policy Res. 2018;7(1):36. doi:10.1186/s13584-018-0219-y.
- Merte JL, Kroll CM, Collins AS, Melnick AL. An epidemiologic investigation of occupational transmission of Mycobacterium tuberculosis infection to dental health care personnel: infection prevention and control implications. J Am Dent Assoc. 2014;145(5):464–471. doi:10.14219/jada.2013.52.
- Freytag I, Bucher J, Schoenberg M, et al. Donor-derived tuberculosis in an anesthetist after short-term exposure: An old demon transplanted from the past to the present. Anaesthesist. 2016;65(5):363–365. doi:10.1007/s00101-016-0162-7.
- Kan B. Tuberculosis in Stockholm: studies on Transmission, Prevention and Control. Stockholm: Department of Medicine in Solna Unit of Infectious Diseases, Karolinska Institute; 2013.
- Townes JM, Hale M, Behm H. Resource-intensive contact investigation resulting from an unrecognized pulmonary tuberculosis case at a rheumatology clinic. Open Forum Infect Dis. 2016;3(suppl 1):1397. doi:10.1093/ofid/ofw172.1100
- Di Quinzio M, Alvarez GG, Stockton K, Roth VR. Effective screening tool to triage recovery rooms for possible tuberculosis patients undergoing bronchoscopy. Int J Tuberc Lung Dis. 2012;16(5):665–669. doi:10.5588/ijtld.11.0555.
- Greenaway C, Menzies D, Fanning A, Canadian Collaborative Group in nosocomial Transmission of Tuberculosis, et al. Delay in diagnosis among hospitalized patients with active tuberculosis-predictors and outcomes. Am J Respir Crit Care Med. 2002;165(7):927–933. doi:10.1164/ajrccm.165.7.2107040.
- Hubad B, Lapanje A. Inadequate hospital ventilation system increases the risk of nosocomial Mycobacterium tuberculosis. J Hosp Infect. 2012;80(1):88–91. doi:10.1016/j.jhin.2011.10.014.
- Public Health Agency of Canada. Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings. Ottawa, Canada: Public Health Agency of Canada; 2013. Available at: https://www.canada.ca/en/public-health/services/infectious-diseases/nosocomial-occupational-infections/routine-practices-additional-precautions-preventing-transmission-infection-healthcare-settings.html. Accessed August 10, 2021.
- Canadian Centre for Occupational Health and Safety. OSH Answers Fact Sheets. Available at: https://www.ccohs.ca/oshanswers/hsprograms/hazard_control.html Published 2021. Updated January 3, 2018. Accessed August 12, 2021.
- Beggs CB, Shepherd SJ, Kerr KG. Potential for airborne transmission of infection in the waiting areas of healthcare premises: stochastic analysis using a Monte Carlo model. BMC Infect Dis. 2010;10(1):247. doi:10.1186/1471-2334-10-247.
- Nardell EA, Keegan J, Cheney SA, Etkind SC. Airborne infection. Theoretical limits of protection achievable by building ventilation. Am Rev Respir Dis. 1991;144(2):302–306. doi:10.1164/ajrccm/144.2.302.
- Centers for Disease Control and Prevention (CDC). Guidelines for Environmental Infection Control in Health-Care Facilities. Atlanta, GA: Centers for Disease Control and Prevention (CDC); 2019. Available at: https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines-P.pdf. Accessed August 12, 2021.
- Canadian Standards Association (CSA). Special requirements for heating, ventilation, and air-conditioning (HVAC) systems in health care facilities (CSA Z317.2.19). 2019.
- American Society of Heating Refrigerating and Air-Conditioning Engineers (ASHRAE), American National Standards Institute (ANSI). ANSI/ASHRAE/ASHE Standard 170-2021: Ventilation of health care facilities. 2021.
- Government of Canada. Canadian Biosafety Standard (CBS) for Facilities Handling or Storing Human and Terrestrial Animal Pathogens and Toxins. Second edition. https://www.canada.ca/en/public-health/services/canadian-biosafety-standards-guidelines/second-edition.html. Updated March 11, 2015. Accessed 2021.
- Menzies D, Fanning A, Yuan L, FitzGerald JM. Hospital ventilation and risk for tuberculous infection in Canadian health care workers. Canadian Collaborative Group in Nosocomial Transmission of TB. Ann Intern Med. 2000;133(10):779–789. doi:10.7326/0003-4819-133-10-200011210-00010.
- Centers for Disease Control and Prevention (CDC). Guidelines for the investigation of contacts of persons with infectious tuberculosis. MMWR. 2005;54(RR-15):1–47.
- Miller SL, Hernandez M, Fennelly K, et al. Efficacy of Ultraviolet Irradiation in Controlling the Spread of Tuberculosis. Centres for Disease Control and Prevention (CDC), National Institute of Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/nioshtic-2/20022472.html. Updated October 2002. Accessed 2021.
- Mphaphlele M, Dharmadhikari AS, Jensen PA, et al. Institutional Tuberculosis Transmission. Controlled Trial of Upper Room Ultraviolet Air Disinfection: A Basis for New Dosing Guidelines. Am J Respir Crit Care Med. 2015;192(4):477–484. doi:10.1164/rccm.201501-0060OC.
- Escombe AR, Moore DA, Gilman RH, et al. Upper-room ultraviolet light and negative air ionization to prevent tuberculosis transmission. PLoS Med. 2009;6(3):e1000043. doi:10.1371/journal.pmed.1000043.
- Whalen J. Environmental control for tuberculosis: basic upper-room ultraviolet germicidal irradiation guidelines for healthcare settings guide. Centers for Disease Control and Prevention (CDC), National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/docs/2009-105/pdfs/2009-105.pdf?id=10.26616/NIOSHPUB2009105. Updated March 2009. Accessed 2021.
- Wheeler PW, Lancaster D, Kaiser AB. Bronchopulmonary cross-colonization and infection related to mycobacterial contamination of suction valves of bronchoscopes. J Infect Dis. 1989;159(5):954–958. doi:10.1093/infdis/159.5.954.
- Ramsey AH, Oemig TV, Davis JP, et al. An outbreak of bronchoscopy-related Mycobacterium tuberculosis infections due to lack of bronchoscope leak testing. Chest. 2002;121(3):976–981. doi:10.1378/chest.121.3.976.
- Brosseau LM, Conroy LM, Sietsema M, et al. Evaluation of Minnesota and Illinois hospital respiratory protection programs and health care worker respirator use. J Occup Environ Hyg. 2015;12(1):1–15. doi:10.1080/15459624.2014.930560.
- Canadian Standards Association (CSA). Selection, use, and care of respirators. In. CAN/CSA Z94.4-18 2018.
- Newberry DM, Robertson Bell T. Congenital Tuberculosis: A New Concern in the Neonatal Intensive Care Unit. Adv Neonatal Care. 2018;18(5):341–349. doi:10.1097/ANC.0000000000000555.
- Schechner V, Lessing JB, Grisaru-Soen G, et al. Preventing tuberculosis transmission at a maternity hospital by targeted screening radiography of migrants. J Hosp Infect. 2015;90(3):253–259. doi:10.1016/j.jhin.2015.03.008.
- Muñoz FM, Ong LT, Seavy D, et al. Tuberculosis among adult visitors of children with suspected tuberculosis and employees at a children’s hospital. Infect Control Hosp Epidemiol. 2002;23(10):568–572. doi:10.1086/501972.
- Cavallazzi R, Wiemken T, Christensen D, Community-Acquired Pneumonia Organization (CAPO) Investigators, et al. Predicting Mycobacterium tuberculosis in patients with community-acquired pneumonia. Eur Respir J. 2014;43(1):178–184. doi:10.1183/09031936.00017813.
- Chaisson LH, Duong D, Cattamanchi A, et al. Association of Rapid Molecular Testing With Duration of Respiratory Isolation for Patients With Possible Tuberculosis in a US Hospital. JAMA Intern Med. 2018;178(10):1380–1388. doi:10.1001/jamainternmed.2018.3638.
- Poonawala H, Leekha S, Medina-Moreno S, et al. Use of a Single Xpert MTB/RIF Assay to Determine the Duration of Airborne Isolation in Hospitalized Patients With Suspected Pulmonary Tuberculosis. Infect Control Hosp Epidemiol. 2018;39(5):590–595. doi:10.1017/ice.2018.25.
- Lippincott CK, Miller MB, Popowitch EB, et al. Xpert MTB/RIF assay shortens airborne isolation for hospitalized patients with presumptive tuberculosis in the United States. Clin Infect Dis. 2014;59(2):186–192. doi:10.1093/cid/ciu212.
- Chaisson LH, Roemer M, Cantu D, et al. Impact of GeneXpert MTB/RIF assay on triage of respiratory isolation rooms for inpatients with presumed tuberculosis: a hypothetical trial. Clin Infect Dis. 2014;59(10):1353–1360. doi:10.1093/cid/ciu620.
- Dharmadhikari AS, Mphahlele M, Stoltz A, et al. Surgical face masks worn by patients with multidrug-resistant tuberculosis: impact on infectivity of air on a hospital ward. Am J Respir Crit Care Med. 2012;185(10):1104–1109. doi:10.1164/rccm.201107-1190OC.
- Boparai N, Patterson B, Dekoningh J, et al. P220 Standardising TB incident management across a large UK TB network. Thorax. 2018;73(suppl 4):A220–A221. doi:10.1136/thorax-2018-212555.377
- Henderson M, Howard SJ. Screening for latent tuberculosis in UK health care workers. Occup Med (Lond)). 2017;67(8):641–643. doi:10.1093/occmed/kqx119.
- Cornelissen L. Profile of immigrants in nursing and health care support occupations. The Daily: Statistics Canada; May 2021. 2291‑0840.
- Davidson JA, Fulton N, Thomas HL, et al. Investigating a tuberculosis cluster among Filipino health care workers in a low-incidence country. Int J Tuberc Lung Dis. 2018;22(3):252–257. doi:10.5588/ijtld.17.0620.
- Dobler CC, Farah WH, Alsawas M, et al. Tuberculin skin test conversions and occupational exposure risk in US healthcare workers. Clin Infect Dis. 2018;66(5):706–711. doi:10.1093/cid/cix861.
- Diel R, Niemann S, Nienhaus A. Risk of tuberculosis transmission among healthcare workers. ERJ Open Res. 2018;4(2):00161-2017– 02017. doi:10.1183/23120541.00161-2017.
- Okada RC, Barry PM, Skarbinski J, Chitnis AS. Epidemiology, detection, and management of tuberculosis among end-stage renal disease patients. Infect Control Hosp Epidemiol. 2018;39(11):1367–1374. doi:10.1017/ice.2018.219.
Appendix 1: Summary of Available Evidence
Table 1a. Epidemiological investigations reporting TB exposures in healthcare settings, linked to a health care worker index case
Table 1b. Epidemiological investigations reporting TB exposures in healthcare settings, linked to a patient or long-term care resident (patient) index case
Table 1c. Observational studies reporting on TB exposures in healthcare settings
Appendix 2: Available Supporting Body and Quality of Evidence for Recommendations and Good Practice Statements
Table 2a. Tuberculosis (TB) exposures in healthcare or long-term care settings linked to absent or sub-optimal baseline TB screening in health care workers (HCWs)
Table 2b. Tuberculosis (TB) risk (latent or active TB infection) among health care workers (HCWs) compared to non-HCW populations in low-incidence countries
Table 2c. Tuberculosis (TB) exposures linked to delayed diagnosis and initiation of airborne precautions in patients or residents
Table 2d. Tuberculosis (TB) exposures due to premature discontinuation of airborne precautions
