Abstract
Chronic obstructive pulmonary disease (COPD) patient care must include confirming a diagnosis with postbronchodilator spirometry. Because of the clinical heterogeneity and the reality that airflow obstruction assessed by spirometry only partially reflects disease severity, a thorough clinical evaluation of the patient should include assessment of symptom burden and risk of exacerbations that permits the implementation of evidence-informed pharmacological and nonpharmacological interventions. This guideline provides recommendations from a comprehensive systematic review with a meta-analysis and expert-informed clinical remarks to optimize maintenance pharmacological therapy for individuals with stable COPD, and a revised and practical treatment pathway based on new evidence since the 2019 update of the Canadian Thoracic Society (CTS) Guideline. The key clinical questions were developed using the Patients/Population (P), Intervention(s) (I), Comparison/Comparator (C), and Outcome (O) model for 3 questions that focuses on the outcomes of symptoms (dyspnea)/health status, acute exacerbations and mortality. The evidence from this systematic review and meta-analysis leads to the recommendation that all symptomatic patients with spirometry-confirmed COPD should receive long-acting bronchodilator maintenance therapy. Those with moderate to severe dyspnea (modified Medical Research Council ≥2) and/or impaired health status (COPD Assessment Test ≥10) and a low risk of exacerbations should receive combination therapy with a long-acting muscarinic antagonist/long-acting ẞ2-agonist (LAMA/LABA). For those with a moderate/severe dyspnea and/or impaired health status and a high risk of exacerbations should be prescribed triple combination therapy (LAMA/LABA/ICS) azithromycin, roflumilast or N-Acetylcysteine is recommended for specific populations; a recommendation against the use of theophylline, maintenance systemic oral corticosteroids such as prednisone and mono-ICS is made for all COPD patients.
RÉSUMÉ
Les soins aux patients atteints de maladie pulmonaire obstructive chronique (MPOC) doivent inclure la confirmation d’un diagnostic par spirométrie post-bronchodilatateur. En raison de l’hétérogénéité clinique et du fait que l’obstruction du flux d’air évaluée par spirométrie ne reflète que partiellement la gravité de la maladie, une évaluation clinique approfondie du patient doit inclure une évaluation du fardeau des symptômes et du risque d’exacerbations permettant la mise en œuvre d’interventions pharmacologiques et non pharmacologiques fondées sur des données probantes. Cette ligne directrice formule des recommandations issues d’une revue systématique complète, assortie d’une méta-analyse et d’observations cliniques d’experts, afin d’optimiser le traitement pharmacologique d’entretien pour les personnes atteintes de MPOC stable, de même qu’un parcours de soins révisé et pratique fondé sur de nouvelles données probantes depuis la mise à jour de la Ligne directrice de la Société canadienne de thoracologie (SCT) de 2019. Les questions cliniques clés ont été élaborées à l’aide βdu modèle Patients/Population (P), Intervention(s) (I), Comparaison/Comparateur (C) et Résultat (O) pour trois questions portant sur les résultats des symptômes (dyspnée)/état de santé, les exacerbations aiguës et la mortalité. Les données probantes de cette revue systématique et de cette méta-analyse conduisent à recommander que tous les patients symptomatiques atteints de MPOC confirmée par spirométrie reçoivent un traitement d’entretien bronchodilatateur à action prolongée. Les personnes présentant une dyspnée modérée à sévère (Conseil de recherches médicales modifié ≥2) et/ou un état de santé altéré (test d’évaluation de la MPOC ≥10) et un faible risque d’exacerbations doivent recevoir un traitement combiné avec un antagoniste muscarinique à longue durée d’action/agoniste ẞ2 à longue durée d’action (LAMA/LABA). Pour les personnes présentant une dyspnée modérée/sévère et/ou un état de santé altéré et un risque élevé d’exacerbations, une trithérapie doit être prescrite (LAMA/LABA/CSI). L’azithromycine, le roflumilast ou la N-acétylcystéine sont recommandés pour des populations spécifiques; une recommandation contre l’utilisation de la théophylline, des corticostéroïdes oraux systémiques d’entretien tels que la prednisone et la mono-CSI est faite pour tous les patients atteints de MPOC.
Introduction
Chronic lung diseases, in particular chronic obstructive pulmonary disease (COPD), lead to a high burden of disease reflected in morbidity, mortality and health care costs. COPD is the third leading cause of death worldwide, causing 3.23 million deaths in 2019.Citation1 In 2019, it was associated with 4.7% of global disability-adjusted life years (DALYs) and ranked as the sixth leading cause of DALYs.Citation2 In Canada, all-cause mortality rates were higher among those living with COPD than those without COPD, across all age groups; rate ratios ranged from 3.7 in the 50-54 age group to 1.7 in the 85 and older age group.Citation3 COPD accounts for over 50% of chronic respiratory disease prevalence among males and females,Citation4 and for an astounding 81.7% of the total number of deaths from chronic respiratory diseases.Citation5
The course of COPD over time is characterized by persistent dyspnea and disabilityCitation6 with acute exacerbations that lead to a faster lung function decline,Citation7 worsened health status,Citation8 and increased hospitalizations.Citation9 Exacerbations are the main driver of healthcare costs and the large economic burden in COPD has been documented in a number of studies.Citation10 The 30-day readmission rate for acute exacerbations in different developed countries can be as high as 22%, and poor discharge medication reconciliation is among the factors thought to contribute to early readmissions.Citation11 Severe exacerbations are also associated with increased all-cause mortality.Citation12
There is an urgent need to offer effective and personalized management plans for individuals living with COPD to improve symptoms and health status, prevent acute exacerbations and reduce mortality. An integrative comprehensive approach to COPD management that includes confirming a diagnosis of COPD with spirometry, evaluating symptom burden, health status and risk of exacerbations over time and implementing pharmacological and nonpharmacological treatments is both effective and recommended. Importantly, relevant and evidence-based nonpharmacologic interventions such as smoking cessation counseling, vaccinations, self-management education, and pulmonary rehabilitation aimed at healthy lifestyle behaviors and improved daily management of COPD are vital for effective comprehensive management of COPD.Citation13
Figure 1. Integrated Comprehensive Management of COPD.Integrated comprehensive management of COPD includes confirming COPD diagnosis with postbronchodilator spirometry, evaluation and on-going monitoring of dyspnea/symptom burden and risk of exacerbations and use of both pharmacologic and nonpharmacologic interventions (see ) to alleviate dyspnea/symptoms, improve health status, prevent AECOPD and reduce mortality. The approach should not be viewed as “stepwise” and may not necessarily occur in the order they appear for all patients. Self-Management Education includes optimizing inhaler device technique and [re-]review, assessment and review of medication adherence, breathing and cough techniques, early recognition of AECOPD, written AECOPD action plan and implementation (when appropriate), promoting physical activity and/or exercise, and other healthy habits including diet and smoking cessation.**Inhaled Maintenance/Preventative Pharmacotherapies are long-acting muscarinic antagonists (LAMA) and/or long-acting ẞ2-agonists (LABA) with or without inhaled corticosteroids (ICS). ICS monotherapy should NOT be used in COPD management.*Other pharmacotherapies include oral therapies (prophylactic macrolide, and PDE-4 inhibitor, mucolytic agents for patients with chronic bronchitis), alpha-1-antitrypsin augmentation therapy for documented severe A1AT deficiency, and opioids for severe refractory dyspnea (see prior CTS Guideline).Citation13ǂSurgical therapies may include lung transplantation and lung volume reduction (including with endoscopic valves).Abbreviations. A1AT, alpha-1 antitrypsin; AECOPD, acute exacerbation of COPD; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; CTS, Canadian Thoracic Society; mMRC, modified Medical Research Council; prn, as-needed; NIV, noninvasive ventilation.
![Figure 1. Integrated Comprehensive Management of COPD.Integrated comprehensive management of COPD includes confirming COPD diagnosis with postbronchodilator spirometry, evaluation and on-going monitoring of dyspnea/symptom burden and risk of exacerbations and use of both pharmacologic and nonpharmacologic interventions (see Figure 3) to alleviate dyspnea/symptoms, improve health status, prevent AECOPD and reduce mortality. The approach should not be viewed as “stepwise” and may not necessarily occur in the order they appear for all patients. Self-Management Education includes optimizing inhaler device technique and [re-]review, assessment and review of medication adherence, breathing and cough techniques, early recognition of AECOPD, written AECOPD action plan and implementation (when appropriate), promoting physical activity and/or exercise, and other healthy habits including diet and smoking cessation.**Inhaled Maintenance/Preventative Pharmacotherapies are long-acting muscarinic antagonists (LAMA) and/or long-acting ẞ2-agonists (LABA) with or without inhaled corticosteroids (ICS). ICS monotherapy should NOT be used in COPD management.*Other pharmacotherapies include oral therapies (prophylactic macrolide, and PDE-4 inhibitor, mucolytic agents for patients with chronic bronchitis), alpha-1-antitrypsin augmentation therapy for documented severe A1AT deficiency, and opioids for severe refractory dyspnea (see prior CTS Guideline).Citation13ǂSurgical therapies may include lung transplantation and lung volume reduction (including with endoscopic valves).Abbreviations. A1AT, alpha-1 antitrypsin; AECOPD, acute exacerbation of COPD; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; CTS, Canadian Thoracic Society; mMRC, modified Medical Research Council; prn, as-needed; NIV, noninvasive ventilation.](/cms/asset/5127fbc7-82c9-408c-8b89-3306cea8f469/ucts_a_2231451_f0001_c.jpg)
This clinical practice guideline, informed by a comprehensive systematic review and a meta-analysis: (i) provides an update from the Canadian Thoracic Society (CTS) Clinical Practice Guideline on Pharmacotherapy in Patients with COPD − 2019 for the optimal approach to the pharmacological treatment of individuals with COPD to alleviate symptoms, improve health status and prevent exacerbations;Citation13 and (ii) synthesizes emerging evidence on whether maintenance pharmacotherapy reduces mortality. This guideline has systematically evaluated evidence and formulated corresponding evidence-based recommendations for each key clinical question and outlines a practical clinical treatment pathway based on those recommendations, the quality and strength of the evidence, balance of benefits and harms and perceived patient preferences.
This guideline does not address nonpharmacological interventions (e.g., smoking cessation counseling, vaccines, self-management education, pulmonary rehabilitation), long term oxygen therapy, noninvasive ventilation, interventional bronchoscopy or surgery, respiratory palliative care or management of acute exacerbations.
Objectives
The overall objective of this CTS guideline is to help clinicians match pharmacological treatment to the clinical status of individuals with stable COPD. This is an important step toward personalizing therapy based on individual characterization.
The specific objective is to provide clinical guidance with evidence-based recommendations from a systematic review with a meta-analysis and expert-informed clinical remarks to optimize maintenance pharmacological therapy aimed at alleviating dyspnea and improving health status, preventing exacerbations and reducing mortality for individuals with stable COPD.
Target patient population
The update applies to all individuals with stable COPD.
Target users
Visions to the integrated and comprehensive management of COPD
shows the approach to integrated and comprehensive management in COPD. Integrated and comprehensive clinical care should include: (i) diagnosis of COPD confirmed with postbronchodilator spirometry, clinical evaluation, and routine follow-up and assessment; and (ii) comprehensive management, which comprises evidence-informed nonpharmacological and pharmacological interventions, and patient supportive healthcare system practices.
Spirometry is essential for the diagnosis of COPD. A postbronchodilator FEV1/FVC ratio <0.70 confirms the diagnosis, although some have proposed using <5th percentile (LLN) of a reference population. Evidence supports the use of a fixed ratio less than 0.70 versus the <5th percentile LLN of a reference population, as more appropriate to identify individuals at risk of clinically significant COPD.Citation14–Citation16 However, the fixed ratio approach may under or over-estimate presence of airflow obstruction at the extremes of age. While the diagnosis of COPD is confirmed by a reduced post-bronchodilator FEV1/FVC ratio <0.7, the severity of airflow obstruction in COPD should be evaluated by the magnitude of reduction in the post-bronchodilator FEV1. Many individuals with COPD remain undiagnosed; although they may be symptomatic, have poor overall health status, and have an increased risk of exacerbations, pneumonia and death.Citation17 At the same time, due to underuse of spirometry to confirm the diagnosis of COPD, symptomatic patients, especially those with a smoking history, may receive unneeded inhaled therapy.Citation18
Essential COPD management goals include improving lung function, reducing dyspnea and other symptoms, enhancing health status, and reducing acute exacerbations of COPD (AECOPD), which are strongly associated with increased mortality.
The approach outlined in should NOT be viewed as a “stepwise” approach, but rather an expanding menu of effective therapies addressing increasing impairment and disability, risk of adverse clinical outcomes, and providing significant clinical benefits.
Methodology
This guideline was developed in accordance with the CTS guideline development process,Citation19 including the GRADE methodologyCitation20 and use of the AGREE II checklist throughout the guideline process.Citation13
Guideline panel composition
The COPD guideline panel comprised 16 experts: 13 respirologists with experience in COPD management, research and research methodology; 1 primary care physician; 1 pharmacist, knowledge mobilization expert and 1 methodologist. All author conflicts of interests are available at www.cts-sct.ca/guideline-library/. There were no patients participating in the guideline panel although “patient value COPD outcomes” were also substantiated from the published literature (see the following section).
Key clinical questions
The key clinical questions were developed using the Patients/population (P), Intervention(s) (I), Comparison/comparator (C), and Outcome (O)—the PICO model. Three PICO questions were included in this guideline. PICO 1 and 2 were based on the previously published CTS Position Statement on Pharmacotherapy in Patients with Stable COPD – An Update, 2017Citation21 and CTS Clinical Practice Guideline on Pharmacotherapy in Individuals with COPD − 2019 Update of Evidence.Citation13 Stable COPD excludes patients with acute worsening of dyspnea or acute exacerbations. We used evidence/studies from our previous published guidelinesCitation13,Citation21 and incorporated newly identified evidence after their search date limits. PICO 3 was added in light of new evidence surrounding the impact of inhaled maintenance agents on mortality in COPD. It evaluated the role of pharmacotherapeutic agents compared to other agents in preventing mortality in individuals with COPD. After development of these PICOs and before evidence review, the clinical importance of the outcomes of each PICO was rated by experts, on a graded scale of 1–9, 1 being low and 9 as high defined in the GRADEpro workbook.Citation22 Scores were ascribed based on perceived patient and clinical relevance. The rating of the outcomes as 7–9 was considered “critical,” 4-6 as “important” and 1-3 as “limited importance.” The outcomes considered for this guideline were primarily (1) dyspnea, health status and exercise tolerance; (2) exacerbations; and (3) mortality (score 7-9); other outcomes included were physical activity, lung function and adverse events (score 4-6). “Patient value COPD outcomes” were also assessed based on a recent systematic review on how patients value COPD outcomes.Citation23 This study showed that exacerbation and hospitalization are the outcomes that COPD patients rate as most important. Patients rated adverse events as important but on average, less so than symptom relief. Furthermore, this quantitative evaluation was complemented by a recent qualitative study that reported that patients and carers considered that COPD associated breathlessness took over their lives, and saw their worlds shrink physically and socially due to chronic breathlessness.Citation24
Literature search and screening of abstracts
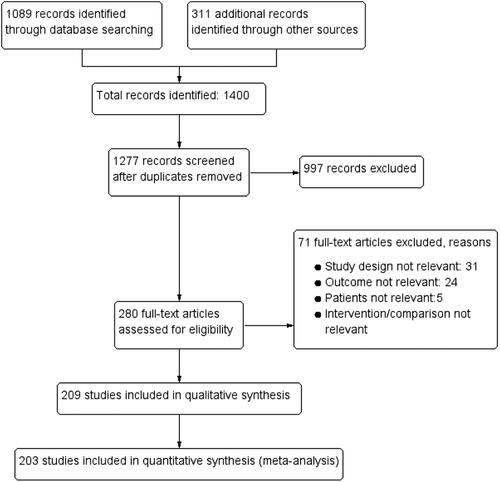
In addition to the studies included in our previous guidelines,Citation13, Citation21 a comprehensive search of literature was performed from MEDLINE, EMBASE and COCHRANE libraries from the end date of the 2019 guideline search (October 18, 2018 to June 9, 2022) for PICO 1 and 2, and from 1974 to June 9, 2022 for PICO 3. Relevant studies and review articles were hand-searched to identify further articles. See Online Supplement 3 for details of the search strategy, additional studies identified, and the study selection process (predefined criteria, titles and abstracts, full text screening). The PRISMA Diagram () presents the records identified, included and excluded, and reasons for exclusion.
Study design and risk of bias
We included only randomized controlled trials (RCTs) for this guideline. The risk of bias of these RCTs was assessed by (JW/JM) and verified by the methodologist using Cochrane Risk of Bias Tool for RCTs.Citation25 This tool assessed various components of the RCTs, such as risk of selection bias (randomization, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of outcome assessment), attrition bias (incomplete outcome data) and reporting bias (selective reporting). See Online Supplement 3 for more details.
Meta-analysis
Meta-analysis of each outcome was performed if more than one study reported an outcome (Online Supplement 2). This was performed by AL using the Review Manager (RevMan, version 5.4) Cochrane Collaboration software. The risk ratios (RRs), rate ratio, mean difference (MD) and their 95% confidence intervals (CIs) were calculated. A random effects model was used for meta-analysis of all the outcomes. The I2 statistics and p values of Q statistics were determined to assess heterogeneity among the studies. A p value of the Q statistic <0.05 was considered to indicate statistically significant heterogeneity. Heterogeneity was classified as moderate (I2 ≥ 30-49%), substantial (I2 ≥ 50-74%) or considerable (I2 ≥ 75%).Citation25 Where appropriate, subgroup meta-analyses based on timing of measurement of outcomes were performed.
The forest plots list intervention and comparator data in columns 2 and 3. The vertical line of “no effect” indicates no difference in effect of an intervention over control and has a value of 0 for continuous variables and 1 for binary variables. For outcomes such as transitional dyspnea index (TDI) and FEV1, when the diamond of effect size lies on the right side of the line of no effect, there is improvement in these outcomes with intervention compared to control, favoring intervention. On the other hand, for outcomes such as St George’s Respiratory Questionnaire (SGRQ), exacerbations, mortality and adverse events (eg, pneumonia), when the diamond of effect size lies on the left side of the line of no effect, there is a reduction in these scores/events with intervention compared to control, favoring intervention (see Online Supplement 2 for details).
GRADE
Grading of the quality of the evidence was performed by the Methodologist using the GRADE process. Components considered when evaluating the certainty of an outcome included study design, risk of bias, inconsistency of results, indirectness of evidence, imprecision and other factors such as publication bias, magnitude of effect, confounding and dose-response gradient. See Online Supplement 3 and GRADEpro handbookCitation22 for additional details on the GRADE methodology.
Synthesis of evidence-base and clinical judgment of risk-versus-benefit
For each PICO question, we considered the overall certainty of evidence for the critical outcomes. In addition to the quality of the evidence, for each therapeutic approach, the panel considered: the balance between the potential health benefits and harms to individual patients and the overall COPD population; the perceived importance of each outcome to patients; and the burden placed on the patient (these considerations are part of the “Contextualization and Deliberations” domain of guidelines and are explicated in “Clinical Remarks” attached to recommendation, where appropriate).Citation26 During panel discussions, members also considered resource use, feasibility and acceptability to all stakeholders. The strength of each recommendation (strong or weak) was determined according to the aforementioned factors. To enable this, the evidence (summary of findings tables, forest plots, quality of evidence assessments) was presented to the entire guideline panel. In the situation where there was lack of data, the panel indicated and employed expert consensus opinion.
Recommendations and classification
The final summary of findings tables, quality of evidence assessments, and corresponding strength for each recommendation (for each PICO question) were discussed by the whole group. Following open and extensive discussions, the entire panel proposed wording and/or updates to prior recommendations, and where applicable, any required change to the strength of the recommendation. They based the strength of each recommendation on the GRADE quality of evidenceCitation20 and synthesis of clinical judgment. The CTS Canadian Respiratory Guidelines Committee (CRGC) executive then vetted the recommendations to optimize language with a view to improving likelihood of uptake.Citation27,Citation28 Recommendations were then voted upon using a six-point voting scale, whereby it was defined a priori that a recommendation would only be accepted if each panel member voted for option 1, 2 or 3 (“wholeheartedly agree,” “agree” or “can support”). For a recommendation to be accepted, it had to be voted on by 75% of the eligible panel members and achieve ratings 1, 2 or 3 by 80% of the voting panelists. In the event of a failure to reach 80% of votes with ratings 1, 2 or 3, another period of discussion ensued, whereby dissenting opinions were heard and considered. The recommendation was revised as necessary and followed by a second round of voting using a three-point scale, for which acceptance of a recommendation required a majority (80%) of panelists to choose option 1 or 2. Throughout this process all recommendations achieved acceptance. We also included practical clinical advice within “Clinical Remarks” attached to recommendations. This advice represents the consensus opinion of panel members based on their expertise.
Review and approval process
The CTS independently invited formal review of this guideline by five external (non-CTS) content experts (Canada, Japan, Spain and United States) and two internal (CTS) members. The lead author responded to the comments and made corresponding changes. The Chair and Vice-Chair of the CRGC then completed their own review and provided further feedback for consideration. Upon acceptance, the CRGC recommended approval of the guideline to the CTS Executive committee for final approval.
Living guideline/future updates
The guideline will be reviewed annually to determine the need for and nature of any updates, in accordance with the CTS Living Guideline Model.
2023 Summary of evidence-based recommendations
- present the recommendations for optimal pharmacotherapy and represents a practical clinical treatment pathway for stable COPD. The following recommendations reflect the strength and quality of evidence and high importance to patients and clinicians as key treatment goals. The “evidence based” recommendations of the CTS guideline help inform treatment decision-making, with reference to similar population of patients that were included in these clinical trials, and population-level health risks included our meta-analysis. Note that not all trials of these interventions characterized study populations’ disease severity by CAT, mMRC score and lung function exactly as we have below, but the panel believes that this description closely matches the cohorts represented in corresponding studies.
Table 1. 2023 recommendations for PICO 1
How does a clinician choose appropriate maintenance pharmacotherapies in individuals with stable COPD to reduce symptom burden, for example, dyspnea and exercise intolerance, and improve health status?
Table 2. 2023 Recommendations for PICO 2
How does a clinician choose appropriate maintenance pharmacotherapies in individuals with stable COPD to reduce the risk of AECOPD?
Table 3. 2023 Recommendations for PICO 3
How does a clinician choose appropriate maintenance pharmacotherapies in individuals with stable COPD to reduce mortality?
Summary PICO 1: Alleviating symptom burden (e.g., dyspnea and exercise intolerance, improving health status)
For PICO 1, lists all the recommendations, their strength and certainty based on the evidence from meta-analysis (summary in Online Supplement Table 1), along with clinical remarks (where applicable). This section presents the optimal use of inhaled and oral pharmacologic maintenance therapies shown to alleviate dyspnea, and to improve exercise tolerance and health status in individuals with stable COPD. Note that this document is not intended to guide the treatment of acute dyspnea.
Clinical remarks
Symptom burden of COPD, notably dyspnea and exercise intolerance, negatively affect patient health status. Reduced health status is thought to be related to COPD symptoms and functional impairments.Citation29 Although symptoms and health status worsen due to acute exacerbations, the relationship between symptom burden and health status has been demonstrated to be independent from confounding variables, which importantly included patients’ exacerbation history.Citation30 Symptoms are also known to be heterogeneous among patients and across disease severity.Citation31 It has been shown that patients receiving bronchodilator therapy still have a high symptom burden that negatively affected their health status and sleep, and a substantial proportion of individuals with a high symptom burden may not be receiving optimal bronchodilation.Citation30 Thus, along with exacerbation risk assessment, monitoring COPD symptom burden consistently and tailoring treatment accordingly should be given more attention, aiming to optimize symptom control and ensure improved health status.
Patient values and preferences
We placed high value on alleviating dyspnea and improving health status as treatment goals.
Review of evidence by outcomes
Dyspnea
Dyspnea is the most common and disabling symptom reported by individuals living with COPD, negatively affecting their performance of activities of daily living. Increasing dyspnea severity is also associated with a greater negative psychological impact.Citation32 Alleviating dyspnea is a key treatment goal of COPD management. Whereas the modified MRC (mMRC) dyspnea scale is simple-to-use and a validated tool for categorizing disability related to dyspnea and COPD disease severity, it is unresponsive to change.Citation33 However, other instruments such as the transitional dyspnea index (TDI) have been demonstrated in RCTs to be responsive to both pharmacological and nonpharmacological interventions.Citation34
Recommendations and changes from last CTS COPD Guideline in 2019 with respect to dyspnea (): There is change to the recommendation from the last CTS COPD guideline in 2019 that for individuals with low symptom burden and health status impairment (mMRC 1), and only mildly impaired lung function (FEV1 ≥ 80% predicted), treatment is recommended starting with an inhaled long-acting bronchodilator (LABD) (rather than a short-acting bronchodilator (SABD)), with no significant difference between inhaled LAMA or LABA monotherapy (rec. PICO P.1.A.). In individuals with moderate to high symptoms (mMRC ≥2) and impaired lung function (FEV1 < 80% predicted), based on updated evidence, there is a change from 2019 with a strong recommendation, with LAMA/LABA dual therapy now being recommended as initial maintenance therapy (rec. PICO P.1.B.). This revised recommendation is based on several RCTs and meta-analyses consistently showing superior efficacy of dual versus monobronchodilator therapy with a similar safety profile.Citation35–39 Based on a single study, there is no difference in dyspnea response to treatment between ICS/LABA combination therapy and LAMA monotherapy between 6 and 24 months,Citation40 and from a number of studies there is no significant difference in dyspnea response to treatment between ICS/LABA combination therapy and either LABA monotherapy or LAMA/LABA dual therapy; however, ICS/LABA combination therapy was associated with higher rates of adverse effects (e.g., pneumonia). Two large studies, The Efficacy and Safety of Triple Therapy in Obstructive Lung Disease (ETHOS)Citation41 and The Informing the Pathway of COPD Treatment (IMPACT),Citation42,Citation43 performed in symptomatic individuals with COPD at a high risk of future exacerbations, have provided strong evidence for an improvement in dyspnea with LAMA/LABA/ICS triple combination therapy compared to either LAMA/LABA dual therapy or ICS/LABA combination therapy, leading to strong recommendations, accordingly (rec. PICO P.1.C.). Of note, we extrapolated this to a population at low risk of exacerbations but with likely a similar symptom burden.
Health status
Health status is often impaired in individuals with COPD and relates to impaired lung function, high psychological and physical symptom burden, low physical activity levels, frequent exacerbations and comorbidities.Citation44,Citation45 Health status may be improved in COPD through both pharmacological and nonpharmacological (eg, pulmonary rehabilitation) interventions. A simple, validated tool such as CAT can be used clinically to routinely assess health status;Citation46 whereas, other reliable and validated disease-specific health status questionnaires (e.g., St. George’s Respiratory Questionnaire) are frequently used in RCTs.Citation47
Recommendations and changes from last CTS COPD Guideline in 2019 with respect to health status (): There is change to the recommendation from the last CTS COPD guideline in 2019 that individuals with low symptom burden and health status impairment (CAT < 10), and only mildly impaired lung function (FEV1 ≥ 80% predicted), treatment is recommended to start with an inhaled long-acting bronchodilator (LABD) (rather than a short-acting bronchodilator [SABD]), with no significant difference between inhaled LAMA or LABA monotherapy (rec. PICO P.1.A.). In individuals with moderate to high symptoms (mMRC ≥ 2, CAT ≥ 10) and impaired lung function (FEV1 < 80% predicted, based on updated evidence, there is a change from 2019 with a strong recommendation, with LAMA/LABA dual therapy now being recommended as initial maintenance therapy (rec. PICO P.1.B.). Although there was no significant difference in health status seen in RCTs comparing LAMA/LABA dual therapy and ICS/LABA combination therapy, LAMA/LABA dual therapy is preferred due to greater improvements in lung function and lower rates of pneumonia, unless a patient has been diagnosed with concomitant asthma. We also extrapolated data from two large studiesCitation41,Citation42 performed in symptomatic individuals with COPD at high risk of future exacerbations, which add to previous evidence of a significant improvement in health status (whether looking at mean change from baseline or a responder analysis) with LAMA/LABA/ICS triple combination therapy compared to either LAMA/LABA dual therapy or ICS/LABA combination therapy, leading to a strong recommendation (rec. PICO P.1.C.)
Other recommendations with respect to dyspnea and health status (): In individuals with moderate to high health status impairment (CAT ≥ 10) and/or FEV1 < 80% predicted, based on updated evidence, there is a change from 2019, with a weak recommendation to continue LAMA/LABA/ICS triple combination therapy rather than stepping down to LAMA/LABA dual therapy (rec. PICO P.1.D.). Withdrawing ICS may result in worsening of health status and lung function. Stepping down may be considered in patients when there are concerns that the step-up may not have been justified in the first place or because of adverse effects. No studies of step-down have assessed the impact on dyspnea. Based on evidence, we do not suggest adding any of the oral medications to improve dyspnea, exercise tolerance, physical activity levels and/or health status (rec. PICO P.1.E.). Additionally, in all individuals with stable COPD and a low risk of exacerbations, we recommend against treatment with ICS monotherapy (rec. PICO P.1.F.).
Exercise tolerance and physical activity
Physical activity (PA) is any bodily movement that results in energy expenditure above the resting state and is associated with reduced health resource use and all-cause mortality in COPD; whereas exercise is a planned, structured, repetitive sub-set of PA.Citation48 Regular physical activity reduces hospital admission and mortality in COPD according to a population-based cohort study.Citation49 PA can be assessed with questionnaires or objectively through validated wearable devices (eg, step counter, accelerometer).Citation50 Exercise tolerance can be assessed using field tests (eg, 6-min walk test, shuttle walk test) or laboratory tests (eg, cardiopulmonary exercise test).Citation51 In patients with COPD, symptoms including dyspnea as well as limb (peripheral muscle) fatigue and discomfort limit exercise tolerance can improve with both pharmacological (eg, LABD) and nonpharmacological interventions (eg, pulmonary rehabilitation).Citation52 However, improvements in exercise tolerance may not result in maintained improvement in PA without a behavioral intervention.Citation53,Citation54
No new evidence assessing the impact of maintenance pharmacotherapy on exercise tolerance or physical activity in COPD was available since the last CTS update in 2019;13 therefore, there were no changes to recommendations from the last update specifically in relation to these two outcome measures.
PICO 2: Preventing AECOPD
For PICO 2, lists all the recommendations, their strength and certainty based on the evidence from meta-analysis (summary in Online Supplement Table 1), along with clinical remarks (where applicable). This section discusses the optimal use of inhaled and oral pharmacologic maintenance therapies shown to prevent AECOPD in individuals with stable COPD. Note that this document is not intended to guide the treatment of acute exacerbations.
Clinical remarks
In Canada, AECOPD is the most frequent cause of acute hospitalization in adults related to chronic conditions.Citation7 From a patient perspective, AECOPD contributes to a decline in lung function,Citation7 poor health statusCitation8 and increased susceptibility to repeated exacerbations. This results in increased morbidity and mortality associated with COPD,Citation12 and COPD accounts for more than 80% of total deaths from chronic respiratory disease globally.Citation5 A primary goal of the outpatient management of COPD is to prevent future AECOPD. A treatment approach that is proactive and prevents future AECOPD will improve health status, reduce healthcare utilization and reduce mortality.Citation41,Citation42
Patient values and preferences
We placed high value on the prevention of AECOPD as a treatment goal. Given the significance that AECOPD has on an individual (both short- and long-term outcomes) and the healthcare system, a pro-active approach to reduce exacerbation is necessary.
Review of evidence by outcomes
Moderate to severe exacerbations
COPD exacerbation is either symptom-based requiring a change of at least one major symptom (dyspnea, sputum purulence, sputum volume), or event-based requiring a change of at least one major symptom and use of antibiotics and/or systemic corticosteroids (moderate exacerbation) or event based requiring a change of hospital admission (severe exacerbation).Citation55 The best way of identifying subjects susceptible to exacerbations is through their exacerbation history, where frequent exacerbations predict risk of future events.Citation56 Exacerbations of COPD have profound impact on patients’ health status, functional capacity and lung function.Citation57,Citation58 The frequency and severity of exacerbations varies, and high risk exacerbations which has been used as operational definition in many large clinical trials has been defined as either frequent moderate exacerbations (exacerbations requiring antibiotic and/or prednisone treatment) or severe exacerbations (resulting in emergency department or hospital admission).Citation29 Severe exacerbations have a poor prognosis with increased mortality,Citation59 significantly impaired health status and increased risk of further exacerbations.Citation60,Citation61 Patients who have been admitted to hospital for a severe exacerbation of COPD are at substantial risk of rehospitalization.Citation9 Therefore, targeted interventions aimed at preventing or reducing the frequency and severity of exacerbations should be a priority for improving patients’ prognoses.
Recommendations and changes from last CTS COPD Guideline in 2019 with respect to moderate to severe exacerbations (): In stable individuals at a low risk of exacerbations and with moderate to high symptom burden, health status impairment (mMRC ≥ 2, CAT ≥ 10) and FEV1 < 80% predicted, based on updated evidence, there is a change from 2019, with a recommendation to start LAMA/LABA dual therapy as initial maintenance therapy (rec. PICO P.2.A.). This aligns with the recommendation P.1.B made in PICO 1. Furthermore, LAMA/LABA dual therapy is preferred to ICS/LABA combination therapy due to significant improvement in lung function and lower rates of adverse effects (eg, pneumonia). However, ICS/LABA combination therapy is preferred to LAMA/LABA dual therapy in individuals with both COPD and concomitant asthma.
Additionally, in stable individuals with COPD at a high risk of exacerbations, with a high symptom burden, health status impairment (mMRC ≥ 2, CAT ≥ 10) and FEV1 < 80% predicted, based on updated evidence as previously described, there is a change from 2019, with a strong recommendation to start LAMA/LABA/ICS triple combination therapy as initial maintenance therapy (rec. PICO P.2.B.). For symptomatic individuals with impaired health status meeting the definition of having a high AECOPD risk (see ), two large RCTs, IMPACTCitation42 and ETHOS,Citation41 demonstrated the benefits of triple inhaled LAMA/LABA/ICS (administered in a single inhaler) versus dual inhaled LAMA/LABA or ICS/LABA. In IMPACT, LAMA/LABA/ICS triple combination therapy was associated with a significantly lower annual rate of moderate or severe exacerbations during treatment than ICS/LABA combination therapy or LAMA/LABA dual therapy (0.91 vs 1.07 vs 1.21 exacerbations/year, respectively). LAMA/LABA/ICS triple combination therapy was also associated with a significantly lower risk of severe exacerbations compared to LAMA/LABA dual therapy (0.13 vs 0.19 severe exacerbations/year; rate ratio 0.66, 95%CI 0.56-0.78). In the ETHOS trial, the annual rate of moderate or severe exacerbations was 24% lower with 320 μg budesonide LAMA/LABA/ICS triple combination therapy compared with LAMA/LABA dual therapy, and 13% lower compared to ICS/LABA combination therapy. Similarly, the annual rate of moderate or severe exacerbation was significantly lower with 160 μg budesonide LAMA/LABA/ICS triple combination therapy compared with LAMA/LABA dual therapy and ICS/LABA combination therapy. No difference in annual rate of moderate or severe exacerbation (or time to first moderate or severe exacerbation) was observed between LAMA/LABA/ICS groups with differing ICS doses (rate ratio 1.00; 95%CI, 0.91-1.10).
Step down from LAMA/LABA/ICS triple combination therapy to dual combination therapies is not suggested (rec. PICO P.2.C.) for individuals at high risk of exacerbations. Withdrawing ICS, in addition to the possibility of lowering health status and lung function, can be associated with an increased risk of moderate-severe AECOPD; this could be more harmful in individuals with blood eosinophils counts ≥300 cells/µL. In COPD symptomatic individuals with impaired health status, at high risk of AECOPD, who continue to exacerbate despite being on LAMA/LABA/ICS triple combination therapy, we recommend the addition of macrolide maintenance therapy in appropriate patients who have normal QT interval on electrocardiograms (ECGs), no significant drug interactions with concomitant medications and no evidence of either indolent or active infection with atypical mycobacteriaCitation62 (rec. PICO P.2.D).
In individuals with COPD, with a chronic bronchitic phenotype at high risk of exacerbations, with a moderate to high symptom burden and/or health status impairment who continue to exacerbate despite being on LAMA/LABA/ICS triple combination therapy, we suggest the addition of either roflumilast or N-acetylcysteine (rec. PICO P.2.E.). We continue to recommend against the use of theophylline or systemic oral corticosteroids such as prednisone for maintenance treatment in COPD. There is no role for ICS monotherapy and ICS should only be used in combination with inhaled long-acting bronchodilators.Citation13 Administering ICS with LAMA/LABA in separate inhalers has not been well studied in COPD, but evidence to date demonstrates incremental benefit with single-inhaler triple therapy compared to multiple-inhaler triple therapy.Citation63
Other considerations: When combination ICS/LABA or triple LAMA/LABA/ICS is used, high doses of ICSCitation64 are not typically necessary to achieve optimum benefit in COPD, as shown by a relatively flat dose-response curveCitation65,Citation66 and greater incidence of adverse effects with higher inhaled ICS doses. In regard to moderate to severe exacerbations, the ETHOS study demonstrated no significant difference in exacerbation reduction between the moderate and low dose ICS, but did demonstrate a mortality benefitCitation41 favoring the moderate dose of inhaled ICS triple combination therapy. As noted, the incidence of pneumonia is higher with inhaled ICS-containing maintenance therapy, especially in individuals with severe and very severe disease. However, these are also the individuals who benefit most from ICS-containing regimens. It is also important to acknowledge that there are many other factors associated with increased risk of pneumonia in individuals with COPD.Citation67 The clinical significance of increased pneumonia in individuals with COPD who use ICS-containing inhaled maintenance therapy must be balanced against concurrent documented improvements in lung function, health status and a reduction in exacerbations. The number needed to treat (NNT) has been established at 4 patients for 1 year to prevent 1 moderate to severe exacerbation with Triple Therapy versus combined inhaled long-acting dual bronchodilator therapy, and the number needed to harm (NNH) at 33 patients for 1 year to cause 1 pneumonia,Citation68 thus highlighting the risk-benefit ratio. We also note that pneumonia has been recognized as a class effect of ICS-containing therapies in individuals with COPD, with no conclusive evidence of intra-class differences.
PICO 3: Reducing mortality
For PICO 3, lists all the recommendations, their strength and certainty based on the evidence from meta-analysis (summary in Online Supplement Table 1), along with clinical remarks (where applicable). This section presents the optimal use of pharmacologic maintenance therapies shown to reduce mortality in individuals with stable COPD.
Clinical remarks
Following a severe exacerbation of COPD, not only does the rate of subsequent exacerbations increase and time between exacerbations decrease, but there is an increased risk of mortality.Citation59 Mortality among patients discharged from hospital can be as high as 6.1% (5.8% in men and 6.8% in women) within 90 days of admission, 11.1% when mortality combines in-hospital plus a 90 day follow upCitation69 and an increased risk of death that persists to one year.Citation70 Moreover, the impact of an exacerbation goes beyond the lungs. An AECOPD also increases the risk of cardiovascular (CV) events, including acute coronary syndrome and stroke in the first 30 days and up to 1 year following the AECOPD.Citation71 CV events increase quickly, in the first 10 days following a moderate exacerbation,Citation72 and the risk persists for up to a year.Citation71 As the third leading cause of death, a critical COPD treatment goal should be to reduce the risk of COPD-related mortality.
Patient values and preferences
We placed high value on reducing mortality as a treatment goal.
Review of evidence by outcome
Mortality
Pharmacologic maintenance therapy comparing monotherapy (LABA, LAMA or ICS) to placebo, dual therapy (LAMA/LABA) to monotherapy (LABA or LAMA), combination therapy (ICS/LABA) to monotherapy (LABA or LAMA) have not shown a reduction in mortality (summary in Online Supplement Table 1). ICS/LABA combination therapy has shown decrease mortality compared to LAMA/LABA dual therapy; these results are mainly derived from two major RCTs, IMPACT and ETHOS.Citation41,Citation42 Some oral therapies have not shown a reduction in mortality (Vitamin D, roflumilast, mucolytic agents, theophyllines, statins) and for others there is a lack of data (N-acetylcysteine, macrolides) ().
Two large RCTs, IMPACT and ETHOS, have helped to inform the role of triple inhaled LAMA/LABA/ICS versus dual inhaled LAMA/LABA or ICS/LABA.Citation41,Citation42 Eligible patients could have a prior diagnosis of asthma, but not current asthma, and the RCTs were enriched for individuals with high symptom burden, impaired health status (score CAT ≥10) and exacerbations in the previous year (≥2 moderate exacerbations and/or ≥1 severe exacerbation requiring hospital admission). ETHOS and IMPACT assessed the risk of all-cause mortality as a prespecified secondary endpoint or other endpoint. In ETHOS, the risk ratio of all-cause mortality for triple inhaled LAMA/LABA/ICS with 320 μg budesonide (but not for 160 ug budesonide), vs LAMA/LABA dual therapy was 0.58 (95%CI, 0.34-0.99), and in IMPACT it was 0.64 (95%CI, 0.42-0.97). In ETHOS, the Hazard Ratio (HR) of all-cause mortality for triple inhaled LAMA/LABA/ICS 320 ug budesonide (but not for 160 ug budesonide) versus LAMA/LABA was 0.54 (95%CI, 0.34-0.87). Neither IMPACT nor ETHOS, demonstrated a difference when triple inhaled LAMA/LABA/ICS was compared to ICS/LABA combination therapy. In both studies, in a separate analysis, the robustness of the mortality findings was assessed after additional data retrieval for patients missing week 52 vital status in the original analyses.Citation73,Citation74 Vital status data were reported for over 99% of the intention-to-treat population. For triple inhaled LAMA/LABA/ICS, all-cause mortality was still statistically reduced versus LAMA/LABA dual therapy. Independent adjudication confirmed lower rates of respiratory and cardiovascular death. Results were similar when the first 30 days of treatment were excluded from the analysis.
Recommendations with respect to mortality (): In individuals at high risk of exacerbations, with a high symptom burden, health status impairment (mMRC ≥ 2, CAT ≥ 10) and FEV1 < 80% predicted, based on this new evidence, we make a strong recommendation for use of LAMA/LABA/ICS triple combination therapy over LABA/LAMA dual therapy (rec. PICO P.3.A.) and over ICS/LABA combination therapy (rec. PICO P.3.B.) to reduce mortality. The greater benefit of combination triple therapy over LABA/LAMA dual therapy and ICS/LABA combination therapy is not only of reducing mortality but also for improving dyspnea, health status, lung function and preventing moderate to severe AECOPD in this particular and well-defined population of patients.
Revision to the COPD pharmacotherapy
Recommended COPD pharmacotherapy promotes an evidence-informed approach that aligns proven effective treatment with symptom burden, risk of future exacerbations and mortality risk (). Since the 2019 CTS Guideline, there has been much clinical research that has informed this current guideline.
Figure 3. COPD Pharmacotherapy.This figure promotes an evidence-informed approach that aligns proven effective treatments with spirometry, symptom burden, risk of future exacerbations and mortality risk. Because of the clinical heterogeneity in COPD, spirometry should not be used in isolation to assess disease severity and this is why it is also important to perform a thorough clinical evaluation of the patient, including symptom burden and risk of exacerbations that permits the implementation of treatments that are specific for subpopulations. SABD prn (as needed) should accompany all recommended therapies across the spectrum of COPD.†Symptom burden encompasses shortness of breath, activity limitation, and impaired health status.††Individuals are considered at “Low Risk of AECOPD” if ≤1 moderate AECOPD in the last year (moderate AECOPD is an event with prescribed antibiotic and/or oral corticosteroids) and did not require hospital admission/ED visit. Individuals are considered at “High Risk of AECOPD” if ≥2 moderate AECOPD or ≥1 severe exacerbation in the last year (severe AECOPD is an event requiring hospitalization or ED visit).*LAMA/LABA single inhaled dual therapy is preferred over ICS/LABA inhaled combination therapy considering the additional improvements in lung function and the lower rates of adverse events such as pneumonia. ICS/LABA combination therapy should be used in individuals with concomitant asthma. There is no universally accepted definition of concomitant asthma. The 2017 CTS Position Statement on COPD Pharmacotherapy provides guidance on the assessment of patients who may have concomitant asthma.**Triple inhaled ICS/LAMA/LABA combination therapy should preferably be administered in a single inhaler triple therapy (SITT), and not in multiple inhalers (see text), although we acknowledge that some patients continue to prefer separate inhalers. +Oral pharmacotherapies in this group include prophylactic macrolide, and PDE-4 inhibitor and mucolytic agents for patients with chronic bronchitis.Abbreviations. CAT, COPD assessment test; mMRC, Modified Medical Research Council; SABD prn, short-acting bronchodilator as needed; AECOPD, acute exacerbation of COPD; ED, emergency department; LAMA, long-acting muscarinic antagonist; LABA, long-acting ẞ2-agonist; ICS, inhaled corticosteroid.
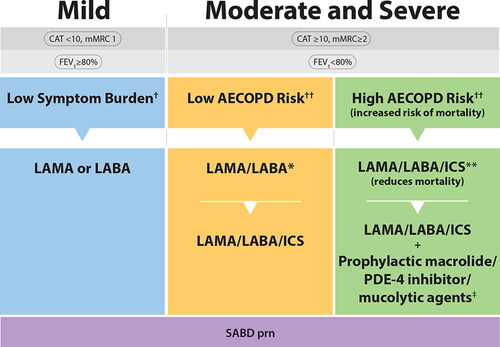
An important change since 2019 is the recommendation for use of long-acting inhaled bronchodilator maintenance therapy in all symptomatic individuals with COPD, including those with mild symptom burden, acknowledging that as-needed short-acting bronchodilator therapy should also be utilized by all individuals across the spectrum of COPD severity.
For individuals with moderate and severe disease with a low risk of future AECOPD, single inhaled LAMA/LABA dual therapy is now indicated given its proven superiority over inhaled LAMA or LABA monotherapy in this setting. In addition, individuals with moderate and severe disease who are at high risk of future AECOPD (definition unchanged from 2019 – ≥2 moderate AECOPD and/or ≥1 severe AECOPD in the past year) should be treated with single inhaled LAMA/LABA/ICS triple combination therapy because of proven superiority and benefits, including most importantly, significant reduction in mortality. Triple inhaled LAMA/LABA/ICS therapy, in a single inhaler triple therapy (SITT), is favored over multiple inhalers, because of potential increased benefits, increased adherence and reduced chance of errors in inhaler technique.Citation63,Citation75,Citation76
Discussion
This comprehensive guideline provides updated pharmacotherapeutic recommendations for COPD, based on newly published literature since the 2019 Guideline UpdateCitation13 on Pharmacotherapy in Individuals with Stable COPD. Our PICO-driven questions elicit evidence for the best pharmacological therapy to alleviate symptoms and to improve health status, to reduce exacerbations and its complications such as hospital admissions and to reduce mortality. The novelty of this guideline, it being the first to our knowledge to do this, is to report on the impact of maintenance pharmacotherapies on mortality in COPD. This guideline also proposes a revised and practical treatment pathway based on the recommendations. As per guideline standards, our approach is based on a careful systematic review and meta-analysis (Online Supplementary document), and takes into account the quality of the evidence and the multifaceted balance between the benefits and harms of treatment.
In this new guideline, we place a high value on the alleviation of symptoms, in particular dyspnea, which is the most debilitating symptom in COPD, and improvement of health status as treatment goals (PICO 1). Inhaled maintenance therapy with LABD is superior to short-acting bronchodilators in achieving these goals. An important change since 2019 is the recommendation for use of LABD maintenance therapy in all individuals who have persistent symptoms, even mild, with COPD. Also, in individuals with moderate to severe dyspnea, and similarly, in individuals with moderate to severe reduced health status, LAMA/LABA dual therapy is strongly recommended over LAMA or LABA monotherapy. However, we have not recommended prescribing LABD treatment in symptomatic persons who currently or formerly smoked cigarettes but have preserved lung function as assessed by spirometry (ie, patients who do not meet criteria for COPD). Although some have advocated for treatment in these individuals, it has been demonstrated that inhaled LABD therapy does not decrease respiratory symptoms in subjects not proven to have COPD by spirometry.Citation77
There are many effective therapies that prevent AECOPD and the choice of therapy should be determined based on the risk of future AECOPD (PICO 2). The goal of preventing AECOPD is to significantly minimize all the negative impacts of AECOPD such as symptom burden, health status worsening, hospital admission and mortality. It is recommended that individuals with moderate and severe disease who are at high risk of AECOPD be treated with SITT because of its many proven benefits, including the reduction of moderate and severe AECOPD and most importantly, significant reduction in mortality.
To date, demonstrating benefits on mortality in RCTs has been difficult, requiring large and selected populations at high risk of death (PICO 3). From an historical perspective, the TORCHCitation78 and SUMMIT trials,Citation79 both powered for the primary outcome of all-cause mortality, failed to show a statistically significant benefit on survival for ICS/LABA combination therapy compared with placebo. The inclusion criteria for TORCH were based on prebronchodilator FEV1 <60% predicted and no requirement for a history of previous exacerbations. The SUMMIT inclusion criteria were based on a history or risk of cardiovascular disease, moderate COPD with a post-bronchodilator FEV1 of 50–70% predicted (primarily COPD with moderate airflow obstruction or GOLD 2) and no prior history of exacerbations (75% had no exacerbations in the prior year). From IMPACT and ETHOS,Citation41,Citation42 it became evident that the TORCHCitation78 and SUMMITCitation79 trials did not include a population at a sufficiently high risk of death from COPD (ie, those with a history of frequent and/or severe AECOPD). These most recent RCTs were enriched for symptomatic patients (CAT ≥ 10) with a history of frequent (≥2 moderate exacerbations) and/or severe exacerbations (≥1 exacerbation requiring a hospital admission). Evidence of reduction in mortality has been strengthened from a postanalysis using the final retrieved dataset, which included Week 52 vital status for 99.6% of the intent-to-treat population risk of death.Citation73,Citation74 Furthermore, adjudicated causes of death suggest a potential role in reducing mortality that may not only be directly by reducing exacerbation but also cardiovascular outcomes.Citation74 Despite an increased risk of pneumonia with ICS use, the overall clinical benefit of a reduction in mortality outweighed the risk of pneumonia as an adverse event and/or serious adverse event. Furthermore, a meta-analysis of RCTs has demonstrated that mortality from pneumonia was not different with ICS containing regimens compared to non-ICS containing regimens [58/31396 vs 33/22544; relative risk 0.97 (95%CI 0.58-1.60; p = 0.89)].Citation80
While discussed, the use of biomarkers such as blood eosinophil count have not been reviewed in this guideline, which is based on systematic reviews of RCTs. Recommendations are limited to some clinical remarks since most of the data on blood eosinophil count are derived from observational studies or post-hoc analysis. However, there is consensus from experts that a subgroup of COPD patients at risk of exacerbations with blood eosinophils ≥300 cells/μL have a stronger likelihood of reduced exacerbations when patients are treated with ICS containing regimenCitation81–83 or increased exacerbations when ICS is withdrawn.Citation84,Citation85
This guideline has not considered factors that might influence the specific choice of inhaled medication beyond the molecule, side effects, and single-inhaler delivery route. Ensuring proper inhalation technique is one of the most important aspects of COPD care and studies have shown that errors in inhaler handling in this population can lead to increased emergency department (ED) admissions for AECOPD, hospitalizations, systemic corticosteroid requirements and antibiotic use.Citation86,Citation87 Also of importance, use of multiple devices with a similar inhalation technique has been shown to be associated with a lower rate of exacerbations and use of rescue medication compared to those who were prescribed multiple devices requiring different techniques.Citation88 Combination therapies for most of the studies in this guideline used single inhalers; similar efficacy cannot be extrapolated to achieving the same combinations with multiple-inhaler therapy. In the INTREPID study, a pragmatic RCT, SITT for COPD resulted in significantly more patients demonstrating improvements in health status and lung function compared to corresponding multiple-inhaler triple therapy.Citation89
The environmental impact and global warming potential associated with metered dose inhalers (MDIs) have been recognized for decades. Chlorofluorocarbons (CFCs) used originally as propellants in MDI devices were phased out under the Montreal Protocol in 1987 due to their ozone-depleting properties and replaced by two hydrofluoroalkane (HFA) propellants. Unfortunately, HFAs are now recognized as potent greenhouse gases with global warming impact. Not all MDIs have the same carbon footprint;Citation90,Citation91 dry-powder inhalers and emerging devices which use novel propellants constitute a lower carbon footprint option. This is particularly relevant for short-acting beta-agonist (SABA) inhalers, which constitute 71% of total inhaler use in Canada.Citation92 Selection of inhaler device should be a shared provider-patient decision informed by many factors including patient inhaler technique, preference, cost/insurance coverage and clinical course. The environmental impact of otherwise equivalent inhaler options should also be a relevant consideration.
Comparison to other recent guidelines
The 2023 CTS Guideline recommendations are consistent to other recent guidelines such as NICECitation93 and ATS,Citation94 and the GOLD report 2023Citation15 but based on the meta-analysis the Canadian guidelines are more progressive. It is well recognized in all guidelines that treatment should not only be based on lung function alone but taking into consideration other measures such as symptoms, health status and risk of exacerbations. All the recent guidelines have recognized the superiority of the LAMA/LABA dual therapy versus monotherapy in patients with high symptom burden, poor health status and low risk of future exacerbations. The 2023 CTS Guideline, ATS and GOLD all recommended starting with dual therapy in this patient population. In symptomatic patients with previous history of recurrent moderate or severe exacerbations, all recommended using single inhaler triple therapy (LAMA/LABA/ICS). CTS is more proactive recommending upfront triple therapy for these patients. The GOLD recommendations make the distinction for patients who have blood eosinophil count of <100 cells/µL not to be increased from LABA/LAMA dual therapy to triple therapy but to add oral therapies such as azithromycin or N-acetylcysteine; CTS recommended these oral medications in addition to triple therapy. CTS and GOLD, which rely on the most recent and larger clinical trials, do not recommend withdrawing ICS in patients with moderate-high symptom burden and high risk of exacerbations unless there are adverse effects of importance; it is also not recommended in COPD patients with blood eosinophils counts ≥300 cells/µL. All guidelines mention adverse effects, but prioritization should be given to the benefit of the outcomes over the risk of adverse events including pneumonia. Finally, all the guidelines recommend minimizing the number of inhalers and the number of different types of inhalers used by each patient as far as possible.
Conclusion
In conclusion, we present an evidence-based guideline with updated recommendations focused on 3 outcome areas: symptoms (dyspnea)/health status, exacerbations and mortality. We recommend: LABD maintenance therapy in all symptomatic patients with COPD confirmed by spirometry and single inhaler dual therapy LABD in those with moderate to severe dyspnea and/or poor health status, with a step up to single-inhaler triple therapy in those with persistent moderate to severe dyspnea and/or poor health status despite treatment with single inhaler dual therapy with LAMA/LABA or ICS/LABA. Given that SITT reduces mortality in individuals with moderate-severe disease and a high risk of AECOPD, we also suggest SITT in all patients at high risk of AECOPD. Our findings suggest the need to implement targeted case-finding strategies for patients to benefit from these therapeutic options. This 2023 CTS Guideline on the Pharmacotherapy Management of Stable COPD guides clinicians in implementing an exciting new paradigm in COPD management, wherein the goals of treatment include not only alleviating symptoms and preventing exacerbations, but also reducing mortality.
Author contributions
JB, MB, PH, DDM executive committee members who led the guideline and systematic review, wrote the manuscript, responded to the reviewers and made the final review; SDA, MFB, SBK, AU, FM, JDM, SM, EP, DS, JW, BLW committee members; AL methodologist; AVD guideline coordinator. Every author contributed in the review evidences from the meta-analysis, committee meetings and recommendations; every author reviewed the manuscript.
Author Note
This guideline was developed by the Canadian Thoracic Society and is jointly published by Taylor & Francis, LLC and Elsevier Inc in the Canadian Journal of Respiratory, Critical Care, and Sleep Medicine and the journal, CHEST, respectively. The articles are identical except for minor stylistic and spelling differences in keeping with each journal’s style. Either citation can be used when citing this article.
Editorial independence
The CTS COPD guideline panel is accountable to the CTS CRGC and the CTS Board of Directors. The CTS COPD guideline panel is functionally and editorially independent from any funding sources of the CTS and does not receive any direct funding from external sources. The CTS receives unrestricted grants that are combined into a central operating account to facilitate the knowledge translation activities of the CTS Assemblies and its guideline panels. No funders played a role in the collection, review, analysis or interpretation of the scientific literature or in any decisions regarding the key messages presented in this document.
Supplemental Material
Download Zip (3.3 MB)Acknowledgments
The authors would like to thank the CRGC Executive Members (Samir Gupta and Sanjay Mehta), and the CTS Executive Members (Richard Leigh, Donna Goodridge and Melinda Solomon) for their input and guidance. We would also like to acknowledge with sincere appreciation our expert reviewers who made valuable contributions to the manuscript: Gerard J. Criner, Chair and Professor, Thoracic Medicine and Surgery, Lewis Katz School of Medicine, Temple University, Philadelphia, Pennsylvania, USA; Miriam Barrecheguren, Department of Pneumology, Hospital Universitari Vall d’Hebron, Vall d’Hebron Institut de Recerca (VHIR), Barcelona, Spain; Jun Ueki, Clinical Research Unit of Respiratory Pathophysiology, Juntendo University Graduate School of Health Care and Nursing, Takasu, Urayasu City, Japan; Christine Jenkins, Head and professor of respiratory medicine, UNSW, Sydney, Australia; Barry Powers, Editor-in-Chief, Canadian Pharmacists Association, Ottawa, Ontario, Canada; Kevin Bussey, Clinical Editor, Canadian Pharmacists Association, Ottawa, Ontario, Canada; Alan Kaplan, Family Physicians Airways Group of Canada, Richmond Hill, Ontario, Canada; and Roger Goldstein, West Park Healthcare Centre, University of Toronto, Toronto, Ontario, Canada.
Disclosure statement
Members of the CTS COPD Guideline Panel declared potential conflicts of interest at the time of appointment, and these were updated throughout the process in accordance with the CTS Conflict of Interest Disclosure Policy.
J. Bourbeau reports grants from McGill University, the McGill University Health Centre Foundation, the Canadian Institute Health Research, Grifols, Novartis, Sanofi, and the Respiratory Health Network of the Fonds de la recherche en santé du Québec; grants and personal fees from Astra Zeneca Canada Ltd, Boehringer Ingelheim Canada Ltd, GlaxoSmithKline Canada Ltd, and Trudell Canada Ltd; and personal fees from Pfizer Canada Ltd, and COVIS Pharma Canada Ltd, outside the submitted work.
M. Bhutani reports personal fees and grants outside the submitted work from AstraZeneca Canada Ltd, Boehringer Ingelheim Canada Ltd, GlaxoSmithKline Canada Ltd, Novartis, Sanofi-Genzyme, the Canadian Institute Health Research, CHEST, The Lung Association of Alberta, The University of Alberta Hospital Foundation, Alberta Innovates Health Solutions, Valeo, and Covis.
P. Hernandez reports grants from the Canadian Institute Health Research, the Lung Association of Nova Scotia, the Nova Scotia Health Authority Research Fund, Astra Zeneca Canada Ltd, Boehringer Ingelheim Canada Ltd, Cyclomedica, Grifols, Respivant, and Vertex; and personal fees from Astra Zeneca Canada Ltd, Boehringer Ingelheim Canada Ltd, GlaxoSmithKline Canada Ltd, Janssen, Merck, Novartis, Sanofi-Aventis, and Trudell, outside the submitted work.
S. D. Aaron receives grants from CIHR. He has received speaking honoraria or has participated on advisory boards for GSK, AZ, Chiesi, and Sanofi.
M-F. Beauchesne reports grants and personal fees from the Cercle du doyen (Faculté de pharmacie, Université de Montréal) and Astra Zeneca Canada Ltd.
A. D’Urzo reports receiving research, consulting and lecturing fees from GlaxoSmithkline, Sepracor, Schering Plough, Altana, Methapharma, AstraZeneca, ONO pharma, Merck Canada, Forest Laboratories, Novartis Canada/USA, Boehringer Ingelheim (Canada) Ltd, Pfizer Canada, SkyePharma, and KOS Pharmaceuticals and Almirall, Sanofigenzyme and TEVA Canada, Valeopharma Canada.
F. Maltais reports grants from AstraZeneca and GlaxoSmithKline, Boehringer Ingelheim, GSK, Sanofi, and Novartis, and personal fees for serving on speaker bureaus and consultation panels from GlaxoSmithKline, Grifols, and Novartis. He is financially involved with Oxynov, a company which is developing an oxygen delivery system.
S. Mulpuru reports grants outside the submitted work from Canadian Institute of Health Research and Canadian Lung Association in partnership with Boehringer Ingelheim Canada Ltd and Astra Zeneca Canada Ltd.
E. Penz reports personal fees from COVIS Pharma, Sanofi Genzyme, Boehringer Ingelheim Canada Ltd, Astra Zeneca Canada Ltd, GlaxoSmithKline Canada Ltd, and Novartis; and grants from the Canadian Institute Health Research, the Saskatchewan Research Foundation, the Respiratory Research Centre, and Astra Zeneca Canada Ltd, outside the submitted work.
D. D. Sin reports personal fees from GlaxoSmithKline Canada Ltd, Boehringer Ingelheim, and AstraZeneca outside of the submitted work.
J. Wald reports personal fees from GlaxoSmithKline Canada Ltd and Astra Zeneca Canada Ltd and a grant from Fisher & Paykel, outside the submitted work.
B. L. Walker reports personal fees from AstraZeneca Canada Ltd, Boehringer Ingelheim Canada Ltd, GlaxoSmithKline Canada Ltd, Novartis, Sanofi-Genzyme and Covis Pharma Canada, outside of the submitted work.
D. D. Marciniuk reports consultancy work with Alberta Health Services, Health Canada, Lung Saskatchewan, Ontario Ministry of Health and Long-Term Care, Saskatchewan Health Authority, Saskatchewan Ministry of Health, Yukon Health and Social Services; reports grants (managed by University of Saskatchewan) from AstraZeneca, Boehringer Ingelheim, Canadian Institute of Health Research, GlaxoSmithKline, Grifols Therapeutics, Lung Saskatchewan, Novartis, Sanofi-Aventis, Saskatchewan Health Research Foundation, Syneos Health, Schering-Plough; employee/roles with University of Saskatchewan, Deputy Editor - CHEST Journal, Board Member - Saskatchewan Health Research Foundation; outside the submitted work.
None declared (A. Lal, A. Van Dam, S. B. Kermelly, and J. D. Marciniuk).
Additional information
Funding
References
- WHO. WHO Global Health Estimates, the Top 10 Causes of Death. Geneva: World Health Organization. https://www.who.int/data/global-health-estimates
- Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/s0140-6736(20)30925-9.
- Asthma and Chronic Obstructive Pulmonary Disease (COPD) in Canada, 2018. Report from the Canadian Chronic Disease Surveillance System. https://www.canada.ca/en/public-health/services/publications/diseases-conditions/asthma-chronic-obstructive-pulmonary-disease-canada-2018.html. Accessed March 21, 2023.
- GBD Chronic Respiratory Disease Collaborators. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2020;8(6):585–596. doi:10.1016/s2213-2600(20)30105-3.
- Levine S, Marciniuk DD. Global impact of respiratory disease - what can we do, together, to make a difference? Chest. 2022;161(5):1153-4. doi:10.1016/j.chest.2022.01.014.
- van Manen JG, Bindels PJ, Dekker FW, et al. The influence of COPD on health-related quality of life independent of the influence of comorbidity. J Clin Epidemiol. 2003;56(12):1177–1184. doi:10.1016/s0895-4356(03)00208-7.
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi:10.1136/thorax.57.10.847.
- Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi:10.1164/ajrccm.157.5.9709032.
- CIHI. Hospitalization rates for COPD across Canadian cities. https://www.cihi.ca/en/hospitalization-rates-for-copd-across-canadian-cities. 2023.
- Foo J, Landis SH, Maskell J, et al. Continuing to confront COPD international patient survey: economic impact of COPD in 12 countries. PLoS One. 2016;11(4):e0152618. doi:10.1371/journal.pone.0152618.
- Shah T, Press VG, Huisingh-Scheetz M, White SR. COPD Readmissions: addressing COPD in the Era of Value-based Health Care. Chest. 2016;150(4):916–926. doi:10.1016/j.chest.2016.05.002.
- Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527.
- Bourbeau J, Bhutani M, Hernandez P, et al. Canadian Thoracic Society Clinical Practice Guideline on pharmacotherapy in patients with COPD – 2019 update of evidence. Canadian Journal of Respiratory, Critical Care, and Sleep Medicine. 2019;3(4):210–232. doi:10.1080/24745332.2019.1668652.
- Bhatt SP, Balte PP, Schwartz JE, et al. Discriminative accuracy of FEV1:FVC thresholds for COPD-related hospitalization and mortality. JAMA. 2019;321(24):2438–2447. doi:10.1001/jama.2019.7233.
- Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Eur Respir J. 2017;49(3):1700214. doi:10.1183/13993003.00214-2017.
- Agustí A, Celli BR, Criner GJ, Halpin D, Anzueto A, Barnes P, Bourbeau J, Han MK, Martinez FJ, Montes de Oca M, Mortimer K, Papi A, Pavord I, Roche N, Salvi S, Sin DD, Singh D, Stockley R, López Varela MV, Wedzicha JA, Vogelmeier CF. Global Initiative for Chronic Obstructive Lung Disease 2023 Report: GOLD Executive Summary. Am J Respir Crit Care Med. 2023;207(7):819–837.
- Çolak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5(5):426–434. doi:10.1016/s2213-2600(17)30119-4.
- Gershon AS, Hwee J, Croxford R, Aaron SD, To T. Patient and physician factors associated with pulmonary function testing for COPD: a population study. Chest. 2014;145(2):272–281. doi:10.1378/chest.13-0790.
- CTS. 2021. Canadian Thoracic Society guideline development process and methodology. https://cts-sct.ca/guideline-library/methodology/
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. J Clin Epidemiol. Apr 2011;64(4):380–382. doi:10.1016/j.jclinepi.2010.09.011.
- Bourbeau J, Bhutani M, Hernandez P, et al. CTS position statement: pharmacotherapy in patients with COPD—an update. Can J Respir Crit Care Sleep Med. 2017;1(4):222–241. doi:10.1080/24745332.2017.1395588.
- GRADEPro GDT. McMaster University, 2015. Evidence Prime, Inc.,: https://www.evidenceprime.com/. Accessed February 10, 2023.
- Zhang Y, Morgan RL, Alonso-Coello P, et al. A systematic review of how patients value COPD outcomes. Eur Respir J. 2018;52(1):1800222. Published 2018 Jul 19. doi:10.1183/13993003.00222-2018.
- Ferreira DH, Kochovska S, Honson A, et al. Two faces of the same coin: a qualitative study of patients’ and carers’ coexistence with chronic breathlessness associated with chronic obstructive pulmonary disease (COPD). BMC Palliat Care. 2020;19(1):64. doi:10.1186/s12904-020-00572-7.
- Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928.
- Brouwers MC, Makarski J, Kastner M, Hayden L, Bhattacharyya O. The Guideline Implementability Decision Excellence Model (GUIDE-M): a mixed methods approach to create an international resource to advance the practice guideline field. Implement Sci. 2015;10:36. doi:10.1186/s13012-015-0225-1.
- Gupta S, Rai N, Bhattacharrya O, et al. Optimizing the language and format of guidelines to improve guideline uptake. CMAJ. 2016;188(14):E362–e368. doi:10.1503/cmaj.151102.
- Kastner M, Bhattacharyya O, Hayden L, et al. Guideline uptake is influenced by six implementability domains for creating and communicating guidelines: a realist review. J Clin Epidemiol. 2015;68(5):498–509. doi:10.1016/j.jclinepi.2014.12.013.
- GOLD. Global Initiative for Chronic Obstructive Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of COPD. 2023. GOLD website. https://goldcopd.org/
- Marvel J, Yu TC, Wood R, Higgins VS, Make BJ. Health status of patients with chronic obstructive pulmonary disease by symptom level. Chronic Obstr Pulm Dis. 2016;3(3):643–652. doi:10.15326/jcopdf.3.3.2015.0177.
- Agusti A, Calverley PM, Celli B, et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010;11(1):122. doi:10.1186/1465-9921-11-122.
- Hernandez P, Balter M, Bourbeau J, Hodder R. Living with chronic obstructive pulmonary disease: a survey of patients’ knowledge and attitudes. Respir Med. 2009;103(7):1004–1012. doi:10.1016/j.rmed.2009.01.018.
- Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. doi:10.1136/thx.54.7.581.
- Lewthwaite H, Jensen D, Ekström M. How to assess breathlessness in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2021;16:1581–1598. doi:10.2147/copd.S277523.
- Maltais F, Bjermer L, Kerwin EM, et al. Efficacy of umeclidinium/vilanterol versus umeclidinium and salmeterol monotherapies in symptomatic patients with COPD not receiving inhaled corticosteroids: the EMAX randomised trial. Respir Res. 2019;20(1):238. doi:10.1186/s12931-019-1193-9.
- Wedzicha JA, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209. doi:10.1016/s2213-2600(13)70052-3.
- Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–922. doi:10.2147/copd.S130482.
- Calzetta L, Rogliani P, Matera MG, Cazzola M. A systematic review with meta-analysis of dual bronchodilation with LAMA/LABA for the treatment of stable COPD. Chest. 2016;149(5):1181–1196. doi:10.1016/j.chest.2016.02.646.
- Oba Y, Sarva ST, Dias S. Efficacy and safety of long-acting β-agonist/long-acting muscarinic antagonist combinations in COPD: a network meta-analysis. Thorax. 2016;71(1):15–25. doi:10.1136/thoraxjnl-2014-206732.
- Wedzicha JA, Calverley PM, Seemungal TA, Hagan G, Ansari Z, Stockley RA. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26. doi:10.1164/rccm.200707-973OC.
- Rabe KF, Martinez FJ, Ferguson GT, et al. Triple inhaled therapy at two glucocorticoid doses in moderate-to-very-severe COPD. N Engl J Med. 2020;383(1):35–48. doi:10.1056/NEJMoa1916046.
- Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMoa1713901.
- Tabberer M, Jones CE, Kilbride S, et al. Single-inhaler triple therapy and health-related quality of life in COPD: the IMPACT study. Adv Ther. 2020;37(9):3775–3790. doi:10.1007/s12325-020-01409-8.
- Janson C, Marks G, Buist S, et al. The impact of COPD on health status: findings from the BOLD study. Eur Respir J. 2013;42(6):1472–1483. doi:10.1183/09031936.00153712.
- Wilke S, Jones PW, Müllerova H, et al. One-year change in health status and subsequent outcomes in COPD. Thorax. 2015;70(5):420–425. doi:10.1136/thoraxjnl-2014-205697.
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J. 2009;34(3):648–654. doi:10.1183/09031936.00102509.
- Jones PW, Quirk FH, Baveystock CM. The St George’s respiratory questionnaire. Respir Med. 1991;85 Suppl B:25–31. doi:10.1016/s0954-6111(06)80166-6.
- ACSM ACoSM. ACSM’s Guidelines for Exercise Testing and Prescription. Lippincott Williams & Wilkins; 2000.
- Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61(9):772–778. doi:10.1136/thx.2006.060145.
- Pitta F, Troosters T, Probst VS, Spruit MA, Decramer M, Gosselink R. Quantifying physical activity in daily life with questionnaires and motion sensors in COPD. Eur Respir J. 2006;27(5):1040–1055. doi:10.1183/09031936.06.00064105.
- Puente-Maestu L, Palange P, Casaburi R, et al. Use of exercise testing in the evaluation of interventional efficacy: an official ERS statement. Eur Respir J. 2016;47(2):429–460. doi:10.1183/13993003.00745-2015.
- Casaburi R, Kukafka D, Cooper CB, Witek TJ, Jr., Kesten S. Improvement in exercise tolerance with the combination of tiotropium and pulmonary rehabilitation in patients with COPD. Chest. 2005;127(3):809–817. doi:10.1378/chest.127.3.809.
- Bourbeau J, Sedeno M, Li PZ, et al. Mechanisms associated with increased physical activity in patients undergoing self-management behaviour modification in the randomised PHYSACTO trial. ERJ Open Res. 2021;7(1):00533. doi:10.1183/23120541.00533-2020.
- Troosters T, Maltais F, Leidy N, Lavoie KL, Sedeno M, Janssens W, Garcia-Aymerich J, Erzen D, De Sousa D, Korducki L, Hamilton A, Bourbeau J. Effect of bronchodilation and exercise training with behavior modification on exercise tolerance and downstream effects on symptoms and physical activity in COPD. Am J Respir Crit Care Med. 2018;198(8):1021–1032.
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa0909883.
- Celli BR, Fabbri LM, Aaron SD, et al. An updated definition and severity classification of chronic obstructive pulmonary disease exacerbations: the Rome proposal. Am J Respir Crit Care Med. 2021;204(11):1251–1258. doi:10.1164/rccm.202108-1819PP.
- Anzueto A, Leimer I, Kesten S. Impact of frequency of COPD exacerbations on pulmonary function, health status and clinical outcomes. Int J Chron Obstruct Pulmon Dis. 2009;4:245–251. doi:10.2147/copd.s4862.
- Donaldson GC, Wedzicha JA. COPD exacerbations.1: epidemiology. Thorax. 2006;61(2):164–168. doi:10.1136/thx.2005.041806.
- Suissa S, Dell’Aniello S, Ernst P. Long-term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012;67(11):957–963. doi:10.1136/thoraxjnl-2011-201518.
- Esteban C, Quintana JM, Moraza J, et al. Impact of hospitalisations for exacerbations of COPD on health-related quality of life. Respir Med. 2009;103(8):1201–1208. doi:10.1016/j.rmed.2009.02.002.
- Müllerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. doi:10.1378/chest.14-0655.
- Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. doi:10.1056/NEJMoa1104623.
- Alcázar-Navarrete B, Jamart L, Sánchez-Covisa J, Juárez M, Graefenhain R, Sicras-Mainar A. Clinical characteristics, treatment persistence, and outcomes among patients with COPD treated with single- or multiple-inhaler triple therapy: a retrospective analysis in Spain. Chest. 2022;162(5):1017–1029. doi:10.1016/j.chest.2022.06.033.
- O’Donnell DE, Aaron S, Bourbeau J, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease - 2007 update. Can Respir J. 2007;14 Suppl B(Suppl B):5B–32b. doi:10.1155/2007/830570.
- Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–223. doi:10.1016/s2213-2600(13)70040-7.
- Izquierdo JL, Cosio BG. The dose of inhaled corticosteroids in patients with COPD: when less is better. Int J Chron Obstruct Pulmon Dis. 2018;13:3539–3547. doi:10.2147/copd.S175047.
- Williams NP, Coombs NA, Johnson MJ, et al. Seasonality, risk factors and burden of community-acquired pneumonia in COPD patients: a population database study using linked health care records. Int J Chron Obstruct Pulmon Dis. 2017;12:313–322. doi:10.2147/copd.S121389.
- Dransfield MT, Crim C, Criner GJ, et al. Risk of exacerbation and pneumonia with single-inhaler triple versus dual therapy in IMPACT. Ann Am Thorac Soc. 2021;18(5):788–798. doi:10.1513/AnnalsATS.202002-096OC.
- Crinion S, Cotter O, Kennedy B, et al. COPD exacerbations – a comparison of Irish data with European data from the ERS COPD Audit. Ir Med J. 2013;106(9):268, 270–272.
- Rothnie KJ, Müllerová H, Smeeth L, Quint JK. Natural history of chronic obstructive pulmonary disease exacerbations in a general practice-based population with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(4):464–471. doi:10.1164/rccm.201710-2029OC.
- Kunisaki KM, Dransfield MT, Anderson JA, et al. Exacerbations of chronic obstructive pulmonary disease and cardiac events. A post hoc cohort analysis from the SUMMIT randomized clinical trial. Am J Respir Crit Care Med. 2018;198(1):51–57. doi:10.1164/rccm.201711-2239OC.
- Donaldson GC, Hurst JR, Smith CJ, Hubbard RB, Wedzicha JA. Increased risk of myocardial infarction and stroke following exacerbation of COPD. Chest. 2010;137(5):1091–1097. doi:10.1378/chest.09-2029.
- Lipson DA, Crim C, Criner GJ, et al. Reduction in all-cause mortality with fluticasone furoate/umeclidinium/vilanterol in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;201(12):1508–1516. doi:10.1164/rccm.201911-2207OC.
- Martinez FJ, Rabe KF, Ferguson GT, et al. Reduced all-cause mortality in the ETHOS trial of budesonide/glycopyrrolate/formoterol for chronic obstructive pulmonary disease. A randomized, double-blind, multicenter, parallel-group study. Am J Respir Crit Care Med. 2021;203(5):553–564. doi:10.1164/rccm.202006-2618OC.
- Halpin DMG, Rothnie KJ, Banks V, et al. Comparative adherence and persistence of single- and multiple-inhaler triple therapies among patients with chronic obstructive pulmonary disease in an english real-world primary care setting. Int J Chron Obstruct Pulmon Dis. 2022;17:2417–2429. doi:10.2147/copd.S370540.
- Miravitlles M, Marín A, Huerta A, Carcedo D, Villacampa A, Puig-Junoy J. Estimation of the clinical and economic impact of an improvement in adherence based on the use of once-daily single-inhaler triple therapy in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2020;15:1643–1654. doi:10.2147/copd.S253567.
- Han MK, Ye W, Wang D, et al. Bronchodilators in tobacco-exposed persons with symptoms and preserved lung function. N Engl J Med. 2022;387(13):1173–1184. doi:10.1056/NEJMoa2204752.
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789.
- Vestbo J, Anderson JA, Brook RD, et al. Fluticasone furoate and vilanterol and survival in chronic obstructive pulmonary disease with heightened cardiovascular risk (SUMMIT): a double-blind randomised controlled trial. Lancet. 2016;387(10030):1817–1826. doi:10.1016/s0140-6736(16)30069-1.
- Almagro P, Martinez-Camblor P, Soriano JB. Inhaled corticosteroids and pneumonia mortality in COPD patients. Eur Respir J. 2019;54(3):1901035. doi:10.1183/13993003.01035-2019.
- Singh D, Agusti A, Martinez FJ, et al. Blood eosinophils and chronic obstructive pulmonary disease: a global initiative for chronic obstructive lung disease science committee 2022 review. Am J Respir Crit Care Med. 2022;206(1):17–24. doi:10.1164/rccm.202201-0209PP.
- Suissa S, Dell’Aniello S, Ernst P. Comparative effectiveness of LABA-ICS versus LAMA as initial treatment in COPD targeted by blood eosinophils: a population-based cohort study. Lancet Respir Med. 2018;6(11):855–862. doi:10.1016/S2213-2600(18)30368-0.
- Suissa S, Dell’Aniello S, Ernst P. Single-inhaler triple versus dual bronchodilator therapy in COPD: real-world comparative effectiveness and safety. Int J Chron Obstruct Pulmon Dis. 2022;17:1975–1986. Published 2022 Aug 30. doi:10.2147/COPD.S378486.
- Watz H, Tetzlaff K, Wouters EF, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4(5):390–398. doi:10.1016/S2213-2600(16)00100-4.
- Chapman KR, Hurst JR, Frent SM, et al. Long-term triple therapy de-escalation to indacaterol/glycopyrronium in patients with chronic obstructive pulmonary disease (SUNSET): a randomized, double-blind, triple-dummy clinical trial. Am J Respir Crit Care Med. 2018;198(3):329–339. doi:10.1164/rccm.201803-0405OC.
- Melani AS, Bonavia M, Cilenti V, et al. Inhaler mishandling remains common in real life and is associated with reduced disease control. Respir Med. 2011;105(6):930–938. doi:10.1016/j.rmed.2011.01.005.
- Molimard M, Raherison C, Lignot S, et al. Chronic obstructive pulmonary disease exacerbation and inhaler device handling: real-life assessment of 2935 patients. Eur Respir J. 2017;49(2):1601794. doi:10.1183/13993003.01794-2016.
- Bosnic-Anticevich S, Chrystyn H, Costello RW, et al. The use of multiple respiratory inhalers requiring different inhalation techniques has an adverse effect on COPD outcomes. Int J Chron Obstruct Pulmon Dis. 2017;12:59–71. doi:10.2147/copd.S117196.
- Halpin DMG, Worsley S, Ismaila AS, et al. INTREPID: single- versus multiple-inhaler triple therapy for COPD in usual clinical practice. ERJ Open Res. 2021;7(2):00950-2020. doi:10.1183/23120541.00950-2020.
- Fulford R, Mezzi K, Aumônier S, Finkbeiner M. Carbon footprints and life cycle assessments of inhalers: a review of published evidence. Sustainability. 2022;14(12):7106. doi:10.3390/su14127106.
- Stocker TF, Qin GK, Plattner M, et al. Intergovernmental Panel on Climate Change Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2013.
- Janson C, Maslova E, Wilkinson A, et al. The carbon footprint of respiratory treatments in Europe and Canada: an observational study from the CARBON programme. Eur Respir J. 2022;60(2):2102760. doi:10.1183/13993003.02760-2021.
- Chronic Obstructive Pulmonary Disease in Over 16s: Diagnosis and Management. London: National Institute for Health and Care Excellence (NICE); 2019.
- Nici L, Mammen MJ, Charbek E, et al. Pharmacologic management of chronic obstructive pulmonary disease. An official American thoracic society clinical practice guideline. Am J Respir Crit Care Med. 2020;201(9):e56–e69. doi:10.1164/rccm.202003-0625ST.