Abstract
A significant number of patients with asthma have poor control on their current inhaled therapies, typically a combination of inhaled corticosteroids (ICS) and long-acting beta-2 adrenergic bronchodilators (LABA). Adding a long-acting antimuscarinic agent (LAMA) has been shown to improve asthma control and the availability of triple therapy formulations (ICS/LABA/LAMA) in a single inhaler device or single inhaler triple therapy (SITT) mitigates the adherence concerns associated with use of multiple inhaler devices. Here, we provide an overview of the pivotal data concerning the use of triple asthma therapy in patients with poor control on ICS-LABA treatment, and present our expert approach to their application in the routine clinical management of such patients as well the appropriate sequencing of initiating triple therapy and seeking a referral for consideration of more advanced therapies.
RÉSUMÉ
Un nombre important de patients asthmatiques ont un faible contrôle sur leurs traitements inhalés actuels, généralement une combinaison de corticostéroïdes inhalés (CSI) et de bronchodilatateurs bêta2 adrénergiques à action prolongée (LABA). Il a été démontré que l'ajout d'un agent antimuscarinique à action prolongée (LAMA) améliore la maitrise de l'asthme et que la disponibilité de formulations de trithérapie (ICS/LABA/LAMA) dans un seul inhalateur ou une trithérapie à inhalateur unique (SITT) atténue les problèmes d'observance associés à l'utilisation de plusieurs inhalateurs. Nous donnons ici un aperçu des données pivots concernant l'utilisation de la trithérapie de l'asthme chez les patients ayant un faible contrôle sur le traitement ICS-LABA, et présentons notre vision d’experts concernant leur application dans la prise en charge clinique de routine de ces patients ainsi que le séquençage approprié de l'initiation de la trithérapie et de la recherche d'une référence pour envisager des thérapies plus avancées.
Introduction
For the more than 3.8 million Canadians who live with asthma, achieving and maintaining well-controlled asthma, as defined in the Canadian Thoracic Society (CTS) guideline, is a critical therapeutic goal.Citation1,Citation2 Unfortunately, poorly controlled asthma () is commonplace despite the availability of highly effective inhaled medications. In a 2007 survey of Canadians with asthma, over half reported symptoms of uncontrolled asthma and almost all reported at least one exacerbation in the previous year.Citation3 Of the 494 patients enrolled in the Canadian Economic Burden of Asthma (EBA) study, only 69 (14%) were considered to have controlled asthma.Citation4
Table 1. Characteristics of poor asthma control.a
For some, poor control may be a minor, if disruptive, day-to-day occurrence; however, uncontrolled asthma is associated with excess mortality, a greater risk for severe exacerbations (which is in itself a risk factor for increased mortality), a higher likelihood of developing anxiety and depression, and poor sleep quality.Citation2,Citation4–6 Use of both in-patient and Emergency Room services are higher for Canadians with uncontrolled asthma compared to age-matched controls;Citation7 unsurprisingly, uncontrolled asthma impairs quality-of-life (QoL), and is associated with increases in both direct and indirect costs associated with absenteeism, presenteeism and health care delivery.Citation1,Citation4,Citation8,Citation9 The total direct and indirect costs associated with suboptimal asthma control are projected to exceed $1.3 billion and $14 billion, respectively, by 2033.Citation9
Inhaled corticosteroids (ICS) remain the foundation of asthma treatment.Citation2,Citation10 However, a dose-response plateau is observed in most patients at a daily dose equivalent to 200-250 µg of fluticasone propionate (FP). The addition of a second agent, typically a long-acting beta-2 agonist (LABA), improves their effectiveness.Citation2,Citation11,Citation12 For patients who continue to experience poor control, potential add-on therapies to ICS/LABA include long-acting antimuscarinic agents (LAMAs, also known as anticholinergic agents), macrolides and leukotriene receptor antagonists, as well as the biologics anti-interleukin (IL)-4, anti-IL-5/5 receptor, anti-immunoglobulin (Ig)E and anti-thymic stromal lymphopoietin (TSLP). Oral corticosteroids (OCS), although effective, should be reserved as urgent therapy. The adverse consequences of systemic corticosteroids are cumulative and their use should prompt referral for specialist care.Citation2,Citation13 This article reviews the role of ICS-LABA-LAMA triple therapy in asthma by providing an overview of the pivotal data, supplemented by the clinical perspectives of the authors regarding their application in routine clinical practice.
First steps
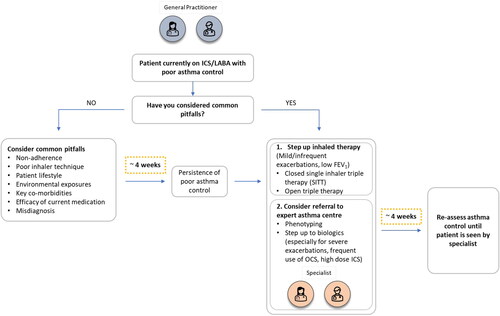
When faced with a patient with poor asthma control or exacerbations despite being prescribed an ICS/LABA, before stepping up therapy physicians should consider some common pitfalls that may compromise the effectiveness of treatment (). These include, but are not limited to: the wrong diagnosis, poor adherence to effective medication prescribed but not taken, poor inhaler technique, exposure to important allergens or workplace sensitizers and untreated co-morbidities.Citation2
Figure 1. Schematic to assist decisions regarding whether to initiate triple inhaled therapy in patients with poor asthma control.
Misdiagnosis
When Canadians with physician-diagnosed asthma were objectively assessed, asthma as a diagnosis was excluded in approximately 30% of them.Citation14 Patients may not be responding to anti-asthma therapy because asthma is not the source of their symptoms. Conditions most commonly misdiagnosed as asthma are chronic obstructive pulmonary disease (COPD) and vocal cord dysfunction.Citation15
Adherence
Over 70% of Canadian adults with asthma are poorly-adherent, the highest proportion of any therapy class,Citation16 and nonadherence is recognized as one of the main reasons for suboptimal asthma management and poor clinical outcomes.Citation17 Symptom driven (as opposed to preventative) management strategies in asthma may contribute to both unintentional and intentional nonadherence;Citation18 however, financial barriers for patients with asthma who do not have drug benefit insurance and those who experience lower socio-economic status are also significant factors for nonadherence.Citation18 According to a recent government report, one in five Canadians struggle to pay for their prescription medicines and three million do not fill their prescriptions because they cannot afford to.Citation19
Inhaler technique
Suboptimal inhaler technique is seen in up to 70% of patients and is associated with poor asthma control and increased exacerbations as well as increased risk for hospitalization and ER visits.Citation2,Citation5 Co-prescribing of pressurized metered dose inhalers and dry powder inhalers has been shown to adversely affect the patient’s ability to use either type of inhaler optimally with resultant impacts on clinical outcomes.Citation18,Citation20
Environmental exposures
Factors that trigger asthma should be identified and avoided, if possible. These include smoking and vaping, both first- and secondhand, indoor allergens such as dust mites or pet dander, and medications that increase the risk for bronchospasm (e.g., NSAIDs, beta-blockers).Citation2 As about one third of adult-onset asthma cases may have a work-related component, it is important to perform a thorough medical and occupational history to identify any such triggers.Citation2
Key co-morbidities
Co-morbidities such as chronic rhinosinusitis with nasal polyps, allergic rhinitis, obesity, gastro-esophageal reflux, paradoxical vocal fold motion, anxiety and depression can contribute to or mimic the burden of lower respiratory symptoms. Good asthma care includes screening for and managing such conditions.Citation2
If any of the aforementioned issues are identified, the authors strongly recommend a re-assessment of asthma control at around four weeks following intervention. In cases of exacerbation, a longer window prior to re-assessment may be warranted.
When two becomes three
The 2021 Global Initiative for Asthma (GINA) recommends adding a LAMA in patients aged ≥18 years who, despite being adherent to inhaled LABA combined with medium-or high-dose ICS, still experience symptoms or exacerbations. The 2021 guidelines of the CTS advocate tiotropium as a step-up therapy ().Citation2,Citation13
Antimuscarinic agents ensure bronchodilation by blocking acetylcholine signaling through airway muscarinic receptors, a separate and complementary mechanism of action from LABAs which act via β2 adrenoreceptors.Citation21 Inhaled antimuscarinic agents were the first effective inhaled agents used in Western medicine;Citation22 however, the introduction of the more effective and better tolerated beta-adrenergic agents decreased their use in asthma. However, anticholinergic agents have added bronchodilator benefit when added to adrenergic agents, a property that once saw the commonplace use of short-acting antimuscarinic agents in ambulatory asthma care and which persists in the setting of acute severe asthma in the emergency room.Citation23,Citation24
In their long-acting formulations, inhaled antimuscarinic agents have been widely regarded as the agents of choice for patients with COPD.Citation25 Recent randomized clinical trials have highlighted their potential as a component of triple therapy to achieve control in people with asthma. In patients with uncontrolled asthma despite treatment with high-dose ICS plus LABA, the addition of a LAMA improved lung function, reduced the risk for exacerbations and the need for oral corticosteroid use, with no discernible impact on safety.Citation26–28 In the opinion of the authors, any patient who has poor asthma control despite appropriate use of an ICS/LABA should be considered for step up to triple inhaled therapy and referral to a specialist center for an additional assessment.
The addition of a LAMA to an ICS/LABA combination may be achieved via a separate inhaler (as is the case with tiotropium: an open triple therapy combination),Citation29 or through the use of a closed single inhaler triple therapy (SITT) combination of ICS/LABA/LAMA. Currently, two formulations of triple therapy in a single inhaler are available in Canada ().Citation30 Both are fixed dose inhalers, to be taken once daily.Citation31,Citation32 A fixed dose combination of ICS/LABA/LAMA containing beclomethasone dipropionate formoterol fumarate and glycopyrronium bromide (Chiesi Group) has been approved by the European Medicines Agency but is not currently licensed in Canada.Citation33
Table 2. Potential inhaled-onlyTable Footnotea step-up therapy for Canadians with poor asthma control treated with ICS/LABA combination.
Open triple therapy combination
Tiotropium (Boehringer Ingelheim Pharmaceuticals, Inc.) is indicated as add-on maintenance bronchodilator treatment in adults with uncontrolled asthma who are already taking ICS/LABA combination therapy, and was the first LAMA approved for treatment of asthma in Canada.Citation29,Citation36 Tiotropium is delivered as a soft mist using the Respimat® inhaler as two inhalations of 2.5 µg, taken once daily. Other LAMAs are approved for use in Canada for the management of COPD, and while not approved for the treatment of asthma, the authors recognize that factors and patient’s preference may make tiotropium a less preferred choice when opting for open triple therapy. As such, other LAMAs may be used in replacement, as the benefits are likely to be a class effect.
In the PrimoTinA-asthma 1 and 2 trials (NCT00772538 and NCT00776984), the addition of tiotropium to existing ICS/LABA therapy for 48 weeks in patients with uncontrolled asthma significantly improved FEV1 at 24 weeks compared with placebo (by a difference of 86 ± 34 mL in the first and by 154 ± 32 mL in the second trial), increased the time to the first severe exacerbation (time to first quartile had a severe exacerbation: 282 days for the tiotropium group vs. 226 days for the placebo group) and reduced the risk for a severe exacerbation by 21%. Adverse events were similar between the groups.Citation28
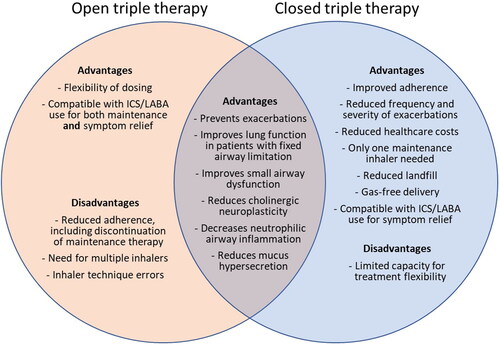
Challenges with open triple therapy include treatment adherence and persistence: The evidence supporting greater adherence with a single inhaler compared with multiple inhalers in asthma is compelling and translates into reduced healthcare resource costs.Citation30,Citation37 Adherence is also typically higher for once-daily dosing, when compared with twice-daily dosing.Citation38 One U.S.-based real-world analysis reported that within 6 months of initiating triple therapy with tiotropium, about half of patients had discontinued ICS/LABA therapy and used only their LAMA bronchodilator therapy (a behavior associated with worsening asthma control and increased risk for mortality).Citation39 In patients using long-term maintenance medication for obstructive lung disease, inhaler technique errors were less common when patients used one inhaler device as compared with two different inhalation devices.Citation40 Given the availability of two closed SITTs in Canada, the authors recommend that the benefits and risks associated with open triple therapy requiring at least two separate scheduled inhalers should be considered carefully. The authors note that it is still possible to use an ICS/LABA inhaler for symptom relief (referred to as MART therapy) and, in combination with a LAMA, as maintenance therapy (although evidence to support this strategy remains lacking).Citation13 Other combinations of inhalers (for example, LAMA/LABA + ICS or LAMA + LABA + ICS) could also be used for open triple therapy, if the benefit outweighed the established risks of nonadherence, poor inhaler technique and environmental impact.
Single inhaler triple therapies in people with uncontrolled asthma
The two closed SITTs available in Canada are: mometasone (MF)/indacaterol (IND)/glycopyrronium (GLY) (Valeo Pharma Inc., Novartis Pharmaceuticals Canada Inc.), in high-dose ICS formulation with the Breezhaler® dry powder device;Citation31 and fluticasone (FF)/vilanterol (VI) umeclidinium (UMEC) (GlaxoSmithKline, Inc.) with the Ellipta® dry powder device in either a medium-dose or high-dose ICS combination ().Citation32
The IRIDIUM pivotal randomized controlled trial (NCT02571777) compared the effects of once-daily closed SITT (medium- or high-dose MF/IND/GLY) with either once-daily ICS-LABA (medium- or high-dose MF/IND), all administered via the Breezhaler®, or twice-daily high-dose fluticasone 500 µg/salmeterol 50 µg [FP/SAL]) administered via the Diskus® device, for 52 weeks in patients with uncontrolled asthma (Patient demographics and baseline characteristics are presented in ).Citation41 Of note, only the high-dose version of MF/IND/GLY is commercially available in Canada. Both the medium- and high-dose SITT improved lung function more effectively than MF/IND (Difference in FEV1 at 26 week of treatment: 76 mL and 65 mL for medium- and high-dose, respectively) or the twice-daily FP/SAL (99 mL and 119 mL, respectively). Of even greater importance, the high-dose SITT reduced the rate of exacerbations by 21% compared with the corresponding dose of once daily ICS/LABA (MF/IND) and by 40% compared with the high-dose twice-daily FP/SAL. The incidence of adverse events was similar across treatment groups.
Table 3. Patient demographics and baseline characteristics for pivotal trials.
Closed and open triple therapies were compared in the ARGON trial (NCT03158311). This noninferiority trial compared the same medium- or high-dose once-daily closed SITT (MF/IND/GLY) with the open combination of twice-daily high-dose FP/SAL (administered via the Diskus®) plus once-daily tiotropium (administered via the Respimat® inhaler), in patients with uncontrolled asthma.Citation42 High-dose MF/IND/GLY achieved greater improvements in lung function and health status than the open combination of high-dose FP/SAL plus tiotropium (Mean treatment difference in: FEV1, 96 mL; St George’s Respiratory Questionnaire, −2.0). Rates of exacerbations, and adverse events, were similar across treatment groups.Citation42
The development trials for the FF/UMEC/VI formulations were more complex and involved the testing of not only two doses of the ICS moiety but also two doses of the anticholinergic. The pivotal CAPTAIN trial (NCT02924688) compared the effects of once-daily medium- or high-dose FF/VI with 4 different dose combinations of FF/UMEC/VI (combinations of medium- or high-dose FF and medium- or high-dose UMEC), administered for between 24 and 52 weeks to patients with uncontrolled asthma.Citation43 At Week 24 medium- and high-dose FF/high-dose UMEC/VI were associated with greater improvements in FEV1 than the corresponding doses of FF/VI (Mean improvement in FEV1: medium dose, 110 mL; high-dose, 92 mL). However, in the CAPTAIN trial, the new closed once-daily SITT did not significantly reduce the annualized rate of moderate or severe exacerbations. Improvements in QoL and incidence of adverse events were similar across treatment groups.
Additional considerations
This article has focused on triple therapy as an ICS/LABA/LAMA combination. Although alternative combinations are possible (e.g., ICS/LABA + montelukast, theophylline or sublingual immunotherapy), evidence from randomized controlled trials is lacking for the addition of montelukast (although an open-label observational study did report improved asthma control following 8 weeks of add-on montelukast therapy in patients with uncontrolled asthma on ICS or ICS/LABA therapy at baseline).Citation44 Evidence for theophylline in this setting is lacking, and most studies of sublingual immunotherapy were performed in patients with mild or intermittent asthma, often with comorbid allergic rhinitis.Citation45
Which patients are most likely to benefit most from the addition of an anticholinergic to their treatment regimen? Given the history of anticholinergic bronchodilators in COPD care, investigators have speculated that patients might be more likely to benefit if they are older, if they have been tobacco smokers, if they have persistent obstruction or if they do not have Type 2 markers such as a raised blood eosinophil count. However, none of these characteristics have been shown to be consistently predictive of better response to the use of LAMAs in asthma.Citation46,Citation47 A recent review highlighted the effectiveness of SITT in patients with fixed and variable airflow limitation, as well as those with other treatable traits in asthma.Citation48 Again, re-assessment of asthma control following around 4 weeks of triple therapy, or longer in cases of exacerbation, is strongly recommended in the experience of the authors (). From the authors’ perspectives, triple therapy is a valuable step-up for patients with mild or infrequent exacerbations for symptom control, and for patients with low FEV1 (especially reversible low FEV1) regardless of phenotype. It is also worth trialing in patients with asthma-COPD overlap before stepping up to biologics. Given the financial challenges some Canadians face when filling their prescriptions, it is worth noting that SITTs are markedly less costly than biologics.
In the management of chronic diseases, shared decision-making is helpful for best long-term results, and this includes asthma management. Patient preference in terms of device(s), and Provincial reimbursement of treatment options, may influence the chosen management strategy (the price for a 30-day supply of open and closed combinations in Ontario and Quebec are represented in ). Patients should, of course, understand the potential benefit of better asthma outcomes as they weigh their options. Today, many patients will be influenced by environmental factors when making their choices. The newer closed SITTs deliver their medications without aerosol gases, an environmentally friendly approach as compared with the older pMDI devices. As well, by delivering triple therapy in one inhaler rather than two, the new closed SITTs reduce landfill waste ().Citation49
Figure 2. Potential advantages and disadvantages of open and closed triple inhaled therapies in patients with poor asthma control. Abbreviations: ICS, inhaled corticosteroid; LABA, long-acting beta-agonist.
For patients with severe exacerbations, who rely upon frequent short courses of prednisone or who regularly use high-dose ICS, our experience is that triple therapy will not obviate the need for expensive biologic therapy. It would seem wise to consider referral to an expert asthma center for phenotyping and potentially stepping up to a biologic, while simultaneously assessing if, with once-daily triple therapy, the patient can enjoy good day-to-day symptom control and freedom from exacerbations. Biologics would seem the preferred treatment for patients with significant concomitant diseases such as nasal polyps or chronic respiratory syndrome, as well as profound T2 inflammation.
As with all medications and in particular those which may be used as chronic therapy, patient safety should be a primary consideration both in terms of the product under consideration (potential consequences of taking action) and the relative risk of alternative strategies (potential consequences of taking no action, or prescribing a different product). All anticholinergic products should be used with caution in patients with narrow-angle glaucoma or urinary retention.Citation29,Citation31,Citation32,Citation50 Tiotropium also carries a warning concerning use in patients with prostatic hyperplasia.Citation29 That being said, adverse events typically associated with anticholinergic medications (e.g., dry mouth, urinary retention, prostatic hyperplasia) were rarely, if at all, reported in the pivotal trials for open or closed triple therapy in asthma.Citation28,Citation41–43 A recent meta-analysis of randomized clinical trials comparing triple versus dual therapy in patients with moderate to severe asthma found triple therapy to be associated with a lower incidence of side effects overall when compared with dual therapy, and dry mouth and dysphonia to be the only side effects associated with triple therapy.Citation46 A pairwise meta-analysis reported closed triple therapy was associated with an increased risk of serious vascular side effects, but not serious cardiac side effects or serious side effects in general, in people with asthma.Citation51 This relatively benign and predicted safety profile must be weighed against the risk associated with serious exacerbations, the risks of continued increased use of reliever therapy, and the consequences of biologic therapy.
Conclusion
Although most asthma patients can achieve good symptom control with currently available ICS/LABA therapies, a subset of patients with more severe disease benefit from triple therapy with the addition of a LAMA. Once daily formulations of triple therapy in a single inhaler make this step feasible in the primary practice setting (and more likely to be adhered to and, therefore, effective). In conjunction with step up to triple therapy, the advice of an expert asthma center should be sought.
Author contributions
All authors contributed to the conceptualization, writing, review and editing of this manuscript.
Acknowledgment
The authors would like to thank John Howell PhD of John Howell Consulting, Inc., for providing medical writing and editorial support.
Disclosure statement
M. Balter has served on advisory boards for AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, Merck, Novartis, Sanofi-Genzyme and Valeo Pharma and has received honoraria as a speaker for AstraZeneca, Boehringer-Ingelheim, GlaxoSmithKline, ICEBM, Novartis and Valeo Pharma.
S. Bhinder has received honoraria as a speaker from Boehringer-Ingelheim, AstraZeneca, Merck/Organon, Novartis, Bayer, GlaxoSmithKline, Covis, Sanofi and Valeo; has served on advisory boards for AstraZeneca, Boehringer-Ingelheim, Novartis, GSK, Covis and Sanofi; and has received consulting fees from AstraZeneca, Boehringer-Ingelheim, Novartis, GlaxoSmithKline, Covis and Sanofi.
K. R. Chapman has received honoraria as a speaker for AstraZeneca, Boehringer-Ingelheim, Grifols, GlaxoSmithKline, Merck Frosst, Novartis, Sanofi, Takeda and Valeo Pharma; consulting fees from AstraZeneca, Boehringer Ingelheim, CSL Behring, GlaxoSmithKline, Grifols, InhibRx, Kamada, Merck Frosst, Novartis, Regeneron, Roche, Sanofi, Takeda and Valeo Pharma; and research grants from Amgen, AstraZeneca, CSL Behring, Bellus, Bristol Meyers Squibb, Genentech, GlaxoSmithKline, Gossamer, Grifols, Kamada, Novartis, Regeneron, Roche and Sanofi.
K. Godbout has received honoraria as a speaker from Astrazeneca, Covis, GlaxoSmithKline, Merck, Novartis and Sanofi; consulting fees from Astrazeneca, Covis, GlaxoSmithKline, Novartis, Sanofi and Valeo; and research grants from Astrazeneca, Novartis and Sanofi.
A. Kaplan has received speaking engagement, honoraria and consulting fees from Astra Zeneca, Boehringer Ingelheim, Cipla, Covis, Eisai, GlaxoSmithKline, Pfizer, NovoNordisk, Novartis, Grifols, Teva, Trudell, Valeo, Sanofi and Valeo Pharma, and research grants from Novartis, Boehringer Ingelheim.
A. McIvor has received honoraria as a speaker from AstraZeneca, Boehringer Ingelheim, Grifols, Merck, Novartis, Takeda, Teva and Sanofi Valeo Pharma. He has participated in advisory boards and received consulting fees from AstraZeneca, Boehringer Ingelheim, GSK, Pfizer, Merck, Novartis, Takeda, Teva and Valeo and received research grants from AstraZeneca, Novartis.
P. Papadopoulos is a former employee of Valeo Pharma Inc.
Additional information
Funding
References
- Asthma Canada. Asthma facts and statistics. www.asthma.ca
- Yang CL, Hicks EA, Mitchell P, et al. Canadian Thoracic Society 2021 Guideline update: Diagnosis and management of asthma in preschoolers, children and adults. Can J Respir Crit Care Sleep Med. 2021;5(6):348–361. doi:10.1080/24745332.2021.1945887.
- McIvor RA, Boulet LP, FitzGerald JM, Zimmerman S, Chapman KR. Asthma control in Canada: no improvement since we last looked in 1999. Can Fam Phys. 2007;53(4):672–677.
- Sadatsafavi M, McTaggart-Cowan H, Chen W, Mark Fitzgerald J. Quality of life and asthma symptom control: room for improvement in care and measurement. Value Health. 2015;18(8):1043–1049. doi:10.1016/j.jval.2015.07.008.
- Busse WW, Kraft M. Current unmet needs and potential solutions to uncontrolled asthma. Eur Respir Rev. 2022;31:210176. doi:10.1183/16000617.0176-2021.
- de Marco R, Locatelli F, Cazzoletti L, Bugianio M, Carosso A, Marinoni A. Incidence of asthma and mortality in a cohort of young adults: a 7-year prospective study. Respir Res. 2005;6(1):95. doi:10.1186/1465-9921-6-95.
- To T, Zhu J, Williams DP, et al. Frequency of health service use in the year prior to asthma death. J Asthma. 2016;53(5):505–509. doi:10.3109/02770903.2015.1064949.
- Sadatsafavi M, Chen W, Tavakoli H, Rolf JD, Rousseau R, FitzGerald JM. Saving in medical costs by achieving guideline-based asthma symptom control: a population-based study. Allergy. 2016;71(3):371–377. doi:10.1111/all.12803.
- Zafari Z, Sadatsafavi M, Chen W, Fitzgerald JM. The projected economic and health burden of sub-optimal asthma control in Canada. Respir Med. 2018;138:7–12. doi:10.1016/j.rmed.2018.03.018.
- Vathenen AS, Knox AJ, Wisniewski A, Tattersfield AE. Time course of change in bronchial reactivity with an inhaled corticosteroid in asthma. Am Rev Respir Dis. 1991;143(6):1317–1321. doi:10.1164/ajrccm/143.6.1317.
- Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The gaining optimal asthma ControL study. Am J Respir Crit Care Med. 2004;170(8):836–844. doi:10.1164/rccm.200401-033OC.
- Newton R, Giembycz MA. Understanding how long-acting β2-adrenoceptor agonists enhance the clinical efficacy of inhaled corticosteroids in asthma – an update. Br J Pharmacol. 2016;173(24):3405–3430. doi:10.1111/bph.13628.
- Global Initiative for Athma. Global strategy for asthma management and prevention. 2022. www.ginasthma.org
- Aaron SD, Vandemheen KL, Boulet LP, et al. Overdiagnosis of asthma in obese and nonobese adults. CMAJ. 2008;179(11):1121–1131. doi:10.1503/cmaj.081332.
- Tilles SA. Differential diagnosis of adult asthma. Med Clin North Am. 2006;90(1):61–76. doi:10.1016/j.mcna.2005.08.004.
- Express Scripts Canada. Prescription Drug Trend Report. 2019. https://www.express-scripts.ca/sites/default/files/2020-12/Express_Scripts_Canada_2018_Prescription_Drug_Trend_Report_FINAL.pdf. Accessed July 12, 2022.
- Amin S, Soliman M, McIvor A, Cave A, Cabrera C. Understanding patient perspectives on medication adherence in asthma: a targeted review of qualitative studies. Patient Prefer Adherence. 2020;14:541–551. doi:10.2147/PPA.S234651.
- D'Ancona G, Weinman J. Improving adherence in chronic airways disease: are we doing it wrongly? Breathe (Sheff). 2021;17(2):210022. doi:10.1183/20734735.0022-2021.
- Advisory Council on the Implementation of National Pharmacare. A Prescription for Canada: Achieving Pharmacare for All. Ottawa, ON: Government of Canada; 2019.
- Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012;4(4):184–191. doi:10.4168/aair.2012.4.4.184.
- Rogliani P, Matera MG, Facciolo F, Page C, Cazzola M, Calzetta L. Beclomethasone dipropionate, formoterol fumarate and glycopyrronium bromide: synergy of triple combination therapy on human airway smooth muscle ex vivo. Br J Pharmacol. 2020;177(5):1150–1163. doi:10.1111/bph.14909.
- Chapman KR. The history of antimuscarinic bronchodilator therapy. In: Spector SL, ed. Anticholinergics in Clinical Allergy Practice Lung Biology in Health and Disease. New York: Marcel Dekker; 1999:155–169.
- Rebuck AS, Chapman KR, Abboud R, et al. Nebulized anticholinergic and sympathomimetic treatment of asthma and chronic obstructive airways disease in the emergency room. Am J Med. 1987;82(1):59–64. doi:10.1016/0002-9343(87)90378-0.
- Rebuck AS, Gent M, Chapman KR. Anticholinergic and sympathomimetic combination therapy of asthma. J Allergy Clin Immunol. 1983;71(3):317–323. doi:10.1016/0091-6749(83)90086-6.
- Bourbeau J, Bhutani M, Hernandez P, et al. Canadian Thoracic Society Clinical Practice Guideline on pharmacotherapy in patients with COPD – 2019 update of evidence. Can J Respir Crit Care Sleep Med. 2019;3(4):210–232. doi:10.1080/24745332.2019.1668652.
- Kew KM, Dahri K. Long-acting muscarinic antagonists (LAMA) added to combination long-acting beta2-agonists and inhaled corticosteroids (LABA/ICS) versus LABA/ICS for adults with asthma. Cochrane Database Syst Rev. 2016;2016(1):CD011721. doi:10.1002/14651858.CD011721.pub2.
- Chari VM, McIvor RA. Tiotropium for the treatment of asthma: patient selection and perspectives. Can Respir J. 2018;2018:3464960. doi:10.1155/2018/3464960.
- Kerstjens HA, Engel M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. N Engl J Med. 2012;367(13):1198–1207. doi:10.1056/NEJMoa1208606.
- SPIRIVA Product Monograph. 2022. https://pdf.hres.ca/dpd_pm/00065560.PDF.
- Zhang S, King D, Rosen VM, Ismaila AS. Impact of single combination inhaler versus multiple inhalers to deliver the same medications for patients with asthma or COPD: a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2020;15:417–438. doi:10.2147/COPD.S234823.
- Enerzair Product Monograph. 2021. https://pdf.hres.ca/dpd_pm/00063748.PDF
- Trelegy Product Monograph. 2022. https://pdf.hres.ca/dpd_pm/00068965.PDF
- European Medicines Agency. Trimbow: EPAR - medicine overview. https://www.ema.europa.eu/en/medicines/human/EPAR/trimbow. 2017. Accessed April 5, 2023.
- Bibliothèque et Archives nationales du Québec. RAMQ list of medications. https://www.ramq.gouv.qc.ca/sites/default/files/documents/non_indexes/liste_med_2023-05-25_en_maj.pdf. Published May 25, 2023. Accessed March 31, 2023.
- Ontario Ministry of Health. Ontario drug benefit: formulary/comparative drug index (edition 43). https://www.health.gov.on.ca/en/pro/programs/drugs/odbf_mn.aspx. 2023. Accessed May 31, 2023.
- Kaplan A, Chang K-L. Tiotropium in asthma – perspectives for the primary care physician. Postgrad Med. 2021;133(5):552–564. doi:10.1080/00325481.2020.1816329.
- Busse WW, Abbott CB, Germain G, et al. Adherence and persistence to single-inhaler versus multiple-inhaler triple therapy for asthma management. J Allergy Clin Immunol Pract. 2022;10(11):2904–2913.e6. doi:10.1016/j.jaip.2022.06.010.
- Averell CM, Stanford RH, Laliberté F, Wu JW, Germain G, Duh MS. Medication adherence in patients with asthma using once-daily versus twice-daily ICS/LABAs. J Asthma. 2021;58(1):102–111. doi:10.1080/02770903.2019.1663429.
- Averell CM, Laliberte F, Duh MS, Wu JW, Germain G, Faison S. Characterizing real-world use of tiotropium in asthma in the USA. J Asthma Allergy. 2019;12:309–321. doi:10.2147/JAA.S216932.
- Collier DJ, Wielders P, Van Der Palen J, et al. Critical error frequency and the impact of training with inhalers commonly used for maintenance treatment in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2020;15:1301–1313. doi:10.2147/COPD.S224209.
- Kerstjens HAM, Maspero J, Chapman KR, et al. Once-daily, single-inhaler mometasone-indacaterol-glycopyrronium versus mometasone-indacaterol or twice-daily fluticasone-salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study. Lancet Respir Med. 2020;8(10):1000–1012. doi:10.1016/S2213-2600(20)30190-9.
- Gessner C, Kornmann O, Maspero J, et al. Fixed-dose combination of indacaterol/glycopyrronium/mometasone furoate once-daily versus salmeterol/fluticasone twice-daily plus tiotropium once-daily in patients with uncontrolled asthma: a randomised, Phase IIIb, non-inferiority study (ARGON). Respir Med. 2020;175:106186. doi:10.1016/j.rmed.2020.106186.
- Lee LA, Bailes Z, Barnes N, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respir Med. 2021;9(1):69–84. doi:10.1016/S2213-2600(20)30389-1.
- Keith PK, Koch C, Djandji M, et al. Montelukast as add-on therapy with inhaled corticosteroids alone or inhaled corticosteroids and long-acting beta-2-agonists in the management of patients diagnosed with asthma and concurrent allergic rhinitis (the RADAR trial). Can Respir J. 2009;16 Suppl A(Suppl A):17a–31a. doi:10.1155/2009/145071.
- Fortescue R, Kew KM, Leung MT. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2020;9(9):CD011293. doi:10.1002/14651858.CD011293.pub3.
- Kim LHY, Saleh C, Whalen-Browne A, O'Byrne PM, Chu DK. Triple vs dual inhaler therapy and asthma outcomes in moderate to severe asthma: a systematic review and meta-analysis. JAMA. 2021;325(24):2466–2479. doi:10.1001/jama.2021.7872.
- Singh D, Virchow JC, Canonica GW, et al. Determinants of response to inhaled extrafine triple therapy in asthma: analyses of TRIMARAN and TRIGGER. Respir Res. 2020;21(1):285. doi:10.1186/s12931-020-01558-y.
- Cazzola M, Braido F, Calzetta L, et al. The 5T approach in asthma: Triple Therapy Targeting Treatable Traits. Respir Med. 2022;200:106915. doi:10.1016/j.rmed.2022.106915.
- Fulford B, Mezzi K, Whiting A, Aumônier S. Life-cycle assessment of the breezhaler® breath-actuated dry powder inhaler. Sustainability. 2021;13(12):6657. doi:10.3390/su13126657.
- BREZTRI™ AEROSPHERE® Product Monograph. 2021. https://pdf.hres.ca/dpd_pm/00062143.PDF.
- Rogliani P, Cavalli F, Chetta A, Cazzola M, Calzetta L. Potential drawbacks of ICS/LABA/LAMA triple fixed-dose combination therapy in the treatment of asthma: a quantitative synthesis of safety profile. J Asthma Allergy. 2022;15:565–577. doi:10.2147/JAA.S283489.
