ABSTRACT
Background: Myocardial injury (MI) is common with transcatheter aortic valve implantation (TAVI) and may predict poor outcome. We aim: 1) to evaluate the difference in change of high-sensitivity-Troponin-T (hsTnT) within 24h after transfemoral-TAVI between mechanically-expanded (MEV), self-expanding (SEV) and balloon-expandable-valves (BEV); 2) to determine predictors for MI post-TAVI; and 3) to assess whether MI is associated with 30-day mortality.
Methods: This multicenter retrospective observational study included 1208 consecutively treated transfemoral-TAVI patients from three European centers. All patients treated with a MEV, SEV or BEV with available hsTnT measurements at baseline and within 24 h post-TAVI were included. Significant MI was defined as an elevation of hsTnT ≥ 15x the upper-reference-limit.
Results: Overall, the median hsTnT rise was 741 ng/L and was lower with MEV (MEV 335 vs. SEV 901 vs. BEV 649 ng/L, p < 0.001). MI occurred in 925 patients (77%) and was less frequent with MEV (MEV 67%, SEV 79% and BEV 76%, p = 0.007). Occurrence of MI was similar after implantation of first vs. second- generation SEV (79 vs. 80%, p = 0.72) and BEV (77 vs. 76%, p = 0.90). There was no association between frequency of annulus manipulation and MI. On multivariable analysis (OR (95% CI) non-MEV (1.63 (1.06–2.49)), mean aortic gradient (1.02 (1.01–1.03)), left ventricular ejection fraction (1.03 (1.01–1.04)), and previous myocardial infarction (1.62 (1.04–2.56)) were positively associated with MI. There was no association between MI and 30-day mortality.
Conclusion: Transcatheter valve design determines peri-procedural MI and is less frequent with MEV. MI is not associated with 30-day mortality.
KEYWORDS:
Introduction
Transcatheter aortic valve implantation (TAVI) has become the treatment of choice for patients with severe aortic stenosis at elevated operative risk.Citation1,Citation2 Peri-procedural myocardial injury (i.e. cardiac biomarker rise) after TAVI is frequent and may predict the outcome.Citation3–6 The exact patho-mechanism of myocardial injury is not clear yet, however several studies hypothesize that factors such as global myocardial ischemia due to balloon valvuloplasty, acute aortic regurgitation, rapid pacing-induced hypotension, micro-embolization of aortic valve debris in the coronary arteries, myocardial tissue compression by the expansion of the device and coronary obstruction should be considered as potential mechanisms for myocardial injury.Citation3,Citation7–10 We hypothesize that prosthesis expansion mechanism may also affect the occurrence of myocardial injury.
The aims of this study are 1) to evaluate the difference in change of high sensitive Troponin T (hsTnT) within 24 h after transfemoral-TAVI (TF-TAVI) between mechanically expanded (MEV), self-expanding (SEV) and balloon expandable (BEV) transcatheter heart valves, 2) to determine predictors for myocardial injury after TAVI and 3) to assess whether myocardial injury is associated with 30-day mortality.
Materials and methods
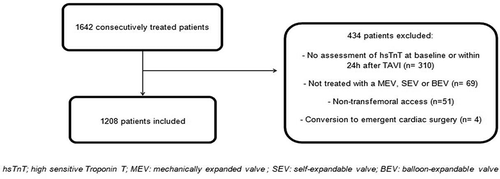
This multicenter retrospective observational study included 1208 consecutively treated TF-TAVI patients from three European centers. All patients underwent coronary angiography (CAG) in preparation for TAVI and were discussed in a multi-disciplinary heart team. The decision to revascularize a coronary vessel with a significant lesion was made by the heart team. All patients treated via transfemoral access with a MEV, SEV or BEV and who had hsTnT measurements at baseline and within 24 h after the index procedure were included. Patients who converted to emergent cardiac surgery were excluded (). All patients consented for treatment. All patients consented for the TAVR procedure and the anonymous use of data for research purposes. Given the retrospective design of the study, the Medical Ethics Committee of the Erasmus University Medical Center waived the need for additional informed consent.
The Lotus valve was considered as a mechanically expanded valve (MEV), Medtronic CoreValve and Evolut R as self-expandable valves (SEV) and Edwards XT and Edwards Sapien 3 as balloon-expandable valves (BEV). Peri-procedural myocardial injury was defined as an elevation of hsTnT ≥ 15x the upper reference limit (URL), within 24 h after the index procedure. The highest hsTnT value within the first 24 h was used for this analysis Tropinin elevation was defined by the following formula: Δ hsTnT = highest troponin level within 24 h post-TAVI – the baseline troponin. The URL for hsTnT was 14 ng/L. All complications were defined according to the VARC-2.Citation11
Statistical analysis
Categorical variables are presented as frequencies and percentages, and compared with the Pearson Chi-Square Test or the Fisher’s exact test, as appropriate. Continuous variables are presented as means (±SD) (in case of normal distribution) or medians (IQR) (in case of skewed distribution) and compared with the use of the Student’s t-test, Mann–Whitney U test or Kruskal Wallis test. Normality of the distributions was assessed using the Shapiro–Wilk test. To study which patient and procedural characteristics are independently associated with myocardial injury after TAVI, logistic regression was performed. Variables were chosen based on etiological considerations. All characteristics judged to be clinically relevant or to have a pathophysiologic role in peri-procedural myocardial injury were included in the multivariable model. To investigate whether valve design is an independent predictor of periprocedural myocardial injury, baseline characteristics that were significantly different between the different valve cohorts were also included in the multivariable logistic regression model. We took into account the observed frequency of the dependent variable y (n/10). The characteristics judged to be clinically relevant were age, gender, previous myocardial infarction, previous PCI, previous CABG, atrial fibrillation at baseline, glomerular filtration rate (GFR), left ventricular ejection fraction (LVEF), mean aortic gradient, PCI during work-up, pre-/post balloon dilatation and valve mechanism (i.e. non-MEV and MEV). Baseline characteristics which were significantly different between the different valve cohorts were body mass index, diabetes mellitus (p = 0.004), hypertension (p = 0.036) and logistic EuroScore. A similar multivariable model was performed with further elaboration regarding valve mechanism (SEV, BEV, and MEV as reference valve mechanism). Survival after TAVI was determined with the use of Kaplan–Meier method. A log-rank test was applied to compare between-group differences. A two-sided alpha level of 0.05 was used to indicate significance. Statistical analyses were performed using SPSS software version 21.0 (SPSS Inc., Chicago, Illinois, USA).
Results
Baseline-procedural characteristics and in-hospital complications
Baseline and procedural characteristics are summarized in and . Overall, 1208 patients were included with a median (IQR) age of 84 (80–87) years and 50% were female. The overall median (IQR) LVEF, mean aortic gradient and Logistic EuroScore were, respectively, 60% (45–65%), 45 (35–54) mm Hg and 17% (11–24%) (). The excluded patients were younger (83 vs. 84 years), more often had peripheral vascular disease (31% vs. 17%), a lower mean gradient (41 vs. 45 mmHg) and a higher logistic EuroScore (20% vs. 17%). During work-up for TAVI, PCI was performed in 252 patients (21%), 18% was staged and 3% concomitant with the index procedure. TAVI was performed in 12% with a MEV, in 56% with a SEV and in 32% with a BEV. Balloon pre- or post-dilatation was performed in 49% of the cases. More than mild residual aortic regurgitation (AR) was seen in 7% ().
Table 1. Baseline characteristics of the total patient population and per valve mechanism
Table 2. Procedural characteristics of the total patient population and per valve mechanism
The overall incidence of myocardial injury was 77% and was less frequent after MEV (MEV 67%, SEV 79% and BEV 76%, p = 0.007). The overall median increase of hsTnT was 741 ng/L and was lower after MEV (MEV 335 ng/L, SEV 901 ng/L and BEV 649 ng/L, p < 0.001). Patients with myocardial injury were older (85 (80–88) vs. 83 (78–85) years, p < 0.001), more often female (53% vs. 40%, p < 0.001), with a lower GFR (42 (30–56) vs. 48 (36–64), p < 0.001), a higher left ventricular ejection fraction (60% (50–65%) vs. 55% (40–60%), p < 0.001) and a higher aortic mean gradient (45 (37–55) vs. 40 (30–50) mm Hg, p < 0.001) compared to patients without myocardial injury.
The incidences of new left bundle branch block (LBBB) and new permanent pacemaker implantation (PPI) were both 15% and were higher after MEV (new LBBB: MEV 42%, SEV 14% and BEV 9%, p < 0.001; new PPI: MEV 24%, SEV 15% and BEV 11%, p = 0.001) (). The development of new LBBB was inversely associated with myocardial injury (63% vs. 80%, p < 0.001; ∆ hsTnT 327 vs. 855 ng/L, p < 0.001) while new PPI was not associated with myocardial injury (77% vs. 77%, p = 1.00; ∆ hsTnT 833 vs. 739 ng/L, p = 0.21).
Table 3. In-hospital complications of the total patient population and divided per valve mechanism
First versus second-generation valves
Medtronic CoreValve versus Evolut R
The baseline characteristics of the patients treated with Medtronic CoreValve (MCV) and Evolut R were similar, except for a higher age (85 vs. 83 years, p < 0.001) and a higher Logistic Euroscore (19 vs. 16, p < 0.002) in the MCV group. From a procedural perspective, patients treated with MCV had more frequent balloon pre- or post-dilatation (62% vs. 36%, p < 0.001) and had more frequent more than mild residual AR (14% vs. 2%, p < 0.001). There was no significant difference in the occurrence of myocardial injury (MCV 79% vs. Evolut R 80%, p = 0.72) and a similar increase in ∆ hsTnT (MCV 900 vs. Evolut R 924 ng/L, p = 0.64) between the two groups.
Repositioning/retrieval data of the Evolut R was available for 127 patients (96.2%). There was no association between the frequency of annulus manipulation (i.e. repositioning/retrieval of the valve) and myocardial injury. Also, there was no difference in the occurrence of myocardial injury between the patients treated with MCS vs. Evolut R regardless of annulus manipulation.
Edwards XT versus Sapien 3
The baseline characteristics were similar for the patients treated with Edwards XT versus Edwards Sapien 3, except for a higher frequency of NYHA class ≥3 at baseline (73% vs. 56%, p = 0.002), pulmonary hypertension (27% vs. 17%, p = 0.027), pacemaker at baseline (19% vs. 9%, p = 0.004) and a higher logistic Euroscore (20 vs. 14, p < 0.001) in the XT group. PCI during work-up for TAVI was less often performed in the XT group while balloon pre- or post-dilatation was more often performed. The occurrence of myocardial injury and increase in hsTnT were similar between the two groups (XT 77% vs. Sapien 3 76%, p = 0.90; ∆ hsTnT (XT 725 vs. Sapien 3 590 ng/L, p = 0.61).
Lotus valve
Baseline and procedural data were similar for patients with versus without annulus manipulation, except for a lower hemoglobin at baseline in the manipulation group (7.7 vs. 7.9 mmol/L, p = 0.048). The occurrence of myocardial injury was similar between the manipulation and no manipulation group (respectively, 71% vs. 60%, p = 0.38; ∆ hsTnT (269 vs. 252 ng/L, p = 0.84).
Repositionable valves
After pooling the data of all the repositionable valves (i.e. Evolut R and Lotus), the occurrence of myocardial injury was similar for the patients with versus without annulus manipulation (73% vs. 73%, p = 1.00; ∆ hsTnT (427 vs. 539 ng/L, p = 0.45). In addition, there was no association between the frequency of annulus manipulation and the occurrence of myocardial injury.
Predictors for myocardial injury
On multivariable analysis non-MEV (OR (95% CI) 1.63 (1.06−2.49), p = 0.025), higher mean aortic gradient (OR (95% CI) 1.02 (1.01–1.03), p < 0.001), left ventricular ejection fraction (OR (95% CI) 1.03 (1.01–1.04), p < 0.001), and previous myocardial infarction (OR (95% CI) 1.62 (1.04–2.56), p = 0.032) were positively associated with myocardial injury, whereas concomitant PCI (OR (95% CI) 0.32 (0.14–0.70), p = 0.004) was inversely associated ().
Table 4. Multivariable logistic regression for the determination of peri-procedural myocardial injury
A similar multivariable analysis, in which further elaboration regarding valve mechanism was performed, is shown in supplemental .
Outcome
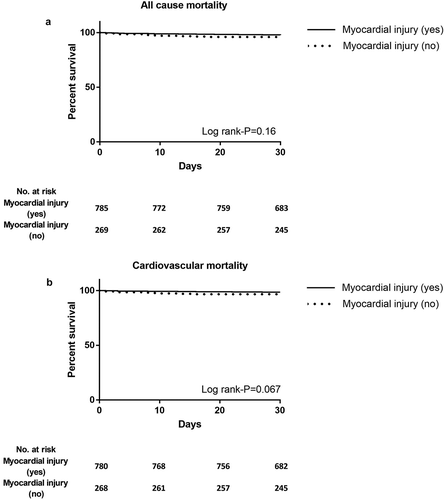
There was no difference in 30-day all-cause and cardiovascular mortality, disabling and non-disabling stroke between the myocardial injury vs. no myocardial injury group (respectively, 3% vs. 4%, p = 0.19; 2% vs. 3%, p = 0.093; 0.2% vs. 0% and 0% vs. 0.4%, p = 0.28). In addition, Kaplan–Meier curves showed no association between myocardial injury and 30-day mortality (,). Similar outcome was observed when the analysis was performed per center (Log-rank p = 0.11; Log-rank p = 0.33; Log-rank p = 0.10). There was also no association between type of prosthesis and the occurrence of myocardial injury with regard to 30-day mortality (Supplemental Figure 1A-C and 2A-C).
Discussion
This is the largest study cohort assessing peri-procedural myocardial injury (i.e. ∆ hsTnT) after TAVI including first and second generation trancatheter heart valves. Key findings:
The occurrence of peri-procedural myocardial injury was 77%;
Myocardial injury was less frequent after MEV;
There was no difference in the occurrence of myocardial injury after implantation of first vs. second generation SEV and BEV;
There were no penalties in terms of myocardial injury for additional manipulations in the annulus;
Myocardial injury was not associated with the need for new PPI; and
Myocardial injury was not associated with 30-day mortality.
The occurrence of peri-procedural myocardial injury after TAVI varies in the literature because different definitions and cardiac biomarkers are used. In our study, the occurrence rate was 77% and was higher than in the study of Sinning et al. (52%).Citation12 This can be explained by the fact that we assessed high sensitivity tropinin T and not troponin I.Citation13 Despite the difference in the occurrence of myocardial injury, both studies demonstrated that the use of SEV and higher left ventricle ejection fraction (LVEF) were associated with peri-procedural myocardial injury. Patients with preserved LVEF may have more viable myocardium than patients with low ejection fraction and are therefore able to release higher troponin levels. Also, SEV typically requires more oversizing, which might lead to greater myocardial tissue compression and trauma and thus more myocardial injury.Citation12
Kahlert et al. suggested that myocardial injury is more related to hypoperfusion-induced ischemia than to peri-procedural microembolization.Citation8 In our study, there was less myocardial injury after the use of MEV than with the other valve mechanisms. We can only hypothesize that there might be more hemodynamic stability during the implantation of a MEV since there is early valve function and no need for rapid pacing. Conversely, a small cardiac magnetic resonance (CMR) study identified myocardial injury in 18% of patients and suggested a coronary embolic pathophysiologic mechanism because of the multifocal distribution and small lesion size.Citation9 Multiple randomized trials on filter-based cerebral embolic protection confirmed cerebral embolization of debris in almost all patients.Citation14,Citation15 Conceivably, embolization is not restricted to the brain but also affects the coronary (micro) circulation. Of note in the US SENTINEL trial, number and overall volume of new brain lesions by DW-MRI was higher with SEV than with BEV suggesting more embolization after the use of SEV.Citation16 In addition, we have shown that a higher aortic valve mean gradient is an independent predictor for peri-procedural myocardial injury. Patients with more severe aortic stenosis (i.e. higher mean gradient) may have more degenerative calcifications that may dislodge and embolize into the coronary vasculature. Also, they may have a larger myocardial muscle mass, which may result in higher troponin release.
First versus second-generation transcatheter heart valves
Second generation THV aim to address the limitations of the first generation THV, introducing repositioning/retrievable features and sealing fabric to reduce paravalvular leaks. Our study showed no difference in peri-procedural myocardial injury between first vs. second generation SEV and BEV. In addition, there were no penalties in terms of myocardial injury for manipulating (i.e. repositioning/retrieval of the valve) in the aortic annulus.
New LBBB and new PPI
The incidence of new LBBB and PPI in our study was higher after MEV, and echoes other registries/studies.Citation17–19 Conduction disorders may occur as a result of myocardial ischemia.Citation20 However, we have shown that development of new LBBB post-TAVI was inversely associated with myocardial injury and that PPI was not associated with myocardial injury. The higher rate of new LBBB and PPI after MEV might be explained by the higher radial force during frame expansion which can damage the conduction tissue. Another potential explanation is the extensive contact of the Lotus frame with the left ventricular outflow tract during the implantation process (i.e. Lotus foreshortening and locking), which could damage the conduction system even more.Citation21
Thirty-day mortality
Similar to the study of Sinning et al. we have shown that there is no association between myocardial injury and 30-day mortality.Citation12 Studies with longer follow-up are needed to assess whether the myocardial injury is associated with long-term survival.
Limitations
We acknowledge the fact that patients were not randomly allocated to a specific valve mechanism and that the groups were not equal in size. Although we did capture patient’s history of prior coronary artery disease, information on the completeness of revascularization prior to TAVI was lacking. However, incomplete revascularization did not affect myocardial injury after TAVI in an earlier study.Citation3 The “true” occurrence rate of peri-procedural myocardial injury might be higher since in our study troponin rise was limited to 24 h post TAVI while the VARC-2 recommends assessing troponin rise up to 72 h. Indeed, troponin levels might further increase over time and meet the definition of myocardial injury after 24 h post-TAVI. However, current trends for early discharge would preclude troponin assessments up to 72 h after TAVI. In addition, several studies have shown a peak of cardiac troponin within 24 h after TAVI.Citation3,Citation12
There are several studies to show that new pacemaker implantation, as well as myocardial injury, are associated with worse outcome.Citation3–6,Citation22 In our study, there was no association between myocardial injury and 30-day mortality. One can only speculate on how the finding of less myocardial injury would compare to the higher incidence of new conduction disorders in terms of long-term survival. Further comparative studies should shed further light on the significance of these findings.
Generalizability of our findings should be put in perspective of 1) the cardiac biomarkers used (hsTnT vs. troponin I, CK-MB, etc.), 2) definition of periprocedural myocardial infarction, 3) specific patient population (i.e. risk profile) and 4) access approach (proportion of apical access).
Conclusion
Transcatheter valve design determines peri-procedural myocardial injury, which is less frequent with MEV.
Supplemental Material
Download Zip (1.1 MB)Disclosure statement
Dr Van Mieghem received research grants from Claret Medical, Abbott Vascular, Boston Scientific, Medtronic and Edwards Lifesciences. All the other authors have nothing to disclose.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med. 2011;364(23):2187–2198. doi:10.1056/NEJMoa1103510.
- Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med. 2016;374(17):1609–1620. doi:10.1056/NEJMoa1514616.
- Rodés-Cabau J, Gutiérrez M, Bagur R, et al. Incidence, predictive factors, and prognostic value of myocardial injury following uncomplicated transcatheter aortic valve implantation. J Am Coll Cardiol. 2011 May 17;57(20):1988–1999. doi:10.1016/j.jacc.2010.11.060.
- Yong ZY, Wiegerinck EM, Boerlage-van Dijk K, et al. Predictors and prognostic value of myocardial injury during transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2012 Jun;5(3):415–423. doi:10.1161/CIRCINTERVENTIONS.111.964882. Epub 2012 Jun 5.
- Ribeiro HB, Nombela-Franco L, Muñoz-García AJ, et al. Predictors and impact of myocardial injury after transcatheter aortic valve replacement: a multicenter registry. J Am Coll Cardiol. 2015 Nov 10;66(19):2075–2088. doi:10.1016/j.jacc.2015.08.881.
- Koskinas KC, Stortecky S, Franzone A, et al. Post-Procedural troponin elevation and clinical outcomes following transcatheter aortic valve implantation. J Am Heart Assoc. 2016 Feb 19;5(2):pii: e002430. doi:10.1161/JAHA.115.002430.
- Généreux P, Head SJ, Van Mieghem NM, et al. Clinical outcomes after transcatheter aortic valve replacement using valve academic researchconsortium definitions: a weighted meta-analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012 Jun 19;59(25):2317–2326. doi:10.1016/j.jacc.2012.02.022. Epub 2012 Apr 11.
- Kahlert P, Al-Rashid F, Plicht B, et al. Myocardial injury during transfemoral transcatheter aortic valve implantation: an intracoronary Doppler and cardiac magnetic resonance imaging study. EuroIntervention. 2016 Mar;11(12):1401–1408. doi:10.4244/EIJY15M05_10.
- Kim WK, Rolf A, Liebetrau C, et al. Detection of myocardial injury by CMR after transcatheter aortic valve replacement. J Am Coll Cardiol. 2014 Jul 29;64(4):349–357. doi:10.1016/j.jacc.2014.03.052.
- Saia F, Marrozzini C, Marzocchi A. Displacement of calcium nodules of the native valve as a possible cause of left main occlusion following transcatheter aortic valve implantation. J Invasive Cardiol. May 2011;23(5):E106–9.
- Kappetein AP, Head SJ, Généreux P, et al. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg. 2013 Jan;145(1):6–23. doi:10.1016/j.jtcvs.2012.09.002. Epub 2012 Oct 16.
- Sinning JM, Hammerstingl C, Schueler R, et al. The prognostic value of acute and chronic troponin elevation after transcatheter aortic valve implantation. EuroIntervention. 2016 Apr 20;11(13):1522–1529. doi:10.4244/EIJY15M02_02.
- Weber M, Bazzino O, Navarro Estrada JL, et al. Improved diagnostic and prognostic performance of a new high-sensitive troponin T assay in patients with acute coronary syndrome. Am Heart J. 2011 Jul;162(1):81–88. doi:10.1016/j.ahj.2011.04.007.
- Van Mieghem NM, van Gils L, Ahmad H, et al. Filter-based cerebral embolic protection with transcatheter aortic valve implantation: the randomised MISTRAL-C trial. EuroIntervention. 2016 Jul 20;12(4):499–507. doi:10.4244/EIJV12I4A84.
- Haussig S, Mangner N, Dwyer MG, et al. Effect of a cerebral protection device on brain lesions following transcatheter aortic valve implantation in patients with severe aortic stenosis: the CLEAN-TAVI randomized clinical trial. JAMA. 2016 Aug 9;316(6):592–601. doi:10.1001/jama.2016.10302.
- Kapadia SR, Kodali S, Makkar R, et al. Protection against cerebral embolism during transcatheter aortic valve replacement. J Am Coll Cardiol 2017 Jan 31;69(4):367–377. doi:10.1016/j.jacc.2016.10.023. Epub 2016 Nov 1.
- Meredith IT, Walters DL, Dumonteil N, et al. 1-Year outcomes with the fully repositionable and retrievable lotus transcatheter aortic replacement valve in 120 high-risk surgical patients with severe aortic stenosis: results of the REPRISE II study. JACC Cardiovasc Interv. 2016 Feb 22;9(4):376–384. doi:10.1016/j.jcin.2015.10.024.
- Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010 Oct 21;363(17):1597–1607. doi:10.1056/NEJMoa1008232. Epub 2010 Sep 22.
- Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014 May 8;370(19):1790–1798. doi:10.1056/NEJMoa1400590. Epub 2014 Mar 29.
- Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J. Oct 2007;28(20):2525–2538. doi:10.1093/eurheartj/ehm355.
- van Gils L, Tchetche D, Lhermusier T, et al. Transcatheter heart valve selection and permanent pacemaker implantation in patients with pre-existent right bundle branch block. J Am Heart Assoc. 2017 Mar 3;6(3):pii: e005028. doi:10.1161/JAHA.116.005028.
- Fadahunsi OO, Olowoyeye A, Ukaigwe A, et al. Incidence, predictors, and outcomes of permanent pacemaker implantation following transcatheter aortic valve replacament: analysis from the U.S. Society of thoracic surgeons/American college of cardiology TVT registry. JACC Cardiovasc Interv. 2016 Nov 14;9(21):2189–2199. doi:10.1016/j.jcin.2016.07.026.