Abstract
Radiocaesium being very soft, lustrous, and highly reactive metal is very harmful if present in large quantity in the environment. Here in this chapter, we will discuss the contaminated sites and possible remediation methods of radiocaesium just to eliminate from ecosystem and make our planet green and peaceful. Lots of radionuclides released not only to atmosphere but also into the oceans, rivers, lakes, and other water bodies after global fallout, Chernobyl accident, giant tsunami, and Fukushima nuclear power plant accident in 2011. It damaged all the living on terrestrial and aquatic ecosystems. Many attempts have been taken into account to reduce its dangerous effects but still world is facing this enigma. Many polluted sites and treatments have been discussed in detail.
1. Introduction
Caesium element is found in soil and rocks naturally. In pure state caesium is silvery and very soft. It is not expected to be found in the area because it causes explosion like reactions when react with air and water. Erosion, weathering of rocks and minerals are natural resources of caesium. Radioactive element caesium during explosion of nuclear weapons it usually released to the environment. Radiocaesium is the more prevalent radionuclides in the environment. The 134Cs and 137Cs are two important radioactive isotopes. The 134Cs half-life is about 2 years and 137Cs is about 30 years. Each atom of 134Cs change into non-radioactive particles barium 134 (134Ba) or xenon 134 (134Xe) at the same time as each 137Cs atom decays to barium 137 (137Ba), which is non-radioactive. For so many years, large amounts of radionuclides have been deposited onto the soil and in water due to nuclear power plants but no any exact measure of its risk evaluation have been taken. Throughout the early ages of nuclear weapons manufacture release of radiocaesium to earthly and water ecosystems provides chance to carry out multidisciplinary studies on the transportation methods of this potentially hazardous radionuclide.
2. Contaminated sites of radiocaesium
2.1. Contamination of radiocaesium in Western North Pacific Ocean
Radiocaesium isotopes 134Cs and 137Cs were entering into the atmosphere in 1950s and 1960s by atmospheric nuclear weapon testings, in 1980s by nuclear fuel reprocessing and in 1980s by Chernobyl accident. Great source of radiocaesium contamination in North Pacific was seen Atmospheric deposition as a result of nuclear weapons test which was estimated by using atmospheric model simulations (Kobayashi, Nagai, Chino, & Kawamura, Citation2013; Miyazawa et al., Citation2013; Morino, Ohara, & Nishizawa, Citation2011; Morino, Ohara, Watanabe, Hayashi, & Nishizawa, Citation2013; Stohl et al., Citation2012). In North Pacific surface sea water, activity concentration of 137Cs observed ranged from 1 to 2.5 (Aoyama, Tsumune, & Hamajima, Citation2012). Beside these sources another most important source to the contamination of North Pacific Ocean with radiocaesium is the spoil to the Fukushima Dai-ichi Nuclear Power Plant (FNPP1). Inside the eastern part of Japan, giant tsunamis in March 2011 and massive Tohoku earthquake resulted in harsh spoil to Fukushima Dai-ichi Nuclear Power Plant. Such accidents free big amount of radiocaesium, not only in islands of Japan but also spread to the part of North Pacific Ocean (Yoshida & Kanda, Citation2012). For radiocaesium transfer, two major pathways are considered; one by direct discharging into the sink and other by atmospheric deposition in March and April 2011. It became a very difficult to quantitatively measure the quantity of radiocaesium released in North Pacific Ocean by Fukushima – resulting source due to immediate dilution by water advection and dispersion mechanisms. Many efforts to measure and find data of Fukushima resulting radiocaesium contamination at surface and interior oceanic water are constant and some have been reported which showed transportation of Fukushima-derived radiocaesium into the North Pacific Ocean by surface currents. (Buesseler et al., Citation2012; Aoyama, Uematsu, Tsumune, & Hamajima, Citation2013; Kameník, Dulaiova, Buesseler, Pike, & Stastn, Citation2013; Kaeriyama et al., Citation2014; Kumamoto, Aoyama, Hamajima, Murata, & Kawano, Citation2015; Ramzaev et al., Citation2014). Total calculated amount of 137Cs and 134Cs accumulated on ocean ranges extensively commencing 5–30 PBq.
A scientist expected the vertical distributions of 134Cs and 137Cs concerning 10 months after the Fukushima Dai-ichi Nuclear Power Plant accident in winter 2012 in western part of North Pacific Ocean (Kumamoto et al., Citation2015). Inventory of water column and Fukushima-derived 134Cs action absorption in changeover area between 35 and 40° N owing to direct discharging of impure water from FNPP1 were found highest while the inventory water column of the bomb-derived 137Cs were maximum in south area. Mean values probably may be 1020 ± 80 and 820 ± 120 Bq m−2, in that order for Fukushima-derived 134Cs and bomb-derived 137Cs in the water column inventories. Such data suggests the shock of FNPP1 mishap in western part of North Pacific Ocean almost similar as nuclear weapons trying in winter 2012.
2.2. Bomb-derived action concentrations of 137Cs
Another fact related to FNPP1 accident was addition of Fukushima-derived 137Cs added to bomb-derived 137Cs. In comparison to 137Cs released, 134Cs ahead of the accident had moved out indicating the use of 134Cs as radiocaesium tracer from the FNPP1 accident. Therefore, after FNPP1 and decay alteration, excess 137Cs action concentration in relation to the 134Cs action absorption in seawaters collected indicates bomb-derived 137Cs action application. A small number of studies have previously been calculating bomb-derived 137Cs action application in oceanwaters of north part of Pacific Ocean herein fashion (but they did not evaluate action concentrations and quantitative account for Fukushima-derived and bomb-derived 137Cs (Inoue et al., Citation2012; Kameník et al., Citation2013; Ramzaev et al., Citation2014).
2.3. Measurement of radiocaesium in Japanese Islands
The Japanese Government and the Tokyo Electric Power Company in late March 2011 started assessment of marine biota in the coastal areas of FNPP1 (Nuclear Regulation Authority, Citation2011; Oikawa, Takata, Watabe, Misonoo, & Kusakabe, Citation2013; Tokyo Electric Power Company, Citation2011). The decay-corrected 134Cs/137Cs ratio in those monitoring figures and activity concentrations were approximately one in concord ratio in stagnant water in damaged FNPP1 and equivalent, respectively. Lots of measurements were between 2 and 6 PBq range (Miyazawa et al., Citation2013; Nair et al., Citation2014; Prime Minister of Japan & His Cabinet, Citation2011; Tsumune, Tsubono, Aoyama, & Hirose, Citation2012). But temporary or spatial extrapolation in the calculation ranged 11–27 PBQ facilitating evaluation of the 137Cs (or 134Cs) from the directly discharged radioactive waters (Bailly du Bois et al., Citation2012; Charette et al., Citation2013).
Nuclear Regulation Authority, had discovered that 134Cs and 137Cs resulting since the spoiled FNPP1 caused radioactive contagion of Japan islands and soil of these island has been enormously neglected by such disasters. Derived from ground capacity and the air dose resulting from the air monitoring, support equal distribution of both radioisotopes of caesium. The predictable quantity of 137Cs (134Cs) action in islands of Japan was 2.4 PBQ (1015 Bq) (Morino et al., Citation2013).
2.4. 134Cs vs. 137Cs activity concentrations
Previous studies report shows similarities of the isotopic relationships in the North Pacific Ocean with activity concentrations for 134Cs and 137Cs in winter 2012 approximately in one-to-one relationship (slope ¼ 1.009 ± .006) as shown in Figure (Aoyama et al., Citation2012; Kameník et al., Citation2013; Kofuji & Inoue, Citation2013; Ramzaev et al., Citation2014). Such relationship chains the supposition that approximately equivalent sum radio activities of Fukushima derived 134Cs and 137Cs is on the loose into north part of Pacific Ocean. Average bomb-derived 137Cs action absorption of 1.1 Bq m−3 is in agreement with analysed values in North Pacific (1.0e1.7 Bq m−3).
2.5. Activity concentration in surface water
A researcher shows bomb-derived 137Cs in exterior seawater ranged.4 and 2.2 Bq m−3 in the winter 2012. Average values in tropical and subtropical region were 1.2 ± .2 Bq m−3 (n ¼ 9) and 1.5 ± .1 Bq m−3 (n ¼ 17) (Povinec et al., Citation2013). Another assumption was that the bomb-derived 137Cs action concentration was elevated than Fukushima-derived 134Cs action absorption at many stations in Kuroshio Extension Current. It also suggests that there is a major effect on direct discharge of polluted water on radiocaesium action in western part of North Pacific Ocean.
Katsumi Hirose in his study a summary of the radioactive fallout data by the government of Japanese reported the depositional environment behaviour of Fukushima-derived radionuclides in a large area of South Tohoku and Kanto Plain region of 21–23 March 2011. In their environmental radioactivity monitoring special emphasis was given onto exterior dose rate, radioactivity capacity in ecological samples. Pure water for falling dust, while every day and month wise precipitation samples were unruffled by rain water samplers. These results indicate that Fukushima-derived radioactive blur was more dominant exaggerated in Honshu Island and the apparent atmospheric residence time revealed by temporal change of Fukushima-derived radiocaesium was about 10 d for the Fukushima-derived 137Cs from the Fukushima Dai-ichi NPP.
The distribution of monthly 137Cs accumulation in March, April, and May, respectively was studied. From findings, higher 137Cs deposition was analysed in March at Fukuoka. It is also concluded that the large deposit of Fukushima-derived radionuclides 137Cs occurred in the North Pacific coast and inland areas of eastern Honshu Island and the sea areas of eastern Honshu Island in Japan less affected by Fukushima-derived radionuclides. At the same time, 134Cs found in sample which is exposed to Fukushima-derived radionuclides are to Fukuoka. Figure also express that in the air release of radionuclides from Fukushima Dai-ichi NPP continues until April 2011, but release at fewer rates. The deposition of 137Cs increased in the south-west of Japan and Japan Sea side as compared in March. His study describes monthly 137Cs depositions in May declined in all areas of Japan, but it is also seen within 300 km of the Fukushima Dai-ichi NPP higher 137Cs deposits occurred. To recognize Fukushima Nuclear Accident (FNA) impacts on sea environment. A studied seawater and a composite squid samples and Caesium-134 and Caesium-137 were found high in all samples (Yu et al., Citation2015). Caesium-134 normally undetectable before FNA also found in this sample, with 1.65 ± .13 Bq/kg wet activity level. The Oyashio Current, flowing southwards along the east coast of Japan, joins with the northward Kuroshio Current at 35° N and flows eastwards into the open ocean. For this purpose, Kuroshio Current extension with elevated temperature and the Oyashio Current with low down temperature and salinity were selected. The radiological evaluation showed the radioactive discharge from Fukushima Nuclear Accident would not have a major unfavourable outcome on sea biota at inhabitant’s intensity.
They show that the radioactivity of 137Cs in surface seawater varies widely ranging from 1 to 826 mBq/L. From this, it was seen that 65% data were lower than 100 mBq/L and 1% was higher than 800 mBq/L. For 134Cs, the activity data w distributed in a similar pattern. At the 0 m depth layer, the 137Cs and 134Cs activity ranged from 1 to 82 mBq/L and from below detection limit to 757 mBq/L, and the medians were 51 mBq/L and 45 mBq/L, respectively whereas the activity of 137Cs and 134Cs ranged from 1 to 755 mBq/L at the 20-m depth layer and from below detection limit to 66 mBq/L, and the medians were 39 mBq/L and 35 mBq/L, respectively. While at the depth of 50 m, the activity of 137Cs and 134Cs varied from 2 to 678 mBq/L and from below detection limit to 597 mBq/L, and the medians were 52 mBq/L and 43 mBq/L, respectively.
The horizontal distributions of 137Cs and 134Cs at different depths also studied. The largest value was observed at the station W2-7, over 615 km (332 NM) away from Fukushima. The figure presents same biogeochemical behaviour with similar distribution patterns for 137Cs and 134Cs. The activities of 137Cs and 134Cs were higher in the surface seawater at the middle and east of section W2 than in the other regions whereas the distribution patterns of 137Cs and 134Cs were similar as in the surface seawater at depths of 20 and 50 m.
The outline of 137Cs action in seawater revealed that water part of radionuclide was transported southwards crossways the Kuroshio conservatory with the north Pacific mode Kuroshio extension’s barrier. It put effect on FNA radionuclide transport at Section J4 and described that it was higher at the northern side of the Kuroshio conservatory (35° N) than southern side (Men et al., Citation2015).
2.6. Dispersion of Fukushima radionuclides in the world
A study indicates the dispersion of 137Cs over America and Europe after Fukushima PPA to expect the contamination of radionuclides at international level (Povinec et al., Citation2013). Different studies mainly focused on atmospheric and marine modelling to evaluate the bang of the accident on the environment and to compare, predict, and measure radionuclide contents of 137Cs in the Pacific Ocean. In order to find the pollution levels of 137Cs over America and Europe, atmospheric Lagrangian dispersion modelling was applied. Northern part of Pacific Ocean will be labelled with Fukushima 137Cs 10y after the mishap with meditation less than 1 Bq/m3. The 137Cs amount in surface waters of the open Pacific will be 20 Bq/m3. The spring will reach the US coast 4–5 y after the mishap, however, levels will be under 3 Bq/m3. After a comparison of measured and simulated 137Cs amount in atmospheric aerosols and seawater with global consequences and the Chernobyl mishap, the main sources of pre-Fukushima radionuclide in the area found.
Entire world has been labelled with the Fukushima radionuclides, though extremely small levels. Special convey of air heaps between Fukushima and Europe at 500 hPa (5000ma.s.l.) air heights was indicated by forward and backward trajectories. The horizontal distribution in Europe reaches over 4000 km wide belt as the Lagrange modelling showed. Two prominent radionuclide maxima observed in whole Europe with decreasing amplitude of the North to the South portrayed by temporal and spatial distribution of Fukushima-derived radio nuclides in aerosols accounts for different air masses in European sky. A typical journey time between Fukushima and Europe are estimated to 10–15 days while the travel time of the Fukushima coast to the U.S. west coast estimated to be 4–5 years. But after 10 years the concentration will be similar to that of global consequences. Studies also confirm that the open Pacific radionuclide concentrations danger radiation will hold the world’s population of utilization of seafood.
2.7. Categories of waste containing radiocaesium
There are generally four categories for radioactive wastes containing 134Cs and 137Cs: low-level waste (LLW) and high-level waste (HLW), mixed waste and spent nuclear fuel. Low-level waste not in the sense with low radioactive pollutants but polluted with 134Cs and 137Cs comprise soaked and dried up wastes. Nuclear power plant actions and sustaining fuel cycle operations generate LLW about 64% volume and 70% of radioactivity. Contagion with radiocaesium records for only a minute fraction of the action of LLW in the world. All radiocaesium formed as a consequence of fission reaction leftovers rapt inside spent nuclear fuel rods. Barnwell, South Carolina, Richland, and Washington in the United States are accepting LLW while more than half of the LLW in eastern United States is inclined at the Barnwell site. According Nuclear Act Waste policy, HLW is “the highly radioactive material from the reprocessing of spent nuclear fuel, includes liquid wastes formed in reprocessing and any solid material from such liquid waste containing fission products in sufficient concentration ...” (42 USC 100) and also generated from the production of plutonium. Whereas minute fractions of radioactive particles associated with uranium enriched show recovery from fleet fuel reactor. In Washington, Georgia, South Carolina, and New York, 100 million litres of liquid HLW stored in underground tanks.
Irradiated targets and fuel elements used in nuclear reactors are spent nuclear fuels. Profitable reactors have generated spent fuel >30,000 metric tons. It is more radioactive, due to the huge meditation of fission products. Such fuel is currently stored on their own production sites as lucrative nuclear power plants and DOE services protect and cool the material. Almost all DOE spent fuel is stored at four sites: Hanford, Idaho National Environmental, Savannah River and Engineering Laboratory, and Western-Vallie. Radioactive and chemically dangerous materials are collectively referred to as mixed waste. Dangerous mixed waste containing radioactive isotopes were burned in Oak Ridge, Tennessee. All high and low level waste is classified as mixed waste.
2.8. Radiocaesium accumulation in marine organisms
Many studies on the accumulation of Cs in marine biota using metrics such as ecological half lives and field concentration factors give clues of Cs accumulation levels in fishes and bivalves (Kawai et al., Citation2013). However, these mechanisms give no satisfactory answers to radiocaesium contamination by organisms. There is a need to further investigate such biota. In this regard, a study was conducted on the differential bioaccumulation of 134Cs in tropical marine organisms and the relative importance of exposure pathways (Metian et al., Citation2016). For this purpose, five tropical marine species: three bivalves, one decapod (shrimp Penaeus stylirostris), and one alga (Lobophora variegata) were elected. Marine organisms were irradiated using pathways like: seawater (all of them), food (shrimp and bivalves), and sediment (bivalves). Bioaccumulation competency of the two oysters found similar for all the pathways exposed. A single feeding with radiolabelled food in bivalves and shrimp resulted in assimilation efficiencies of Cs between 7.0 ± .4 and 40.7 ± 4.3%, with a variable retention time (half-life eTb1/2 – ranging from 16 ± 3 to 89 ± 55 d). This confession pathway show small bioaccumulation competence for sediment-bound Cs because the clam lives buried in the sediment and that was lower than the two-oyster species, which are not used to live in this media (.084 ± .003 and .080 ± .005). The alga and the shrimp to a lesser extent dissolved Cs more efficiently than the bivalves (approx. 14 and 7 times higher, respectively). Cs accumulation from sediment was similar to the absorbed (61.6 ± 9.7 to 79.2 ± 2.3%) and retained (Tb1/2: 37 ± 2 to 58 ± 25 d) for the three-bivalve’s species. G. pectinatum species showed Cs distribution in the soft parts whereas most of the Cs taken up from seawater and sediment was principally located on the hard parts of the bivalves and shrimp. Regardless of the reduced relocate effectiveness of Cs from food, the use of a worldwide bioaccumulation representation indicated the tropic pathways were the main uptake route of Cs in the bivalves and shrimp. The study also depict uptake of radiocaesium in three bivalve’s species exposed to radiolabelled sediments and also shows their kinetics by means of easy first-order exponential kinetic model Equation (1).
(1)
where CFt and CFss are the concentration factor at time t (d) and ke is the depuration rate constant (d−1). The depuration kinetics was best built-in using either a simple-part Equation (2) or two-part exponential depuration model Equation (3).
(2)
(3)
where ke is the depuration rate constant (d−1), At and A0 are the remaining activities (%) at time t (d) and 0, respectively; “s” and “l” subscripts stand for the short- and long-lived exponential components, respectively.
An exponential model with a solid-state exposure describes the uptake kinetics of the bivalves exposed to radiolabelled sediment during 35 d reached within the ten first days. The values of the 134Cs transfer factor (TFss) were low (<.1) for all species. Both oysters displayed similar behaviour and retained 62–63% of 134Cs from sediment vs. 79% for the clam. The biological half-life of 134Cs depurated according to this long-lived constituent between 36 and 58 d ranged. Major part of 134Cs action was associated with shell for both oysters while fraction of 134Cs associated with the soft parts of the bivalves represented only <2–22% and TF30d lower than whole-body soft-parts (Table ).
Table 1. Concentration factor and Transfer factor of Cs134 in three bivalve species, seawater, and sediment (Metian et al., Citation2016).
2.8. Bioaccumulation model of radiocaesium
The global bioaccumulation of 134Cs in the bivalves and the shrimp for their comparative input of every exposure path was calculated by means of kinetic parameters resolute in different experiments and other parameters such as the 134Cs Kdf in phytoplankton (8178 for I. galbana; present study) and mussel 100, Kds in sediment and the ingestion rates determined in bivalves and shrimp (IR ¼ .0046–.0149 g g−1 d−1 and .3472 g g−1d−1, respectively; present study) (Dietze & Kriest, Citation2012; International Atomic Energy Agency [IAEA], Citation2004; Wang, Ke, Yu, & Lam, Citation2000). Results indicate that the seawater and sediment contributed for <1–31% and 6–24%, respectively, whereas food pathway was the major contributor (56–83%) to the global bioaccumulation of 134Cs in the different taxa.
The accumulation of Cs in different species from New Caledonia (South Pacific) uncovered via different pathways (seawater, food and sediment) and also by comparing results obtained with published data on Cs accumulation in different temperate species (Metian et al., Citation2016). Results indicate that tropical bivalves did not accumulate 134Cs more than species from temperate regions. In reality, the CF obtained in P. stylistic (8.26) is relatively low compared to CFs from temperate decapod crustaceans.
2.9. Uptake capacity of Japanese soil for radiocaesium
Investigations on Japanese soil for radiocaesium sorption distinctiveness by comparing soil chemical properties after Fukushima PPA were performed (Uematsu et al., Citation2015). Detailed analysis of the radiocaesium interception potential (RIP) of soil was done due to high variability of radiocaesium soil-to-plant transfer factor. For 51 top soils samples from the surrounding area of the Fukushima accident affected area RIP soil and other soil properties were determined. Multiple regression analysis with soil organic matter and cation exchange capacity explained the soil RIP (R2 = .64). The correlation between RIPsoil and clay content suggests lower RIP soil per unit soil clay content for Japanese soils compared to European region, likely related to the soil mineralogy, i.e., lower content of micaceous minerals.
2.10. Contamination of radiocaesium in East coast of Malaysia
South China Sea separates the East and West Malaysia with a coastline of about 9000 km (World Resources Institute) and by Straits of Sulu Sea, Celebes Sea, and Malacca. The Radiocaesium monitoring in aquatic environment of Malaysia after nuclear fallouts of Fukushima Di ichi Nuclear Power Plant is a lot attention not only for the state but also to complete the international record. Main source of protein for most of Malaysians is seafood. Some of researcher carried out a revise to analyse the radioactive contagion in aquatic ecological resources of Sabah and Sarawak in turn to evaluate radiation blow on human ensuing by use of infected seafood (Yii & Zaharudin, Citation2007). The normal action of Sabah near-shore, Sarawak near-shore, Sabah Coastal and Sarawak coastal set-up was 2.36 ± .50 Bq/m3, 2.30 ± .51 Bq/m3, 2.68 ± .27 Bq/m3, and 2.36 ± .43 Bq/m3, in that order.
The 137Cs action was in series of 1.47–3.36 Bq/m3 and 1.69–3.32 Bq/m3 for Sarawak and Sabah correspondingly. 137Cs actions were resolute by measuring the peak area under the photo peak of the gamma spectrum at 661 keV found equal to gamma intensity corrected for finding competence, % of gamma-ray profusion of the radionuclide and revival of 134Cs tracer. No significant difference was seen of 137Cs actions in bottom and surface water samples at 95% confidence level. These results concluded low Cs activity in coastal waters compared to other areas saving seafood for human consumption and economy of the country.
3. Possible techniques for radiocaesium remediation
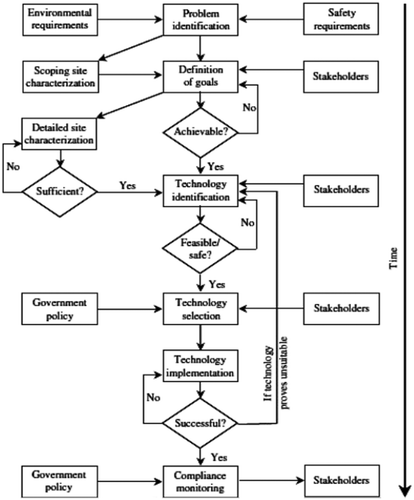
It is well known that our environment is completely spoiled with hazardous pollutants but to clean it a lot of particular technologies and big amounts of assets and times are required for this purpose (Onishi, Citation2014). After a few years of tsunami and nuclear power plant accident in Japan, development and completion of revitalization works are still continuing. While discussing about groundwater contamination in Fukushima two points are mainly focused i.e., the source and removal of pollutants. But in this plan, a lot of strategies devised by the nuclear plant operator Tokyo Electric Power Company (TEPCO), national research institutes and the Japan Atomic Energy Agency (JAEA) to get rid of contagion pathways at that site. In the whole world, few methods are most commonly useful in the clean-up of sites, even as other technologies are in pioneering process. Figure shows some idea about the pathway leading to remediate radiocaesium from the environment.
To reduce radionuclide concentrations, multilayered restraint has set many instant and long-standing goals and addresses the pollution problem at plant. By development of implemented strategies and targets are frequently reassessed and reviewed.
Some scientist applied clean-up works with four different methods to remove the source of contagion and lessen water volumes to be treated in Fukushima Di ichi nuclear power plant and reduce its flow to Pacific Ocean (Marui & Gallardo, Citation2015).
3.1. Seaside impervious wall
To prevent from radiocaesium contamination a plan involves the building of concrete and steel wall at seaside along the seashore ahead of unit 1–4 reactors. This would reach the base of aquifer to a depth of 30 m and intend to disrupt more exterior overflow and groundwater outflows to the ocean. At the same time to depressurize the top soil and to stop dam uphill formation. By installing such a design, it would be possible to collect water into tanks along with that released into the sea following treatment. These results would put forward that radionuclides transport from side to side groundwater may be reserved by the wall and it will contribute to preserve brine concentrations within enviable values. This can also be supported by the levels of 137Cs have reduced from high concentrations of several thousand Bq/L almost immediately after the mishap, to fewer than .55 Bq/L in 2015, well lower than management rank of 10 Bq/L set by the World Health Organization for drinking water (World Health Organization, Citation2011).
3.2. Landward impervious wall (frozen wall)
Previous work indicates that inflow of water to the buildings could be possible when approximately volume of 30 m3/day approaches. Ice wall is also build in region of the reactor units to stop groundwater inflows and resulting contaminates sites. A new technology works well to reduce inflow of water to buildings by placing underground pipes and using brine to circulate to keep refrigerant to them. By keeping such activities sediments will freeze creating a barrier to water flow. In the coal mines in South Wales the same methodology appeared 150 years ago and it is used in mining and civil engineering (Vitel, Rouabhi, Tijani, & Guérin, Citation2015).
3.3. Groundwater bypasses; sub drain
Another tool for further reduction in the accretion of impure water in buildings can be done by constructing underground bypass. If the system would be installed by 12 bores it can abstract normally 300 m3/day of water. After several months of discussion, an agreement was done to permit the discharge of waters with Cs amount less than 1 Bq/L. Main purpose of the plan was to release water in ocean facing burly resistance from neighbouring communities, particularly from the fishing industry which doubts an extensive pollution around the previously exaggerated areas. Though the system helps to improve the pressure for waste storage space and constitutes pace ahead to deal with contagion for its basis.
3.4. Biological remediation method
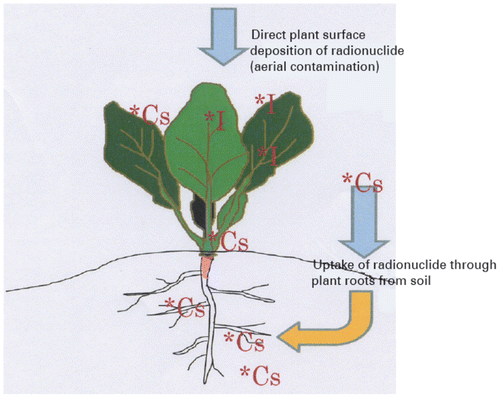
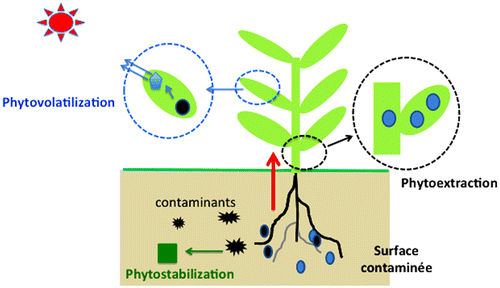
Higher plants and micro-organisms have ability to decrease the mobility of radiocaesium in soil and sediments but cannot directly detached or degrade them like other certain organic compounds. Many factors like cleanup goals, soil properties, depth of contagion, height of radionuclide contagion, removal of harvested biomass, the presence of hazardous materials, and climatic situation all persuade the probability of achievement. The site specific conditions are strongly favoured to the possible flourishing application of phytostabilization and phytoextraction strategies to infected soils. Both phytostabilization (the use of plants to reduce the off-site fatalities of contaminants through erosion and leaching losses) and phytoextraction (contaminants concentration into harvestable portions of plant biomass) includes in phytoremediation. Figure describes how plant can be contaminated by radiocaesium.
3.5. Facings
Starting on the topographic highs Facings are being implemented over 1.45 km2 behind the reactors. This technique predicted that such measures on your own could lessen groundwater inflows into the reactors by upto 110 m3/day. Facings effects would be seen after two to three years finishing point (METI Ministry of Economy of Japan, Citation2013).
3.6. Phytoremediation
A promising, ecological gainful and in situ alternative action technology supposed to be Phytoremediation it relies on the plant capacity and linked micro-organisms to extract radiocaesium contaminants from marine ecosystems (IAEA, Citation2001). The Lemna gibba aquatic plant was utilized to absorb 137Cs from simulated water radioactive dissipate. The uptakes values for Co-60 and Cs-137 recorded values of 1,213 and 872 Bq/g, respectively, from the simulated waste solution containing 6,100 Bq at pH 6.9 after 24 h of contact time (Jasiulionis & Rozkov, Citation2007). The uptakes values for Cs-137 recorded 872 Bq/g from the simulated waste solution containing 6,100 Bq at pH 6.9 after 24 h of contact time. Lemma gibba an aquatic plant showed high efficiently in bioremoving and bioaccumulating 137Cs.
3.7. Phytoextraction and phytostabilization
Recent studies at Oak Ridge National Laboratory propose that woodland flora could be a key source for preservation of radionuclides at contaminated places. Planting and amending soil and phytostabilization of buffer zones can increase the availability of 137Cs for phytoextraction through methods of may be integrated into field designs to reduce losses by leach and erosion of 137Cs subsequent plant yield. Hence, phytostabilization can significantly contribute to environmental management through processes of wind and water erosion at polluted sites by serving to reduce the off-site relocation of particle-reactive radionuclides as shown in Figure (Japanese Ministry of Education Culture Sports Science and Technology Citation2011b). Phytoextraction termed like natural uptake of metals by plants for partial removal of radiocaesium from soils. However, this technique has mostly been applied to reduce the contamination from radiocaesium from soil (Japanese Ministry of Education Culture Sports Science and Technology Citation2011a) as a result of preventing or reducing soil erosion 137Cs that tend to sorbs the soil strongly and can greatly reduce the migration of radionuclides. Such a technique termed as Phytostabilization. Several potential constraints may limit the use of phytoextraction at DOE sites.
3.8. Wetlands
Another useful technique for removal of radiocaesium is the development of wetlands. The uptake of radiocaesium contaminants in developing plants accounts for 5% of total elimination capability in wetlands. In support of this method aquatic plants are more helpful. Their major role is to boost water residence time and enlarge sedimentation of contaminated particles and also supply oxygen to roots to provide positive conditions for microbes. For bioremediation of radiocaesium contaminants, a high volume of water for waterlogged plants is required.
3.9. Biological dilution
The biological dilution method is based on theories connecting organic buffering. Biological dilution by adding different types of fertilizers can reduce the amount of bioavailable radiocaesium (137Cs) in individual fish in lakes (discharge from aqua culture, p-enriched lime and commercial fertilizer). If fish stocks are reduced in the lakes ultimately radiocaesium already uptake by the nutrient web can be removed and influencing the predation pressure to alter the nutrient web to enclose relatively more plankton, that could result in lesser concentrations of 137Cs in the biota (Morino, Ohara, & Nishizawa, Citation2011).
3.10. Microbiological effect
The main purpose of micro-organisms in biological techniques is to breakdown harmful material in the soil to less hazardous forms. Such micro-organisms can be more effective to metal but not fruitful to radioactive particles. However, many researchers found that cysteine is an effective agent for enhancing the discharge of some metals from soil. Pseudomonas putida (soil bacterium) has been used to find out caesium adsorption and other elements and their pressure on the movement of metals in soils (Bossew, Lettner, Hubmer, Erlinger, & Gastberger, Citation2007). The investigators suggest that adsorption as a function of pH and ionic strength for low metal concentrations. Caesium is shown to adsorption exhibited at the bacteria (a Kd of ~ 102–103); however, for other metals adsorption was much higher (like the Kd for mercury adsorption was on the order of 106) (IAEA, Citation2000a). Studies have also investigated that under the influence of active micro-organisms depending on the properties of the soil, metals turn out to be more mobile (Ministry of Land Infrastructure Transport & Tourism, Citation2011).
4. Chemical remediation methods for radiocaesium
Up till now, a small number of chemical remediation techniques are accessible for 137Cs contamination. Few of them are discussed as:
4.1. Application of uranyl citrate complex
Besides, studied the photochemical behaviour of uranyl ion in the presence of organic acids which undergoes oxidation reduction reactions on disclosure to visible light (Tyler, Cartier, Davidson, Long, & Tipping, Citation2001). This mixture with polluted soil treated primarily with bacteria that break down the complex to carbon dioxide and water. Such approach would also aid in 137Cs removal from impure soil and sediments.
4.2. Chemical fertilizers
Harmful special effects of 137Cs on plant accumulation and efficacy of phytoextraction of chemical amendments in the shape of fertilization have been noticed in earlier studies. The best options to minimize 137Cs radioactive in local food chain is the adaptation of land use management and agricultural water management practices with nitrogen and potassium fertilization techniques (Ministry of Education, Culture, Sports, Science, and Technology [MEXT], Citation2011). Many economically applicable methods are present to remediate lakes impure by 137Cs, and they include potash treatment, liming and fertilization of low production lakes. Large amounts of potassium fertilization results in a considerable decrease in 137Cs uptake (Hirose, Citation2012).
4.2.1. Ammonium addition
Many field testing linked with study point out that plants have high biomass and uptake production could be used to remediate a 137Cs-contaminated place. It is generally observed that ammonium-based fertilizers addition increases 137Cs bioavailability and has the potential to enhance the plant uptake of soil 137Cs.
4.2.2. Liming
The regional authorities and National Environmental Protection Agency in Sweden applied a simple “rule-of-the-thumb” system, whereby lakes are usually limed to just about 6.4–6.5 (Bowyer et al., Citation2011). The “normal” choice in mean annual pH in tiny glacial lakes varies from around 5.5 to approximately 7.2, so 6.4. To enhance the proportion of 137Cs in the sediments of a lake bottom, which prevents 137Cs biouptake, lake liming has been performed by many researchers. This is totally based on the hypothesis that the tendency of flocculation 137Cs transport particles is enhanced by increases in the alkalinity and pH of lake water.
4.2.3. Plant growth using fertilizers
A studied the farms lands for crops and agriculture. He found that soil was severely contaminated by direct deposition of radionuclides from FNPP accident Komamura, Tsumura, Yamaguchi, Kihou, & Kodaira, Citation2005). Plant uptake capacity was much higher than 5,000 Bq/kg soil. Due to these facts rice growing and other agriculture were restricted in the area. In order to solve this issue, the application of potassium fertilizers and the addition of adsorbents seen effective for inhibiting any further uptake of radioactive caesium by plants.
4.2.4. Removal of radiocaesium from liquid wastes
For radiocaesium sorption from liquid waste material geared up potassium nickel hexacyanoferrate-granular activated carbon (KNiHCF-GAC) (Dashtinejad, Samadfam, Fasihi, Grayeli Fumeshkenar, & Sepehrian, Citation2014; Kobayashi et al., Citation2013) was utilized. Maximum adsorption capacity of KNiHCF-GAC to 163.9 mg/g was reported. In some studies, modification is based on the precipitation and impregnation technique. Apart from this method these days many carbon nanotudes and carbonaceous material have been applied to sorbs radiocaesium from liquid wastes. These techniques fit well to reduce contamination and pollutants from Fukushima derived radiocaesium.
4.2.5. Removal of radiocaesium from soil by desorption
Desorption kinetics of radiocaesium in the soil after Fukushima NPP accident (Murota, Saito, & Tanaka, Citation2016; Stohl et al., Citation2012; Tyler et al., Citation2001; Wang et al., Citation2000). They found that radiocaesium gradually changed to stable form in the soil. Using three site desorption model for determining desorption half-life of radiocaesium, found it to be about two years even from slowest sorption site. In this study, long-term sorption technique was adopted using batch sorption ion exchange resin as sorbent. Main function of sorbent was to keep the radiocaesium in aqueous phase preventing it from resorption of desorbed Cs+. It was observed that after 139 d in dilute KCl media, up to 60% of 137Cs was desorbed it was also found to be larger than the desorption by conventional short-term extraction with 1 M ammonium acetate.
5. Conclusion
After the world-wide spread of radionuclides, many dump sites we found contaminated with these hazardous particles. North Pacific Ocean found mostly influenced by 2011 disasters. Many islands in Japan found rich with radionuclides which not only deteriorate the water quality, human health, and living biota but induce long-term negative life threatening impacts. Activity concentrations and bomb-derived concentrations for 137Cs prove to be of great importance in order to evaluate total concentration in oceans, islands, rivers etc. Many studies have been discussed on the determination of radionuclides in different organisms which show considerable amounts in different organs. Different physical, chemical, and biological remediation methods reveal the reduction of radiocaesium up to a certain limit. Due to long half-life, even after so many years of natural disaster and nuclear accidents still there is a need to discover new methods and technologies that works better to eliminate these harmful particles from our environment.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aoyama, M., Tsumune, D., & Hamajima, Y. (2012). Distribution of 137Cs and 134Cs in the North Pacific Ocean: Impacts of the TEPCO Fukushima-Daiichi NPP accident. Journal of Radioanalytical and Nuclear Chemistry, 296, 535–539.
- Aoyama, M., Uematsu, M., Tsumune, D., & Hamajima, Y. (2013). Surface pathway of radioactive plume of TEPCO Fukushima NPP1 released 134Cs and 137Cs. Biogeosciences, 10, 3067–3078.10.5194/bg-10-3067-2013
- Bailly du Bois, P., Laguionie, P., Boust, D., Korsakissok, I., Didier, D., & Fiévet, B. (2012). Estimation of marine source-term following Fukushima Dai-ichi accident. Journal of Environmental Radioactivity, 114, 2–9.10.1016/j.jenvrad.2011.11.015
- Bossew, P., Lettner, H., Hubmer, A., Erlinger, C., & Gastberger, M. (2007). Activity ratios of 137Cs, 90Sr and 239? 240Pu in environmental samples. Journal of Environmental Radioactivity, 97, 5–19.10.1016/j.jenvrad.2007.02.008
- Bowyer, T.W., Biegalski, S.R., Cooper, M., Eslinger, P.W., Haas, D., & Hayes, J.C. (2011). Elevated radioxenon detected remotely following the Fukushima nuclear accident. Journal of Environmental Radioactivity, 102, 681–687.10.1016/j.jenvrad.2011.04.009
- Buesseler, K.O., Jayne, S.R., Fisher, N.S., Rypina, I.I., Baumann, H., Baumann, Z., … Yoshida, S. (2012). Fukushima-derived radionuclides in the ocean and biota off Japan. Proceedings of the National Academy of Sciences, 109, 5984–5988.10.1073/pnas.1120794109
- Charette, M.A., Breier, C.F., Henderson, P.B., Pike, S.M., Rypina, I.I., Jayne, S.R., & Buesseler, K.O. (2013). Radium-based estimates of cesium isotope transport and total direct ocean discharges from the Fukushima nuclear power plant accident. Biogeosciences, 10, 2159–2167.10.5194/bg-10-2159-2013
- Dashtinejad, M., Samadfam, M., Fasihi, J., Grayeli Fumeshkenar, F., & Sepehrian, H. (2014). Synthesis, characterization, and cesium sorption performance of potassium nickel hexacyanoferrate-loaded granular activated carbon. Particulate Science and Technology, 32, 348–354. doi:10.1080/02726351.2013.873505
- Dietze, H., & Kriest, I. (2012). 137Cs off Fukushima Dai-ichi, Japan: Model based estimates of dilution and fate. Ocean Science, 8, 319–332.10.5194/os-8-319-2012
- Hirose, K. (2012). 2011 Fukushima Dai-ichi nuclear power plant accident: Summary of regional radioactive deposition monitoring results. Journal of Environmental Radioactivity, 111, 13–17.10.1016/j.jenvrad.2011.09.003
- Inoue, M., Kofuji, H., Hamajima, Y., Nagao, S., Yoshida, K., & Yamamoto, M. (2012). 134Cs and 137Cs activities in coastal seawater along Northern Sanriku and Tsugaru Strait, northeastern Japan, after Fukushima Dai-ichi nuclear power plant accident. Journal of Environmental Radioactivity, 111, 116–119.10.1016/j.jenvrad.2011.09.012
- International Atomic Energy Agency. (2000). Report of the specialists’ meeting on environmental protection from the effects of ionizing radiation: International perspectives (Ref 723-J9-SP-1114.2). Vienna, Austria: Author.
- International Atomic Energy Agency (2001). Report of the second FAO, the classification of soil systems on the basis of transfer factors of radionuclide from soil to reference plants. Vienna: Author.
- International Atomic Energy Agency. (2004). Sediments distribution coefficients and concentration factors for biota in the marine environment (p. 18, Technical Reports Series No. 422). Vienna, Austria: Author.
- Japanese Ministry of Education Culture Sports Science and Technology. (2011a). Readings of environmental radioactivity level by prefecture. Tokyo: MEXT.
- Japanese Ministry of Education Culture Sports Science and Technology. (2011b). Readings of sea area monitoring. Tokyo: MEXT.
- Jasiulionis, R., & Rozkov, A. (2007). 137 Cs activity concentration in the ground-level air in the Ignalina NPP region. Lithuanian Journal of Physics, 47, 195–202.10.3952/lithjphys.47207
- Kaeriyama, H., Shimizu, Y., Ambe, D., Masujima, M., Shigenobu, Y., Fujimoto, K., … Watanabe, T. (2014). Southwest intrusion of 134 Cs and 137 Cs derived from the Fukushima Dai-ichi nuclear power plant accident in the western North Pacific. Environmental Science & Technology, 48, 3120–3127.10.1021/es403686v
- Kameník, J., Dulaiova, H., Buesseler, K.O., Pike, S.M., & Stastn, K. (2013). Cesium-134 and 137 activities in the central North Pacific Ocean after the Fukushima Daiichi nuclear power plant accident. Bio geosciences, 10, 6045–6052.
- Kawai, H., Kitamura, A., Mimura, M., Mimura, T., Tahara, T., Aida, D., … Sasaki, H. (2013). Radioactive cesium accumulation in seaweeds by the Fukushima 1 nuclear power plant accident two years’ monitoring at Iwaki and its vicinity. Journal of Plant Research, 127, 23–42.
- Kobayashi, T., Nagai, H., Chino, M., & Kawamura, H. (2013). Source term estimation of atmospheric release due to the Fukushima Dai-ichi nuclear power plant accident by atmospheric and oceanic dispersion simulations. Journal of Nuclear Science and Technology, 50, 255–264.10.1080/00223131.2013.772449
- Kofuji, H., & Inoue, M. (2013). Temporal variations in 134Cs and 137Cs concentrations in seawater along the Shimokita Peninsula and the northern Sanriku coast in northeastern Japan, one year after the Fukushima Dai-ichi nuclear power plant accident. Journal of Environmental Radioactivity, 124, 239–245.10.1016/j.jenvrad.2013.06.003
- Komamura, K., Tsumura, A., Yamaguchi, N., Kihou, N., & Kodaira, K. (2005). Monitoring 90Sr and 137Cs in rice, wheat, and soil in Japan from 1959 to 2000. Miscellaneous Publication of National Institute for Agro-Environmental Sciences, 28, 1–56.
- Kumamoto, Y., Aoyama, M., Hamajima, Y., Murata, A., & Kawano, T. (2015). Impact of Fukushima-derived radiocesium in the western North Pacific Ocean about ten months after the Fukushima Dai-ichi nuclear power plant accident. Journal of Environmental Radioactivity, 140, 114–122.10.1016/j.jenvrad.2014.11.010
- Marui, A., & Gallardo, A.H. (2015). Managing groundwater radioactive contamination at the Daiichi nuclear plant. International Journal of Environmental Research and Public Health, 12, 8498–8503.10.3390/ijerph120708498
- Men, W., He, J., Wang, F., Wen, Y., Li, Y., Huang, J., & Yu, X. (2015). Radioactive status of seawater in the northwest Pacific more than one year after the Fukushima nuclear accident. Scientific Reports, 5, 6. doi:10.1038/srep07757
- Metian, M., Pouil, S., Hédouin, L., Oberhänsli, F., Teyssié, J.L., Bustamante, P.L., & Warnau, M. (2016). Differential bioaccumulation of 134Cs in tropical marine organisms and the relative importance of exposure pathways. Journal of Environmental Radioactivity, 152, 127–135.10.1016/j.jenvrad.2015.11.012
- METI Ministry of Economy of Japan. (2013). Preventative and multilayered measures for contaminated water treatment at the Fukushima Daiichi Nuclear Power Station of Tokyo Electric Power Company. Retrieved June 15, 2015, from http://www.meti.go.jp/english
- Ministry of Education, Culture, Sports, Science, and Technology (MEXT). (2011). Readings of sea area monitoring. Retrieved from http://www.mext.go.jp/english/incident/1304192.htm
- Ministry of Land Infrastructure Transport and Tourism. (2011). Soil map of Japan. Retrieved from http://tochi.mlit.go.jp/tockok/tochimizu/F3/ZOOMA/0719/index.html
- Miyazawa, Y., Masumoto, Y., Varlamov, S.M., Miyama, T., Takigawa, M., Honda, M., & Saino, T. (2013). Inverse estimation of source parameters of oceanic radioactivity dispersion models associated with the Fukushima accident. Biogeosciences, 10, 2349–2363.10.5194/bg-10-2349-2013
- Morino, Y., Ohara, T., & Nishizawa, M. (2011). Atmospheric behavior, deposition, and budget of radioactive materials from the Fukushima Daiichi nuclear power plant in March 2011. Geophysical Research Letters, 38, L00G11.
- Morino, Y., Ohara, T., Watanabe, M., Hayashi, S., & Nishizawa, M. (2013). Episode analysis of deposition of radiocesium from the Fukushima Daiichi nuclear power plant accident. Environmental Science & Technology, 47, 2314–2322.
- Murota, K., Saito, T., & Tanaka, S. (2016). Desorption kinetics of cesium from Fukushima soils. Journal of Environmental Radioactivity, 153, 134–140.10.1016/j.jenvrad.2015.12.013
- Nair, R.N., Sunny, F., Chopra, M., Sharma, L.K., Puranik, V.D., & Ghosh, A.K. (2014). Estimation of radioactive leakages into the Pacific Ocean due to Fukushima nuclear accident. Environmental Earth Sciences, 71, 1007–1019.10.1007/s12665-013-2501-1
- Nuclear Regulation Authority. (2011). Monitoring information of environmental radioactivity level_readings of sea area monitoring. Retrieved July 1, 2014, from http://radioactivity.nsr.go.%20jp/en/list/205/list-1.html
- Oikawa, S., Takata, H., Watabe, T., Misonoo, J., & Kusakabe, M. (2013). Distribution of the Fukushima-derived radionuclides in seawater in the Pacific off the coast of Miyagi, Fukushima, and Ibaraki Prefectures, Japan. Biogeosciences, 10, 5031–5047.10.5194/bg-10-5031-2013
- Onishi, Y. (2014). Fukushima and Chernobyl nuclear accidents’ environmental assessments and U.S. Hanford Site’s waste management. Procedia IUTAM, 10, 372–381.10.1016/j.piutam.2014.01.032
- Povinec, P.P., Gera, M., Holý, K., Hirose, K., Lujaniené, G., Nakano, M., … Gažák, M. (2013). Dispersion of Fukushima radionuclides in the global atmosphere and the ocean. Applied Radiation and Isotopes, 81, 383–392.10.1016/j.apradiso.2013.03.058
- Prime Minister of Japan and His Cabinet. (2011). Report of Japanese Government to the IAEA Ministerial Conference on Nuclear Safety e the Accident at TEPCO’s Fukushima Nuclear Power Stationse. Retrieved July 1, 2014, from http://www.kantei.go.jp/foreign/kan/topics/201106/iaea_houkokushoe.html
- Ramzaev, V., Nikitin, A., Sevastyanov, A., Artemiev, G., Bruk, G., & Ivanov, S. (2014). Shipboard determination of radiocesium in seawater after the Fukushima accident: Results from the 2011–2012 Russian expeditions to the Sea of Japan and western North Pacific Ocean. Journal of Environmental Radioactivity, 135, 13–24.10.1016/j.jenvrad.2014.03.016
- Stohl, A., Seibert, P., Wotawa, G., Arnold, D., Burkhart, J.F., Eckhardt, S., … Yasunari, T.J. (2012). Xenon-133 and caesium-137 releases into the atmosphere from the Fukushima Dai-ichi nuclear power plant: Determination of the source term, atmospheric dispersion, and deposition. Atmospheric Chemistry and Physics, 12, 2313–2343.10.5194/acp-12-2313-2012
- Tokyo Electric Power Company. (2011). Press releases. Retrieved July 1, 2014, from http://www.tepco.co.jp/en/%20press/corp-com/release/index1103-e.html
- Tsumune, D., Tsubono, T., Aoyama, M., & Hirose, K. (2012). Distribution of oceanic 137Cs from the Fukushima Dai-ichi nuclear power plant simulated numerically by a regional ocean model. Journal of Environmental Radioactivity, 111, 100–108.10.1016/j.jenvrad.2011.10.007
- Tyler, A.N., Cartier, S., Davidson, D.A., Long, D.J., & Tipping, R. (2001). The extent and significance of bioturbation on Cs-137 distribution in upland soils Catena. Biological and Environmental Sciences Journal Articles, 43, 81–99.
- Uematsu, S., Smolders, E., Sweeck, L., Wannijn, J., Van Hees, M., & Vandenhove, H. (2015). Predicting radiocaesium sorption characteristics with soil chemical properties for Japanese soils. Science of The Total Environment, 524–525, 148–156.10.1016/j.scitotenv.2015.04.028
- Vitel, M., Rouabhi, A., Tijani, M., & Guérin, F. (2015). Modeling heat transfer between a freeze pipe and the surrounding ground during artificial ground freezing activities. Computers and Geotechnics, 63, 99–111.10.1016/j.compgeo.2014.08.004
- Wang, W.X., Ke, C., Yu, K.N., & Lam, P.K. (2000). Modeling radiocesium bioaccumulation in a marine food chain. Marine Ecology Progress Series, 208, 41–50.
- World Health Organization. (2011). Guidelines for drinking water-quality (4th ed.). Geneva: WHO Press.
- Yii, M.W., & Zaharudin, A. (2007). Concentration of 137Cs in seawater surrounding East Malaysia. Journal of Radioanalytical and Nuclear Chemistry, 274, 323–329.10.1007/s10967-007-1118-9
- Yoshida, N., & Kanda, J. (2012). Tracking the Fukushima radionuclides. Science, 336, 1115–1116.10.1126/science.1219493
- Yu, W., He, J., Lin, W., Li, Y., Men, W., Wang, F., & Huang, J. (2015). Distribution and risk assessment of radionuclides released by Fukushima nuclear accident at the northwest Pacific. Journal of Environmental Radioactivity, 142, 54–61.10.1016/j.jenvrad.2015.01.005