ABSTRACT
Oral anticoagulation reduces the ischemic stroke risk in patients with nonvalvular atrial fibrillation (NVAF) but inherently increases the bleeding risk. Percutaneous left atrial appendage occlusion (LAAO) offers an alternative to long-term anticoagulation in patients at risk for cardioembolic events due to underlying atrial fibrillation. Although long-term clinical data remain limited, percutaneous LAAO appears to be a safe and effective means of reducing hemorrhagic stroke and non-procedural-related bleeding events compared to warfarin. Incomplete closure of the LAA resulting in a peri-device leak (PDL) is common after interventions targeting the LAA and has been variably associated with an increased risk of embolic stroke in patients following LAAO. However, the clinical significance of PDL remains unclear, in part, due to variability in the definitions used for PDL, modalities used for the detection of PDL, and the confounding impact of continuing therapeutic anticoagulation in patients found to have PDL on surveillance imaging. In the current review, we examine the association of PDL with ischemic stroke and systemic embolization and explore mechanistic considerations in the development of PDL as they pertain to the unmet need for improving the diagnostic assessment of PDL from an anatomic and physiologic perspective.
Introduction
Arterial thromboembolism resulting in ischemic stroke is the most common and serious complication of nonvalvular atrial fibrillation (NVAF). The risk of ischemic stroke in non-anticoagulated patients with NVAF may be as high as 9% per year in those with a prior history of a stroke or transient ischemic attack and ranges from 1.5% to 3% per year in elderly patients and those with diabetes or hypertension.Citation1 Oral anticoagulation reduces ischemic stroke risk by approximately 60% but also imparts a 0.2–0.5% annual risk of serious, life-threatening bleeding complications.Citation2–6 Approximately 30% of patients with NVAF do not receive therapeutic anticoagulation due to relative or absolute contraindications related to bleeding risk and, even when appropriately administered, anticoagulant therapy is associated with increased bleeding and a residual risk of ischemic stroke.Citation7,Citation8
The left atrial appendage (LAA) is the most common site of thrombus formation and cardioembolism in patients with NVAF.Citation9 Percutaneous left atrial appendage occlusion (LAAO) has emerged as a viable approach for patients with NVAF who cannot tolerate long-term anticoagulation by providing a mechanical seal of the appendage, thereby preventing embolization of LAA thrombi.Citation10 The Watchman device (Boston Scientific, St. Paul, Minnesota) is the most commonly implanted LAAO device in the U.S. and has the most robust clinical data having been studied in two randomized controlled trials and accompanying continued access registries.Citation11–14 Data from the Watchman trials, registries, and meta-analysis suggest that the Watchman device reduces post-procedural bleeding (particularly hemorrhagic stroke) and mortality, however with an increased risk of ischemic stroke compared to warfarin that may be related in part to peri-procedural factors around the time of implantation.Citation12 Of note, a larger proportion of the ischemic strokes after LAAO were nondisabling compared with warfarin, such that the outcomes relating to hemorrhagic and disabling/fatal strokes are more favorable after LAAO.Citation14
Incomplete closure of the LAA resulting in peri-device leak (PDL) is one such factor that is often recognized at the time of device implantation but may also be identified later on follow-up surveillance imaging.Citation15 The clinical significance of PDL is not well understood and has been postulated to be a mediator of increased stroke risk following LAAO however definitive clinical data linking PDL to stroke risk has remained elusive.Citation15,Citation16 Here we briefly review the incidence and clinical implications of PDL, advantages, and limitations of existing modalities used to identify PDL, and discuss the absence of a clear gold standard technique capable of addressing the unmet need of definitive identification and quantitation of PDL.
Discussion
Peri-device leak after LAAO
Incidence of PDL: impact of diagnostic modality
A wide range of devices are available or in development, including Watchman (Boston Scientific, St. Paul, Minnesota), Amulet (Abbott Vascular, St. Paul, Minnesota), WaveCrest (Biosense Webster, Irvine, California), Occlutech LAA occluder (Occlutech International AB, Helsingborg, Sweden), LAmbre closure system (LifeTech), Ultraseal (Cardia), and LARIAT (SentreHEART, Redwood City, California). Comprehensive discussions of the efficacy, technical aspects, and regulatory status of the devices used for LAAO are available in several recent reviews.Citation17,Citation18 Many, but not all, studies of these devices have systematically defined and reported rates and mechanisms of PDL ().Citation17 The incidence of PDL likely depends on the interaction between the LAAO device, anatomical characteristics of the LAA including variability in the morphology of the LAA ostium and body, and possibly tissue characteristics of the underlying atrial tissue (e.g., fibrotic remodeling) (). The occurrence of any degree of PDL occurs in approximately 30–40% of patients following LAAO at 12 months post-implantation as detected by transesophageal echocardiogram (TEE), and any PDL as detected by cardiac computed tomography (CT) has been reported to be as high as 50–60%.Citation19–21 The definition of “significant” PDL remains elusive as a definitive link to adverse clinical outcomes has not been established. An arbitrary definition of significant PDL that was utilized in the Watchman trials and has generally been accepted in other studies is a ≥5 mm diameter gap between the device edge and the LAA tissue with the residual flow by TEE using a Nyquist limit set around 25cm/s. Using this definition and modality, significant PDL occurs in 10−20% of patients after endovascular or epicardial LAA closure.Citation16,Citation17
Table 1. Summary of definitions, frequency, modality for detection, and impact on outcomes of PDL in percutaneous LAAO studies
Existing modalities for the detection of PDL provide different information about the nature and degree of the leak and, in the case of TEE, may be operator-dependent in the sensitivity for PDL detection. Three-dimensional (3D) TEE may be more sensitive than two-dimensional (2D) TEE for the detection of PDL.Citation22 A recent study of leak following LARIAT found that in patients who had experienced an ischemic stroke, 3D TEE identified patients with small leaks (<3 mm) not seen on 2D TEE.Citation16 Both 2D and 3D TEE provide information regarding the mechanism of PDL () including peri-device flow and the associated structural gap between device and LAA, and TEE may also identify low-velocity flow within the LAA suggestive of incomplete endothelialization of the LAAO device particularly when no leak is detected between the LAA and device (i.e., no PDL per se).Citation23 Cardiac CT is more sensitive for the detection of PDL as has been observed by multiple investigators and may provide greater insight into the mechanisms of PDL as discussed below.Citation15,Citation20,Citation21,Citation24 However, the detection of contrast in the LAA following LAAO is not proof of PDL per se because residual device permeability after device implantation may also account for contrast opacification of the LAA. Furthermore, 3D reconstructions from cardiac CT may improve LAAO device sizing which may translate to improved procedural success and reductions in PDL.Citation22,Citation25 However, neither TEE nor CT provides quantitative information regarding the degree of PDL or residual device permeability which may be a critical factor in the prognostic significance of PDL on thrombotic risk.
Figure 2. Examples of current LAAO devices and possible device-specific mechanisms of PDL at the time of device implantation. (a) Single lobe, membrane-coated devices such as the Watchman (Boston Scientific) interact with LAA in one circumferential zone at the level of the LAA landing zone where PDL may occur with these devices. In lobe and disc devices such as the Amplatzer Amulet (Abbott) interact with the LAA at two circumferential territories including the landing zone and then more distally in the body of LAA. These contact sites and the area between the lobe and disc represent potential regions where PDL can occur. The uniformity of LAA-device contact can be influenced by the presence of an additional lobe that can result in off-axis implantation and resultant PDL of both device types. (b) Leak following epicardial LAAO with the LARIAT device is always centrally located and could be modulated by the morphology of the LAA body and ostium. Purple and blue arrows represent (turbulent in some cases) flow into and out of the LAA , respectively. Abbreviations: LA, left atrium; LAA, left atrial appendage; LAAO, LAA occlusion; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; LLR, left lateral ridge; MV, mitral valve; LV, left ventricle; PDL, peri-device leak; Cx, circumflex artery
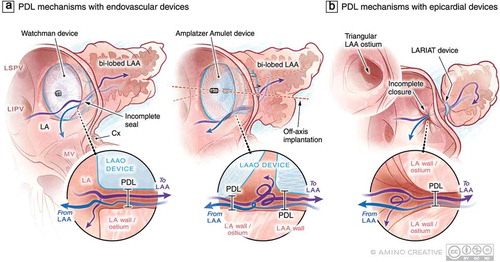
PDL and stroke: impact of study heterogeneity
In addition to the variability of the definitions and modalities used for the identification of PDL, small sample sizes, incomplete follow-up, physician discretion on the management of PDL (i.e., continue anticoagulation or not), and small numbers of clinical events have hampered the ability of investigators to characterize the clinical significance of incomplete percutaneous closure of the LAA. In contrast to a study of surgical LAA closure showing an increased risk of stroke in those with incomplete LAA closure (detected using cardiac CT), most studies of percutaneous LAAO have not demonstrated an association between PDL and stroke risk.Citation15,Citation24,Citation26,Citation27 A retrospective study of 98 patients who underwent LAAO with the pericardial LARIAT device observed that in 3 of 5 patients who experienced a recurrent embolic stroke and who had a TEE at the time of the event were found to have a leak of <5 mm.Citation16 The investigators also noted that 2 of 3 leaks were not detected by 2-dimensional TEE but as noted above were only observed on 3D TEE, and further noted that PDL increased over time in 5%, 15%, and 20% of patients with significant PDL detected by TEE at the time of implantation, at 6 and 12 months, respectively. In a large and comprehensive study of PDL, Tzikas et al. examined 1,001 patients who underwent successful implantation of the AMPLATZER™ Cardiac Plug (ACP).Citation28 The investigators observed that 98.1% of patients had complete closure and the presence of TEE-detected significant PDL (>3 mm in this case) was observed in 1.9% of patients but was not associated with adverse events at follow-up in their study. In addition, device-related thrombus (DRT) was also not associated with adverse events in their study however patients identified with DRT were treated with anticoagulation which may have obscured any relationship of DRT with ischemic events in this study. In another study of 344 patients treated with ACP, the presence of severe PDL (>5 mm) detected by TEE was observed in 0.6% of patients and, along with DRT, was not associated with adverse events at follow-up.Citation29 In a sub-study of the Watchman PROTECT AF (Percutaneous Closure of the Left Atrial Appendage Versus Warfarin Therapy for Prevention of Stroke in Patients With Atrial Fibrillation) trial, investigators observed “major” PDL defined as >3 mm in 32.4% of patients but did not observe any association with PDL and an increased risk of thromboembolism.Citation30
In a recent study of patients treated with different LAAO endovascular devices, cardiac CT-detected PDL (defined as any contrast in the LAA) was common and decreased from 3 to 12 months (68.5% to 56.7%, P = 0.02). The only identified predictor of PDL was a device compression ratio of less than 10%. At a median follow-up of 236 days, 9.1% of patients experienced a major adverse cardiac event (stroke, DRT, or cardiovascular death).Citation24 Although not statistically significant, patients with MACE had a greater frequency of PDL compared to patients without MACE (12% vs 4.3%, P = 0.3). The three mechanisms observed to contribute to PDL included the absence of device sealing presumed to be due to incomplete endothelialization, presence of a gap between the device and LAA, and an off-axis position of the device. These mechanisms were also previously described in another study of cardiac CT in the identification of PDL following LAAO with Watchman and ACP devices.Citation20
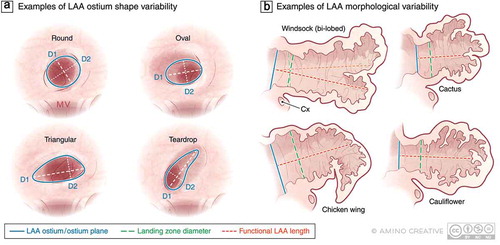
Figure 1. Examples of variability in LAA anatomy that may present challenges to imaging and device sizing and selection. (a) Various shapes of the LAA ostium have been described including round, oval, triangular, and teardrop however the association between LAA ostial shape and successful closure is not well established. (b) Morphologic variation in the body of the LAA are well-recognized including the so-called windsock, cactus, chicken wing, and cauliflower variants as well as the presence of more than one LAA lobe which may impact the success of endovascular device-based closure. Abbreviations: D1, D2 maximum diameters in 2 dimensions; MV, mitral valve; Cx, circumflex artery
PDL and putative mechanisms of thrombotic risk
Despite the uncertainties surrounding the association between PDL and thrombotic risk, it is generally accepted that complete sealing of the LAA is preferred due to the possibility that a residual leak following LAAO could permit embolization (“escape”) of LAA thrombus and/or prevent endothelialization of the device and thereby contribute to DRT which in contrast to PDL has more directly been linked to the risk of stroke.Citation46 However, it is unclear if small size leaks would permit the escape of an LAA thrombus of sufficient size to result in a clinically meaningful embolic event.
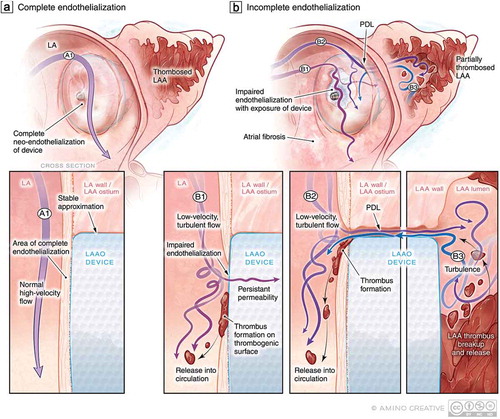
Different mechanisms of PDL including off-axis device implantation, device under-sizing, and incomplete closure due to appendage morphologic variations () may each be associated with unique flow disturbances and thus portend differential risks of impaired endothelialization. Turbulent flow around the device could activate platelets and clotting factors resulting in thrombus formation and left atrial flow may be modulated by the coexistence of mitral regurgitation which is commonly seen in patients with atrial fibrillation.Citation47 Furthermore, the risk of PDL may be distinct for different devices, underscoring the need for further investigation into the prognostic significance of PDL in larger studies with systematic ascertainment of PDL and outcomes. In patients with PDL, persistent flow around the device may delay or inhibit device endothelialization and thus render the device susceptible to device-related thrombus (DRT) formation – an effect that may be modulated by the underlying tissue substrate and degree of fibrosis (). The notion that perturbed and residual flow detected by TEE may be a risk factor for DRT is supported by the observation that residual LAA flow following surgical LAA closure is associated with thrombus formation and embolic events.Citation27 Although PDL may be associated with an increased risk of DRT, this association has not been uniformly established which may reflect the variability in the diagnostic modality, timing, and definition used for identifying PDL.Citation46,Citation48
Figure 3. PDL as a precursor to DRT: Putative mechanisms of impaired endothelialization following implantation. (a) Following endovascular LAAO, over time, the LAAO device should ideally be covered by healthy endothelium to prevent thrombogenicity and resultant PDL. (a1) The nature of LA flow (high-velocity laminar vs. low-velocity turbulent) may impact the efficacy of endothelialization. (b) In the context of (b1) low-velocity turbulent flow in the LA, impaired endothelialization may result thereby exposing the thrombogenic surface of the LAAO device to the coagulation system at a time when patients may be discontinuing anticoagulation therapy resulting in DRT and risk of cardioembolism. In the context of PDL (b2), persistent flow in and out of the LAA around the device may also lead to impaired endothelialization and PDT but also delay thrombosis of the LAA and allow for the embolization of LAA thrombi
Novel strategies to prevent and detect PDL
As noted above, a causal relationship between PDL following percutaneous LAAO and adverse clinical events has not been clearly demonstrated, possibly because PDL may not be a mediator of stroke risk or because of the arbitrariness of PDL definitions used to-date and variability in study design elements, including LAAO device, sample size, confounding from anticoagulation in patients with PDL, and the timing and modality of the diagnostic imaging tool used to detect PDL. Existing data suggest that PDL is common despite the use of TEE to facilitate procedural planning and device selection. Cardiac CT is a promising modality for procedural planning capable of acquiring high-resolution delineation of cardiac and LAA anatomy, and an emerging consensus exists that pre-procedural insights afforded by cardiac CT in terms of LAA size and shape may be useful in guiding LAAO device type and size.Citation49 Although device selection guided by cardiac CT has been shown to reduce LAAO procedure time, existing data have to-date not demonstrated that pre-procedural cardiac CT guidance reduces PDL following LAAO.Citation50 Lastly, cardiac CT cannot be used intra-procedurally and is thus only suitable as a planning or post-procedure monitoring modality. Furthermore, due to initial device permeability, smaller-sized leaks may not be detected easily or discerned due to the spatial resolution limitations of cardiac CT and contrast in the LAA thus inappropriately attributed to device permeability alone.
Intracardiac echo allows for real-time and high-resolution detection of PDL, typically does not obscure fluoroscopic guidance, and allows for the procedure to be performed under local anesthesia but does not provide full 3D imaging necessary to ensure complete sealing of the LAA, and often does not provide information regarding LAA depth and is associated with a steep learning curve.Citation51 Perhaps the most promising intra-procedural modality for PDL detection is 3D TEE,Citation52 but this approach faces several drawbacks, including the semi-quantitative nature of flow assessment, learning curve, and operator- and patient-related factors affecting the acquisition of high-quality LAA images which can be limited by acoustic shadowing and reverberation artifacts. Furthermore, the frequent use of general anesthesia during LAAO requires additional clinical staff including an anesthesiologist and at times an imaging cardiologist. The need for additional staff requirements significantly impedes the efficiency of the procedural workflow, increases cost, increases the risk of gastrointestinal injury, and may limit the primary operating physician’s ability to collect anatomical and physiological information according to his or her discretion.Citation53
In light of the limitations of existing modalities for PDL detection, novel diagnostic tools capable of providing real-time, high-resolution 3D reconstructions of the LAA obtained by the operating physician and minimally affected by operator- and patient-factors are needed. New tools with the ability to generate 3D anatomical reconstructions may improve the measurement of the LAA, optimize device sizing, and possibly reduce PDL. Any new methods or tools would also benefit from the ability to assess the physiologic significance of PDL. Quantification of PDL and residual device permeability may provide critical information to guide the LAAO procedure and provide additional insights into the clinical significance of incomplete closure.
Conclusions
PDL is an increasingly recognized and likely underreported event following LAA closure. Additional research is needed to characterize the incidence of PDL and the relationship between PDL size, location, and hemodynamics on clinical outcomes. In order to support these efforts – and, most importantly, minimize PDL altogether – standardized and comprehensive imaging approaches are needed to ensure full occlusion of the LAA and/or better quantify leak physiology. There is a need for real-time, high-definition 3D functional imaging not achievable with available imaging devices, and the areas outlined in this review may help to guide improved device design and development of novel imaging tools. Such tools may also be useful in other procedures where residual leaks occur, such as percutaneous atrial septal defect repairs or management of paravalvular leak in TAVR/TMVR patients.
Acknowledgments
We would like to acknowledge the support of Mr. Cassio Lynm of Amino Creative, LLC in the design of our figure graphics.
Disclosure statement
Mazen Albaghdadi MD MSc – Consultant and grant support from CSI, Inc. Grant support from Boston Scientific, Consultant for K2 Medical, Inc. Andrew O. Kadlec, MD, PhD – Consultant to K2 Medical. Andrew Adler – Consultant to K2 Medical. Alexander Romanov, MD PhD, FEHRA, FHRS, FESC – Nothing to disclose. Usman R Siddiqui MD FHRS – Consultant to K2 Medical. Karl-Heinz Kuck, MD – Consultant to K2 Medical. Horst Sievert, MD – Study honoraria to institution, travel expenses, consulting: 4tech Cardio, Abbott, Ablative Solutions, Ancora Heart, Append Medical, Axon, Bavaria Medizin Technologie GmbH, Bioventrix, Boston Scientific, Carag, Cardiac Dimensions, Cardiac Success, Cardimed, Celonova, Comed B.V., Contego, CVRx, Dinova, Edwards, Endologix, Endomatic, Hemoteq, Hangzhou Nuomao Medtech, Holistick Medical, K2, Lifetech, Maquet Getinge Group, Medtronic, Mokita, Occlutech, Recor, Renal Guard, Terumo, Trisol, Vascular Dynamics, Vectorious Medtech, Venus, Venock, Vivasure Medical. Martin B. Leon, MD – Nothing to disclose. Torsten Vahl, MD – Abbott Laboratories: Grant/Research Support, Consulting Fees. Boston Scientific: Grant/Research Support, Consulting Fees. Edwards Lifesciences: Grant/Research Support, JenaValve Technology: Grant/Research Support, Consulting Fees, Medtronic: Grant/Research Support, Siemens Healthineers: Grant/Research Support, Consulting Fees.
Additional information
Funding
References
- Stroke Risk in Atrial Fibrillation Working Group. Independent predictors of stroke in patients with atrial fibrillation: a systematic review. Neurology. 2007;69(6):546–554. doi:10.1212/01.wnl.0000267275.68538.8d.
- Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857–867. doi:10.7326/0003-4819-146-12-200706190-00007.
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi:10.1056/NEJMoa1107039.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi:10.1056/NEJMoa0905561.
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883–891. doi:10.1056/NEJMoa1009638.
- Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369(22):2093–2104. doi:10.1056/NEJMoa1310907.
- Camm AJ, Accetta G, Ambrosio G, et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart. 2017;103(4):307–314. doi:10.1136/heartjnl-2016-309832.
- O’Brien EC, Holmes DN, Ansell JE, et al. Physician practices regarding contraindications to oral anticoagulation in atrial fibrillation: findings from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF) registry. Am Heart J. 2014;167(4):601–609 e1. doi:10.1016/j.ahj.2013.12.014.
- Cresti A, Garcia-Fernandez MA, Sievert H, et al. Prevalence of extra-appendage thrombosis in non-valvular atrial fibrillation and atrial flutter in patients undergoing cardioversion: a large transoesophageal echo study. EuroIntervention. 2019;15(3):e225–e230. doi:10.4244/EIJ-D-19-00128.
- January CT, Wann LS, Calkins H, et al. AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the heart rhythm society. J Am Coll Cardiol. 2019;74(1):104–132. doi:10.1016/j.jacc.2019.01.011.
- Reddy VY, Sievert H, Halperin J, et al. Percutaneous left atrial appendage closure vs warfarin for atrial fibrillation: a randomized clinical trial. JAMA. 2014;312:1988–1998. doi:10.1001/jama.2014.15192.
- Holmes DR Jr., Kar S, Price MJ, et al. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64(1):1–12. doi:10.1016/j.jacc.2014.04.029.
- Holmes DR Jr., Reddy VY, Gordon NT, et al. Long-term safety and efficacy in continued access left atrial appendage closure registries. J Am Coll Cardiol. 2019;74(23):2878–2889. doi:10.1016/j.jacc.2019.09.064.
- Reddy VY, Doshi SK, Kar S, et al. 5-year outcomes after left atrial appendage closure: from the PREVAIL and PROTECT AF trials. J Am Coll Cardiol. 2017;70:2964–2975. doi:10.1016/j.jacc.2017.10.021.
- Raphael CE, Friedman PA, Saw J, Pislaru SV, Munger TM, Holmes DR Jr. Residual leaks following percutaneous left atrial appendage occlusion: assessment and management implications. EuroIntervention. 2017;13(10):1218–1225. doi:10.4244/EIJ-D-17-00469.
- Gianni C, Di Biase L, Trivedi C, et al. Clinical implications of leaks following left Atrial appendage ligation with the LARIAT device. JACC: Cardiovascular Interventions. 2016;9(10):1051–1057. doi:10.1016/j.jcin.2016.01.038.
- Asmarats L, Rodes-Cabau J. Percutaneous left atrial appendage closure: current devices and clinical outcomes. Circulation: Cardiovascular Interventions. 2017;10(11). doi:10.1161/CIRCINTERVENTIONS.117.005359.
- Moussa Pacha H, Al-Khadra Y, Soud M, Darmoch F, Moussa Pacha A, Alraies MC. Percutaneous devices for left atrial appendage occlusion: A contemporary review. World J Cardiol. 2019;11(2):57–70. doi:10.4330/wjc.v11.i2.57.
- Berti S, Santoro G, Brscic E, et al. Left atrial appendage closure using AMPLATZER devices: A large, multicenter, Italian registry. Int J Cardiol. 2017;248:103–107. doi:10.1016/j.ijcard.2017.07.052.
- Saw J, Fahmy P, DeJong P, et al. Cardiac CT angiography for device surveillance after endovascular left atrial appendage closure. European Heart Journal – Cardiovascular Imaging. 2015;16(11):1198–1206. doi:10.1093/ehjci/jev067.
- Granier M, Laugaudin G, Massin F, et al. Occurrence of incomplete endothelialization causing residual permeability after left atrial appendage closure. J Invasive Cardiol. 2018;30:245–250.
- Jaguszewski M, Manes C, Puippe G, et al. Cardiac CT and echocardiographic evaluation of peri-device flow after percutaneous left atrial appendage closure using the AMPLATZER cardiac plug device. Catheter Cardiovasc Interv. 2015;85(2):306–312. doi:10.1002/ccd.25667.
- Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC Cardiovasc Imaging. 2014;7(12):1251–1265. doi:10.1016/j.jcmg.2014.08.009.
- Nguyen A, Gallet R, Riant E, et al. Peridevice leak after left atrial appendage closure: incidence, risk factors, and clinical impact. Cana J Cardiol. 2019;35(4):405–412. doi:10.1016/j.cjca.2018.12.022.
- Prakash R, Saw J. Imaging for percutaneous left atrial appendage closure. Catheter Cardiovasc Interv. 2018;92(2):437–450. doi:10.1002/ccd.26828.
- Aryana A, Singh SK, Singh SM, et al. Association between incomplete surgical ligation of left atrial appendage and stroke and systemic embolization. Heart Rhythm. 2015;12(7):1431–1437. doi:10.1016/j.hrthm.2015.03.028.
- Garcia-Fernandez MA, Perez-David E, Quiles J, et al. Role of left atrial appendage obliteration in stroke reduction in patients with mitral valve prosthesis: a transesophageal echocardiographic study. J Am Coll Cardiol. 2003;42(7):1253–1258. doi:10.1016/S0735-1097(03)00954-9.
- Tzikas A, Shakir S, Gafoor S, et al. Left atrial appendage occlusion for stroke prevention in atrial fibrillation: multicentre experience with the AMPLATZER Cardiac Plug. EuroIntervention. 2016;11(10):1170–1179. doi:10.4244/EIJY15M01_06.
- Saw J, Tzikas A, Shakir S, et al. Incidence and clinical impact of device-associated thrombus and peri-device leak following left atrial appendage closure with the amplatzer cardiac plug. JACC Cardiovasc Interv. 2017;10(4):391–399. doi:10.1016/j.jcin.2016.11.029.
- Viles-Gonzalez JF, Kar S, Douglas P, et al. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation) substudy. J Am Coll Cardiol. 2012;59(10):923–929. doi:10.1016/j.jacc.2011.11.028.
- Holmes DR, Reddy VY, Turi ZG, et al. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374(9689):534–542. doi:10.1016/S0140-6736(09)61343-X.
- Pillarisetti J, Reddy YM, Gunda S, et al. Endocardial (Watchman) vs epicardial (Lariat) left atrial appendage exclusion devices: understanding the differences in the location and type of leaks and their clinical implications. Heart Rhythm. 2015;12(7):1501–1507. doi:10.1016/j.hrthm.2015.03.020.
- Lim YM, Kim JS, Kim TH, et al. Delayed left atrial appendage contrast filling in computed tomograms after percutaneous left atrial appendage occlusion. J Cardiol. 2017;70(6):571–577. doi:10.1016/j.jjcc.2017.04.007.
- Sivasambu B, Arbab-Zadeh A, Hays A, Calkins H, Berger RD. Delayed endothelialization of watchman device identified with cardiac CT. J Cardiovasc Electrophysiol. 2019;30(8):1319–1324. doi:10.1111/jce.14053.
- Freixa X, Abualsaud A, Chan J, et al. Left atrial appendage occlusion: initial experience with the Amplatzer™ Amulet™. Int J Cardiol. 2014;174(3):492–496. doi:10.1016/j.ijcard.2014.03.154.
- Lopez Minguez JR, Asensio JM, Gragera JE, et al. Two-year clinical outcome from the Iberian registry patients after left atrial appendage closure. Heart. 2015;101(11):877–883. doi:10.1136/heartjnl-2014-306332.
- Landmesser U, Schmidt B, Nielsen-Kudsk JE, et al. Left atrial appendage occlusion with the AMPLATZER Amulet device: periprocedural and early clinical/echocardiographic data from a global prospective observational study. EuroIntervention. 2017;13(7):867–876. doi:10.4244/EIJ-D-17-00493.
- Bartus K, Gafoor S, Tschopp D, et al. Left atrial appendage ligation with the next generation LARIAT(+) suture delivery device: early clinical experience. Int J Cardiol. 2016;215:244–247. doi:10.1016/j.ijcard.2016.04.005.
- Lakkireddy D, Afzal MR, Lee RJ, et al. Short and long-term outcomes of percutaneous left atrial appendage suture ligation: results from a US multicenter evaluation. Heart Rhythm. 2016;13(5):1030–1036. doi:10.1016/j.hrthm.2016.01.022.
- Huang H, Liu Y, Xu Y, et al. Percutaneous left atrial appendage closure with the lambre device for stroke prevention in atrial fibrillation: a prospective, multicenter clinical study. JACC: Cardiovascular Interventions. 2017;10(21):2188–2194. doi:10.1016/j.jcin.2017.06.072.
- Park JW, Sievert H, Kleinecke C, et al. Left atrial appendage occlusion with lambre in atrial fibrillation: initial European experience. Int J Cardiol. 2018;265:97–102. doi:10.1016/j.ijcard.2018.02.120.
- Bellmann B, Schnupp S, Kühnlein P, et al. Left atrial appendage closure with the new Occlutech® device: first in man experience and neurological outcome. J Cardiovasc Electrophysiol. 2017;28(3):315–320. doi:10.1111/jce.13141.
- Regueiro A, Bernier M, O’Hara G, et al. Left atrial appendage closure: initial experience with the ultraseal device. Catheter Cardiovasc Interv. 2017;90(5):817–823. doi:10.1002/ccd.260870.
- Asmarats L, Masson JB, Pagnotta PA, et al. Percutaneous left atrial appendage closure with the ultraseal device: insights from the initial multicenter experience. JACC Cardiovasc Interv. 2018;11(19):1932–1941. doi:10.1016/j.jcin.2018.05.023.
- Suradi HS, Hijazi ZM. Left atrial appendage closure: outcomes and challenges. Neth Heart J. 2017;25(2):143–151. doi:10.1007/s12471-016-0929-0.
- Saw J, Nielsen-Kudsk JE, Bergmann M, et al. Antithrombotic therapy and device-related thrombosis following endovascular left atrial appendage closure. JACC: Cardiovascular Interventions. 2019;12(11):1067–1076. doi:10.1016/j.jcin.2018.11.001.
- Delgado V, Bax JJ. Atrial functional mitral regurgitation: from mitral annulus dilatation to insufficient leaflet remodeling. Circulation: Cardiovascular Imaging. 2017;10(3). doi:10.1161/CIRCIMAGING.117.006239.
- Fauchier L, Cinaud A, Brigadeau F, et al. Device-related thrombosis after percutaneous left atrial appendage occlusion for atrial fibrillation. J Am Coll Cardiol. 2018;71(14):1528–1536. doi:10.1016/j.jacc.2018.01.076.
- Korsholm K, Berti S, Iriart X, et al. Expert recommendations on cardiac computed tomography for planning transcatheter left atrial appendage occlusion. JACC: Cardiovascular Interventions. 2020;13(3):277–292. doi:10.1016/j.jcin.2019.08.054.
- Eng MH, Wang DD, Greenbaum AB, et al. Prospective, randomized comparison of 3-dimensional computed tomography guidance versus TEE data for left atrial appendage occlusion (PRO3DLAAO). Catheter Cardiovasc Interv. 2018;92(2):401–407. doi:10.1002/ccd.27514.
- Alkhouli M, Hijazi ZM, Holmes DR Jr., Rihal CS, Wiegers SE. Intracardiac echocardiography in structural heart disease interventions. JACC: Cardiovascular Interventions. 2018;11(21):2133–2147. doi:10.1016/j.jcin.2018.06.056.
- Nucifora G, Faletra FF, Regoli F, et al. Evaluation of the left atrial appendage with real-time 3-dimensional transesophageal echocardiography: implications for catheter-based left atrial appendage closure. Circulation. 2011;4(5):514–523. doi:10.1161/CIRCIMAGING.111.963892.
- Freitas-Ferraz AB, Bernier M, Vaillancourt R, et al. Safety of transesophageal echocardiography to guide structural cardiac interventions. J Am Coll Cardiol. 2020;75(25):3164–3173. doi:10.1016/j.jacc.2020.04.069.
