Abstract
Contamination of lead indicates one of the major threats to soil system. Phytoremediation technique utilized plants which are able to tolerate and accumulate metals within in their tissues. It has recently been suggested that biofuel plants are more suitable for both utilization and remediation of metal contaminated soil. This study reported Pb phytoremediation potential of Cyamopsis tetragonoloba L. in comparison with Sesamum indicum L. in the framework of a pot-experiment. Plants were subjected to seven Pb concentrations (0, 100, 200, 400, 600, 800 and 1000 mg kg-1 soil) for 12 weeks. Our results demonstrated that both C. tetragonoloba and S. indicum were able to tolerate Pb concentrations up to 1000 mg kg-1 which confirms the plant ability to grow well in higher Pb levels. Significant metal accumulation was observed in root along with reduced biomass for both plants species. Furthermore, both plant species could possibly be used for phytostabilization, with success in marginally polluted soils where their growth would not be impaired and decontamination of Pb could be maintained at satisfying levels. However, bioconcentration factor (BCF), bioaccumulation coefficient (BAC) and translocation factor (TF) values proposed that C. tetragonoloba was more efficient for phytoremediation than S. indicum at higher Pb levels.
1. Introduction
Soil pollution with toxic trace metals and their accumulation in soil is of great concern in agricultural production owing to the adverse effects on crop growth i.e., metal phytotoxicity, and soil micro-organisms (Nagajyoti, Lee, & Sreekanth, Citation2010). Toxic trace metals have entered into agricultural soils primarily because of rapid industrialization, inappropriate utilization and disposal of toxic trace metal containing wastes, excessive use of fertilizers and pesticides (Amel et al., Citation2016; Bashmakov, Lukatkin, Anjum, Ahmad, & Pereira, Citation2015), which become hazardous to human and environmental health. Trace metals, such as lead (Pb), cadmium (Cd), nickel (Ni), cobalt (Co), iron (Fe), zinc (Zn), chromium (Cr), iron (Fe) (Nagajyoti et al., Citation2010), are major environmental pollutants, particularly in areas with high anthropogenic activities.
Among soil pollutant, lead (Pb) is one of the toxic metal pollutants (Shahid, Pinelli, Pourrut, Silvestre, & Dumat, Citation2011); widely used in many industrial processes and occurs as a contaminant in all environmental compartments including soil, water, and living organisms (Punamiya et al., Citation2010); known to induce a broad range of toxic effects to morphological, physiological, and biochemical activities of living organisms. Lead impairs plant growth such as root elongation, seed germination, seedling development, transpiration, chlorophyll production, lamellar organization in the chloroplast, and cell division (Gupta, Huang, Yang, Razafindrabe, & Inouhe, Citation2010; Maestri, Marmiroli, Visioli, & Marmiroli, Citation2010). However, the extent of these effects varies and depends on the lead concentrations, the duration of exposure, the intensity of plant stress, and the particular organs studied.
Lead occurs naturally in the earth’s crust (Arias et al., Citation2010) and its natural levels remain below 50 mg kg−1 (Pais & Jones, Citation2000), but anthropogenic activities often modify the amount and nature of lead species present in soil. Anthropogenic sourced lead (Pb) include lead-based paints, lead arsenate pesticide application, gasoline, coal burning, explosives, lead batteries and from the disposal of municipal sewage sludge (Tian, Lu, Yang, et al., Citation2010; Zheng, Liu, Lütz-Meindl, & Peer, Citation2011).
In soils, lead may occur as a free metal ion, complexed with inorganic constituents (e.g., HCO3−, CO32−, SO42−, and Cl−), may exist as organic ligands (e.g., amino acids, fulvic acids, and humic acids); or may be adsorbed on to particle surfaces (e.g., Fe-oxides, biological material, organic matter, and clay particles) (Uzu, Sobanska, Aliouane, Pradere, & Dumat, Citation2009; Vega, Andrade, & Covelo, Citation2010). Generally, lead may accumulates on the surface layer of soil (Cecchi et al., Citation2008) and caused disturbance of soil system. Thus, in order to reduce the level of lead from the natural environment many developed countries has tried to reduce lead emissions using lead-free fuels (Adriano, Citation2001).
Remediation of lead-polluted soils by traditional physical and chemical methods are not suitable for agricultural lands (Oh, Li, Cheng, Xie, & Yonemochi, Citation2013), required large investments and technological resources. Phytoremediation is a promising technique to remediate metal-contaminated soils because it offers in situ advantages, cost effective, and less energy-intensive than high-cost traditional clean-up methods. Phytoremediation involves the use of plants to reclaim high amount of metals from soil into the harvestable parts (i.e., underground roots and aboveground shoots) (Mahmood, Citation2010). The harvested biomass then can be safely processed by drying, ashing, composting, and storage at a landfill, anaerobic digestion, pure plant oil production, microbial, physical, or other chemical means (Ginneken et al., Citation2007).
The capacities of heavy metal uptake and accumulation, mechanisms of metal concentration, exclusion and compartmentation vary among different plant species and also between various parts of plants (Lone, He, Stoffella, & Xe, Citation2008; Sharma, Singh, & Manchanda, Citation2014). Various plant species belongs to botanical families, in particular the Brassicaceae, Asteraceae, Fabaceae, Poaceae, and Chenopodiaceae showing phytoremediation potential, are well documented in the literature (Stanislaw & Gawronski, Citation2007; Anjum, Umar, & Iqbal, Citation2014) but few or less work has been reported on Cyamopsis tetragonoloba L. and Sesamum indicum L.
Guar (Cyamopsis tetragonoloba L.) is one of the important annual legume crops belongs to family Fabaceae. The endosperm of guar seeds contain gum, which is gaining importance as non-food item (Ashraf, Akhtar, Sarwar, & Ashraf, Citation2002). Recently, the oil industry has started using guar gum in hydraulic fracturing in which high pressure is used to crack rock. The increased use of guar gum in oil fracking has boosted the demand of guar woldwide (Abidi et al., Citation2015; Deepak, Sheweta, & Bhupendar, Citation2014). A key feature of legumes as a resource for phytoremediation is their role in providing additional N-compounds to the soil, thus improving soil fertility and ability to support biological growth (Xiuli et al., Citation2013; Hao et al., Citation2014). On the other hand, sesame (Sesamum indicum L.) is an economically important oil seed crop of family Pedaliaceae, planted in arid and semi-arid regions of the world (Elleuch, Besbes, Roiseux, Blecker, & Attia, Citation2007) including Pakistan, used as an alternative feedstock for the production of a biodiesel fuel (Saydut, Duz, Kaya, Kafadar, & Hamamci, Citation2008). The methyl ester extracted from sesame seeds can effectively be utilized as petrodiesel (Ahmad et al., Citation2011).
This study was set up to investigate a plant species that could tolerate high toxicity of Pb treatments in soil. Both the plant species i.e., C. tetragonoloba and S. indicum have fast growth and development rate also high above-ground biomass. It was estimated that plant species with high tolerance could then be utilized for phytoremediation. Considering above facts, the objectives of this research were: (1) to study the phytotoxicity of lead on plant growth parameters; (2) to determine the accumulation and distribution of lead in plant parts growing on a lead-polluted soil, and (3) to identify a potential candidate for effective phytoremediation practice.
2. Material and methods
2.1. Seeds procurement and sterilization
Seeds of the guar variety BR-99, from the Institute of Fodder Research Program and sesame variety TH-6, were collected from the Institute of Oilseeds Research Program, National Agricultural Research Centre (NARC), Islamabad (Table ). In order to avoid any microbial contamination, seeds were surface sterilized with 0.1% HgCl2 for 10 min and washed 7 times with sterilized water (Pourakbar, Khayami, Khara, & Farbidina, Citation2007).
Table 1. Plant species selected for pot experiment.
2.2. Experimental soil collection
Soil samples were collected from Pb-free agricultural fields located in Jamshoro, Sindh, Pakistan, at depth of 0–15 cm using hand shovel. Equidistant (2 m) collected samples were homogenized to prepare one bulk sample. For the greenhouse experiment, soil was air dried at room temperature for 15 days and ground to a final particle size of 2 mm. To enhance soil porosity, sand was mixed in 3:1 proportion with soil samples soil.
2.3. Soil samples measurements
Soil pH was measured with a pH-meter (InoLab-WTB GmbH; Weilheim, Germany) using glass electrode at the 1:2 (w/v) ratio of soil to water suspension (Rachit, Verma, Meena, Yashveer, & Shreya, Citation2016). The electrical conductivity (EC) was measured with an electrical conductivity meter (WTW – 330i) at the 1:2 (w/v) ratio of soil to water suspension (Rachit et al., Citation2016). Organic matter (OM) and organic carbon (OC) (%) were measured according to Walkley and Black (chromic acid titration) method (Fanrong et al., Citation2011). The properties of the soil are shown in Table .
Table 2. The properties of experimental soil.
2.4. Preliminary screening for Pb
In order to select the Pb concentrations for treatment, various doses of Pb(NO3)2 (0, 50, 100, 200, 500, 700, 1000, 1500, and 2000 mg kg−1) were tried in the preliminary screening of the C. tetragonoloba and S. indicum for 20 days. Based on the Pb toxicity symptoms and morphological growth of the seedlings, the following doses (0, 100, 200, 400, 600, 800, 1000 mg kg−1) were finally selected (Table ).
Table 3. Lead treatment levels given in the experiment.
2.5. Pot experiment
Plastic pots were filled with 5 kg sieved soil, after which soil was artificially spiked with Pb (aqueous solution) using Pb(NO3)2 salt to each pot in increasing concentrations (100, 200, 400, 600, 800, 1000 mg kg−1), each with three replicates and kept for 2 weeks to attain equilibrium. The clean soil without Pb spiking was used as control. Pots were arranged in a completely randomized design. After 15 days of equilibration, 20 surface-sterilized seeds were sown per pot. One week after seed germination, plants were thinned to 5 per pot. A plastic tray was kept below the treatment pot to collect any leachate, which was returned to the pots at next watering. The experiment was conducted in a greenhouse for 3 months. Any symptoms of metal toxicity exhibited by plants were visually noted during the experimental period. Plants were harvested 12 weeks after germination. Soil samples (in triplicate) were also collected for analysis of Pb content by an Atomic Absorption Spectrophotometer (Perkin-Elmer, AAnalyst 800). Plant growth and biochemical parameters were also measured.
2.6. Germination percentage (%)
The germination percentage, expressed as percentage of germinated seeds to the total number of viable seeds, calculated by the following equation: (Talebi, Nabavi, & Sohani, Citation2014)(1)
2.7. Morphological parameters
Plants were taken from each replicate pot to measure morphological parameters. Root length, shoot length were measured with the help of scale. Root and shoot fresh weights were also measured with the help of analytical balance. Plant samples were air dried for one week. Then, oven-dried at 80 °C to a constant weight and dry weights were recorded.
2.8. Estimation of photosynthetic pigments
Photosynthetic pigments, in fully expanded leaves from each treatment, were extracted using 0.5 g of fresh material, ground with 10 mL of 80% aqueous acetone. After filtering, 1 mL of the suspension was diluted with a further 2 mL of acetone, and optical density were determined with a UV–visible spectrophotometer (Biochrom Libra S22), using two wavelengths (663 and 645 nm) against blank. Chlorophyll a (Chl a), chlorophyll b (Chl b) and total chlorophyll contents (mg g−1 f.w) were obtained by calculation, following the method of Arnon (Citation1949).(2)
(3)
(4)
2.9. Determination of tolerance index
Tolerance Index (TI) is expressed as the ratio between the growth parameters (root/shoot length, root/shoot fresh, and dry weight) of the plants in contaminated soil in relation to the growth parameters of plants from non-polluted soil calculated according to Chen et al., Citation2011.(5)
2.10. Quality control and quality assurance
All the glassware used during the present experimentation was of high quality, acid resistant Pyrex glass. The analytical grade reagents with a certified purity of 99% and stock metal standard solution (1000 ppm) for AAS analysis were procured from E. Merck (Germany). Working standards were prepared by appropriate dilutions of stock standard solutions with double distilled water.
2.11. Plant samples preparation and Pb determination
To determine Pb accumulation in different plant tissues (i.e., root, stem, leaf, and pod), harvested plant parts were rinsed thoroughly by means of tap water, and after that with deionized (DI) water to clean adhered components of soil, then oven-dried at 80 °C till steady weight. The oven-dried plant tissues were ground carefully using an electric grinder and passed through a 1.0 mm mesh strainer. The ground plant tissue samples (0.5 g) were digested with 12 mL of 3:1 (v/v) HNO3/HClO4 mixtures on a hot plate for 2 h. After cooling, the digested solution was filtered through Whatman’s filter paper and finally makes up the volume up to mark 50 mL by adding deionized (DI) water.
The quantification of lead (Pb) in respective tissues was carried out using atomic absorption spectrometer (Perkin-Elmer, AAnalyst 800) equipped with a lead cathode lamp, under optimum analytical conditions for the estimation of lead. The optimum conditions for AAS used throughout these studies given in Table . The standard calibration method was adopted for the quantification of results and triplicate samples were run to ensure the precision of quantitative results. The Pb concentration and accumulation in plant root and shoot were calculated by the following formula: (Monni, Salemaa, & Millar, Citation2000)(6)
(7)
Table 4. Measurement conditions of AAS for lead (Pb) determination.
2.12. Soil sample preparation and Pb determination
Soil samples were air dried at room temperature, ground, mixed well, and kept in plastic (polyethylene) sealed lock bags used for subsequent metal analysis. Digestions of soil samples were done using aqua regia method. To quantify the Pb content in soil, sample of 1 g soil was digested by means of wet acid digestion method through HNO3 along with HCl in proportion of 3:1 (v/v) and heated on a hot plate for 2 h until the solution becomes clear. After cooling, the volume was completed to 50 mL by adding distilled water. The solution was filtered through Whatman’s filter paper and consequently, examine for Pb contents with Atomic Absorption Spectrophotometer.
2.13. Evaluation of phytoremediation efficiency
To evaluate the phytoextraction/phytostabilization potential of C. tetragonoloba and S. indicum, the following factors were calculated:
2.13.1. Bioconcentration factor (BCF)
The bioconcentration factor (BCF) was represented as the Pb concentration ratio in plant roots to soil, calculated as follow:(8)
2.13.2. Bioaccumulation coefficient (BAC)
The bioaccumulation coefficient (BAC) was expressed as a ratio of Pb in shoot to that in soil, calculated as follow:(9)
2.13.3. Translocation factor (TF)
The translocation factor (TF) was determined as a ratio of heavy metals in plant shoot to that in plant root, calculated as follow:(10)
2.14. Statistical analysis
All experiments were conducted with three replicates and the data collected were analyzed statistically using PASW® Statistics 18 (SPSS Inc., Chicago, IL, U.S.A.). To compare the means of the treatments, analysis of variance (ANOVA) was performed followed by Duncan’s multiple range Post Hoc tests at significance level of p < 0.05, to observe significance difference among means.
3. Results and discussion
3.1. Soil characterization
The soil was sandy loam with slightly acidic to neutral pH (6.89), organic carbon (2.20%), organic matter contents (3.79%), and electrical conductivity (1662 μS cm-1). Among soil properties, soil pH has strong effects on solubility and speciation of metals both in the soil and particularly in the soil solution (Fanrong et al., Citation2011), whereas, organic matter content has a strong influence on cation exchange capacity, buffer capacity as well as on the retention of heavy metals. Thus, metals present in organic rich soils contaminated with heavy metals are less mobile and less bioavailable than metals present in mineral soils (Olaniran, Balgobind, & Pillay, Citation2013). The mobility and availability of heavy metals in the soil are generally low, especially when the soil is high in pH, clay, and organic matter (Rosselli, Keller, & Boschi, Citation2003). Dede et al. (Citation2012) reported that Pb is an immobile metal in soil, since it readily forms a precipitate with a low aqueous solubility within the soil matrix, and in many cases it is not readily bioavailable. So, in accordance with soil properties such factors may act individually or in combination with each other and may alter the soil behaviour of the lead present, as well as the rate of uptake by plants.
3.2. Pb-induced phytotoxic effects
Gradual increase in Pb concentration significantly (p < 0.05) reduced all tested growth and biochemical parameter in two plant species. In the current investigation, the germination percentage of C. tetragonoloba and S. indicum seeds were significantly (p < 0.05) affected at 1000 mg Pb kg−1 as compared to control (Table ). The reduced germination percentages (64.76 and 80.00%) were recorded at 1000 mg Pb kg−1 in C. tetragonoloba and S. indicum, respectively. It has been well documented in the literature that germination is an essential process to determine the effects of Pb toxicity on different plant species. Germination is strongly inhibited at very low concentrations of Pb, even at micromolar levels (Kopittke, Asher, Kopittke, & Menzies, Citation2007). In this study, C. tetragonoloba and S. indicum seeds were able to germinate in the presence of low to moderate level of Pb concentrations in soil. The inhibition of seed germination may also result from the interference of lead with protease and amylase enzymes (Sengar et al., Citation2009).
Table 5. Phytotoxicity of Pb on germination and growth parameters of Cyamopsis tetragonoloba L. and Sesamum indicum L.
Seedling’s height (root and shoot length) is also among primary determinants of plant growth. Addition of Pb was significantly (p < 0.05) inhibited growth in terms of root and shoots length in C. tetragonoloba and S. indicum (Table ). In C. tetragonoloba and S. indicum, the longest roots (17.85 and 13.76 cm) and shoots (134.30 cm and 119.04 cm) were observed in control treatments with 0 mg kg−1 Pb, respectively. Pb is not an essential nutrient and at high concentrations inhibits plant growth. At 1000 mg Pb kg−1 concentration, root length decreased by 13.60 cm and 10.67 cm while shoot length reduced by 100.47 cm and 85.33 cm in both C. tetragonoloba and S. indicum, respectively. Higher concentration of Pb-induced morphological changes in plants i.e., root and shoot elongation in different plants showed a great sensitivity to excessive Pb. Lead exposure in plants strongly limits the growth and development of seedlings (Gopal & Rizvi, Citation2008). At high concentrations, lead inhibits the growth of roots which directly influences root growth and decreasing the capacity of water and nutrients absorption, ultimately leading to reduction in growth of the plant species.
Plant growth is an important parameter used to assess the survival and adaptation of a given species to environmental factors that decisively control biomass production. Addition of Pb-inhibited biomass growth of C. tetragonoloba and S. indicum. Pb contamination showed significant (p < 0.05) negative impacts on both fresh and dry biomass of C. tetragonoloba and S. indicum (Table ). Compared to control treatments, Pb stress at 1000 mg kg−1 reduced root fresh weight (8.15 g plant−1 and 5.92 g plant−1) and shoot fresh weight (21.13 g plant−1 and 9.15 g plant−1) in C. tetragonoloba and S. indicum, respectively. The dry biomass follows the same trend as fresh weight. Compared to control treatments, Pb stress at 1000 mg kg−1 reduced root dry weight from (5.09 g plant−1 and 3.70 g plant−1) and shoot dry weight (10.90 g plant−1 and 5.87 g plant−1) in C. tetragonoloba and S. indicum, respectively. Plant biomass is a significant indicator for characterizing the growth performance of plants in the presence of heavy metal. Under severe lead toxicity stress, plants displayed obvious symptoms of growth inhibition. Plant biomass can be restricted by high doses of lead exposure. Decrease in plant biomass may be associated with disturbed metabolic activities due to reduced uptake of essential nutrients when grown under Pb stress (Gopal & Rizvi, Citation2008; Kopittke et al., Citation2007).
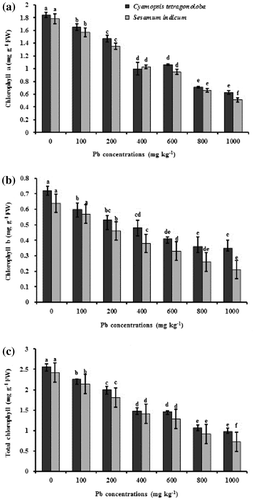
Lead, if present at high levels, can interfere with normal enzyme functions in plants and especially photosynthesis which is one of the plant processes most drastically affected by Pb toxicity. It is evident that chlorophyll content in leaves of C. tetragonoloba and S. indicum were influenced by Pb treatments. Chlorophyll contents decreased significantly (p < 0.05) with gradual increase in Pb concentration from 0 to 1000 mg kg−1 (Figure ). In C. tetragonoloba and S. indicum, the maximum amount of chlorophyll contents were measured at control, while the lowest concentration of chlorophyll a (0.63 and 0.51 mg g−1), chlorophyll b (0.35 and 0.21 mg g−1), and total chlorophyll (0.98 and 0.72 mg g−1) was at 1000 mg Pb kg−1, respectively. A decreased rate of photosynthetic pigment accumulation in association with Pb treatment may be the consequence of peroxidation of chloroplast membranes due to increased level of ROS generation (Srinivasan, Sahi, Paulo, & Venkatachalam, Citation2014). Lead may inhibit chlorophyll biosynthesis by impairing the uptake of essential photosynthetic pigment elements, such as magnesium, potassium, calcium, and iron (Gopal & Rizvi, Citation2008) which disturbed photosynthesis with the substitution of divalent cations by lead.
Figure 1. Effect of Pb stress on photosynthetic pigments chlorophyll-a (a) chlorophyll-b (b), and total chlorophyll (a + b) (c), on C. tertragonoloba and S. indicum after 12-week growth in soil medium with varying concentrations of Pb.
Tolerance indices (TIs) were also affected by Pb toxicty. Plant tolerance to heavy metal stress is estimated based on their root and/or shoot growth inhibition by the metal present in a medium (Srinivasan et al., Citation2014). According to Audet and Charest (Citation2007), if TI values less than 1, this indicates that the plant suffered a stress due to metal pollution with a net decrease in biomass. By contrast, if TI values greater than 1, suggest that plants have developed tolerance with a net increase in biomass (hyper accumulator). If TI values equal to 1, the plant is unaffected by metal pollution, indicate no difference relative to control treatments. In this study, both the plant species (C. tetragonoloba and S. indicum) had different tolerance indices (TIs) under Pb stress (Table ). At 1000 mg kg−1 Pb treatment, C. tetragonoloba and S. indicum had the TIs for root lengths (76.09% and 77.49%) and shoot lengths (74.81% and 71.73%), root fresh weights (57.01% and 48.18%) and shoot fresh weights (39.24% and 22.13%), root dry weights (57.01% and 44.34%) and shoot dry weights (53.63% and 32.28%) respectively. Srinivasan et al. (Citation2014) reported that growth inhibition is a common response to heavy metal stress and is also one of the most important agricultural indices of heavy metal tolerance.
Table 6. Effect of Pb stress on the growth tolerance indices (TIs) of Cyamopsis tetragonoloba L. and Sesamum indicum L.
3.3. Pb concentration in plant tissues
Concentration trends of Pb uptake among the different plant tissues (root, stem leaf, and pod) in both C. tetragonoloba and S. indicum are presented in Table . In C. tetragonoloba, the highest concentration of Pb found in the root: 626.67 mg kg−1 followed by stem: 299.67 mg kg−1, leaf: 89.33 mg kg−1, and pod: 10.40 mg kg−1 at 1000 mg Pb kg−1. However, in S. indicum Pb concentration primarily in the root: 525.67 mg kg−1, with small amount being transferred to leaf: 75.33 mg kg−1 followed by stem: 64.89 mg kg−1 and pod: 8.13 mg kg−1 at 1000 mg Pb kg−1. The high Pb contents in the plant tissues are clearly related to the concentration of metal in the growing environment. Because of strong Pb binding with organic and/or colloidal materials, it is believed that only small amounts of the lead in soil are soluble, and thereby available for plant uptake (Kopittke, Asher, Kopittke, & Menzies, Citation2008). The Pb accumulation capacity, based on their availabilities in the soil, varies greatly among different plants species and cultivars, and is also affected by various soil conditions reviewed by Bertrand, Muhammad, Camille, Peter, and Eric (Citation2011).
Table 7. Pb concentration, bioconcentration factor (BCF), bioaccumulation coefficient (BAC), and translocation factor (TF) of Cyamopsis tetragonoloba L. and Sesamum indicum L.
3.4. Pb accumulation in root and shoot
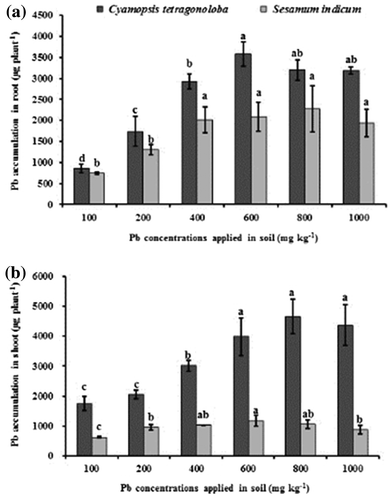
Beside concentrations, a total amount of metals accumulated in the shoots is considered as the most important parameter to evaluate the potential of phytoextraction in plants (Hanen et al., Citation2010). A significant rise in Pb accumulation per plant in root and shoot of both the plant species varied with respect to Pb treatments are expressed in (Figure ). The maximum Pb accumulation in root and shoot of both C. tetragonoloba and S. indicum were observed at 600 and 800 mg Pb kg−1 treatment. The mean Pb accumulation in C. tetragonoloba root were ranged from 856.17 to 3585.17 μg plant−1, while the accumulation of Pb by shoot were ranged from 1749.84 to 4655.07 μg plant−1. On the other hand, in S. indicum root accumulation was ranged from 747.2 to 2280.53 μg plant−1 while, the accumulation of Pb by shoot ranged from 622.99 to 1177.7 μg plant−1. Studies have shown the uptake of metals; their partition and translocation to different plant parts, as well as the degree of tolerance to them are dependent on the metal, its bioavailability, the plant species and its metabolism (D’Souza, Varun, Pratas, & Paul, Citation2013).
Figure 2. Accumulation of Pb in root (a) and shoot (b) of C. tertragonoloba and S. indicum after 12-week growth in soil medium with varying concentrations of Pb.
3.5. Phytoremediation potential
The bioconcentration factor (BCF), bioaccumulation coefficient (BAC), and translocation factor (TF) values (Table ) help to identify the suitability of plants for phytoremediation (i.e., phytoextraction or phytostabilzation) by explaining the accumulation characteristics and translocation behaviours of metals in plants. Plants with BCF, BAC, and TF values > 1 are considered promising phytoextractor, suitable for phytoextraction, while those with BCF and TF < 1 are not suitable for phytoextraction/phytostabilzation (Fitz & Wenzel, Citation2002). However, plants with BCF > 1 and TF < 1 are considered potential phytostabilizers (Mendez & Maier, Citation2008), suitable for phytostabilziation (immobilization).
Between tested plant species, C. tetragonoloba had BCF values > 1 from 100 to 600 mg Pb kg−1 while, BAC and TF values < 1 at all Pb treatments, indicating that C. tetragonoloba could not be a high efficiency plant for Pb translocation from root to the shoot; but suitable for Pb phytostabilzation. In contrast, S. indicum had the BCF > 1 from 100 to 400 mg Pb kg−1, while also have the BAC and TF values < 1 at all Pb treatments, indicating that S. indicum can also be categorized as Pb phytostabilizer.
4. Conclusions
From this study, it has been concluded that none of the plant species were identified as metal hyperaccumulators and not suitable for phytoextraction. Considering the rapid growth, biomass, accumulation efficiency, adaptive properties, tolerance, restoration potential towards Pb, and being leguminous and fast growing crop, C. tetragonoloba can be used as an effective tool to decontaminate Pb-polluted soils in quick and successive flushes than S. indicum. Furthermore, the value of BCFs, BACs, and TFs suggests that both the plant species were suitable for Pb phytostabilization but C. tetragonoloba was more potential candidates for phytoremediation than S. indicum. Moreover, both plants species have economic and ecological values. These plants can both remediate metal-contaminated sites and produce valuable biomass, which can bring income for the owners of the sites. The harvested biomass could then be incinerated and disposed off or the accumulated metal could also be recovered for commercial uses and thus reused as biofuel. Further work is needed to understand the mechanisms of metal absorption and tolerance in plants.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Abidi, N., Liyanage, S., Auld, D., Imel, R.K., Norman, L., Grover, K., … Trostle, C. (2015). Challenges and opportunities for increasing guar production in the United States to support unconventional oil and gas production. In Hydraulic fracturing impacts and technologies (pp. 207–226). Boca Raton, FL: CRC Press.10.1201/b18581
- Adriano, D.C. (2001). Trace elements in terrestrial environments: Biogeochemistry, bioavailability, and risks of metals (2nd ed.). New York, NY: Springer.10.1007/978-0-387-21510-5
- Ahmad, M., Ullah, K., Khan, M.A., Ali, S., Zafar, M., & Sultana, S. (2011). Quantitative and qualitative analysis of sesame oil biodiesel. Energy Sources, Part A: Recovery, Utilization, and Environmental Effects, 33, 1239–1249.10.1080/15567036.2010.531510
- Amel, S.B., Nabil, M., Nadia, A., Hocine, G., Hakim, L., & Nadjib, D. (2016). Phytoremediation of soil contaminated with Zn using Canola (Brassica napus L.). Ecological Engineering, 95, 43–49.
- Anjum, N.A., Umar, S., & Iqbal, M. (2014). Assessment of cadmium accumulation, toxicity, and tolerance in Brassicaceae and Fabaceae plants – Implications for phytoremediation. Environmental Science and Pollution Research, 21, 10286–10293.10.1007/s11356-014-2889-5
- Arias, J.A., Peralta-Videa, J.R., Ellzey, J.T., Ren, M., Viveros, M.N., & Gardea-Torresdey, J.L. (2010). Effects of Glomus deserticola inoculation on Prosopis: Enhancing chromium and lead uptake and translocation as confirmed by X-ray mapping, ICP-OES and TEM techniques. Environmental and Experimental Botany, 68(2), 139–148.10.1016/j.envexpbot.2009.08.009
- Arnon, D.I. (1949). Copper enzymes in isolated chloroplasts: Polyphenol oxidase in Beta vulgaris. Plant Physiology, 24, 1–15.10.1104/pp.24.1.1
- Ashraf, M.Y., Akhtar, K., Sarwar, G., & Ashraf, M. (2002). Evaluation of arid and semi-arid ecotypes of guar (Cyamopsis tetragonoloba L.) for salinity (NaCl) tolerance. Journal of Arid Environments, 52, 473–482.10.1006/jare.2002.1017
- Audet, P., & Charest, C. (2007). Heavy metal phytoremediation from a meta-analytical perspective. Environmental Pollution, 147, 231–237.10.1016/j.envpol.2006.08.011
- Bashmakov, D.I., Lukatkin, A.S., Anjum, N.A., Ahmad, I., & Pereira, E. (2015). Evaluation of zinc accumulation, allocation, and tolerance in Zea mays L. seedlings: Implication for zinc phytoextraction. Environmental Science and Pollution Research, 22(20), 15443–15448.
- Bertrand, P., Muhammad, S., Camille, D., Peter, W., & Eric, P. (2011). Lead uptake, toxicity, and detoxification in plants. Reviews of Environmental Contamination and Toxicology, 213, 113–136.
- Cecchi, M., Dumat, C., Alric, A., Felix-Faure, B., Pradere, P., & Guiresse, M. (2008). Multi-metal contamination of a calcic cambisol by fallout from a lead-recycling plant. Geoderma, 144(1–2), 287–298.10.1016/j.geoderma.2007.11.023
- Chen, L., Long, X.H., Zhang, Z.H., Zheng, X.T., Rengel, Z., & Liu, Z.P. (2011). Cadmium accumulation and translocation in two Jerusalem artichoke (Helianthus tuberosus L.) cultivars. Pedosphere, 21, 573–580.10.1016/S1002-0160(11)60159-8
- D’Souza, R., Varun, M., Pratas, J., & Paul, M.S. (2013). Spatial distribution of heavy metals in soil and flora associated with the glass industry in North Central India: Implications for phytoremediation. Soil and Sediment Contamination: An International Journal, 22, 1–20.10.1080/15320383.2012.697936
- Dede, G., Ozdemir, S., & Dede, O.H. (2012). Effect of soil amendments on phytoextraction potential of Brassica juncea growing on sewage sludge. International Journal of Environmental Science and Technology, 9, 559–564.10.1007/s13762-012-0058-2
- Deepak, M., Sheweta, B., & Bhupendar, S.K. (2014). Guar gum: Processing, properties and food applications—A review. Journal of Food Science and Technology, 51, 409–418.
- Elleuch, M., Besbes, S., Roiseux, O., Blecker, C., & Attia, H. (2007). Quality characteristics of sesame seeds and by products. Food Chemistry, 103, 641–650.10.1016/j.foodchem.2006.09.008
- Fanrong, Z., Shafaqat, A., Haitao, Z., Younan, O., Boyin, Q., Feibo, W., & Guoping, Z. (2011). The influence of pH and organic matter content in paddy soil on heavy metal availability and their uptake by rice plants. Environmental Pollution, 159, 84–91.
- Fitz, W.J., & Wenzel, W.W. (2002). Arsenic transformations in the soil–rhizosphere–plant system: Fundamentals and potential application to phytoremediation. Journal of Biotechnology, 99, 259–278.10.1016/S0168-1656(02)00218-3
- Ginneken, L. V., Meers, E., Guisson, R., Ruttens, A., Elst, K., Tack, F.M.G., … Dejonghe, W. (2007). Phytoremediation for heavy metal contaminated soils combined with energy production. Journal of Environmental Engineering and Landscape Management , 15, 227–236.
- Gopal, R., & Rizvi, A.H. (2008). Excess lead alters growth, metabolism and translocation of certain nutrients in radish. Chemosphere, 70(9), 1539–1544.10.1016/j.chemosphere.2007.08.043
- Gupta D, Huang H, Yang X, Razafindrabe B, Inouhe M (2010) The detoxification of lead in Sedum alfredii H. is not related to phytochelatins but the glutathione. Journal of Hazardous Materials 177(1-3): 437–444.10.1016/j.jhazmat.2009.12.052
- Hanen, Z., Tahar, G., Abelbasset, L., Rawdha, B., Rim, G., Majda, M., … Chedly, A. (2010). Comparative study of Pb-phytoextraction potential in Sesuvium portulacastrum and Brassica juncea: Tolerance and accumulation. Journal of Hazardous Materials, 183, 609–615.
- Kopittke, P.M., Asher, C.J., Kopittke, R.A., & Menzies, N.W. (2007). Toxic effects of Pb2+ on growth of cowpea (Vigna unguiculata). Environmental Pollution, 150(2), 280–287.10.1016/j.envpol.2007.01.011
- Kopittke, P.M., Asher, C.J., Kopittke, R. A., & Menzies, N.W. (2008). Prediction of Pb speciation in concentrated and dilute nutrient solutions. Environmental Pollution, 153(3), 548–554.10.1016/j.envpol.2007.09.012
- Lone, M.I., He, Z.L., Stoffella, P.J., & Xe, Yang (2008). Phytoremediation of heavy metal polluted soils and water: Progresses and perspectives. Journal of Zhejiang University Science B, 9, 210–220.10.1631/jzus.B0710633
- Maestri, E., Marmiroli, M., Visioli, G., & Marmiroli, N. (2010). Metal tolerance and hyperaccumulation: Costs and trade-offs between traits and environment. Environmental and Experimental Botany, 68(1), 1–13.10.1016/j.envexpbot.2009.10.011
- Mahmood, T. (2010). Phytoextraction of heavy metals: The process and scope for remediation of contaminated soils. Soil Environ., 29, 91–109.
- Mendez, M.O., & Maier, R.M. (2008). Phytostabilization of mine tailings in arid and semiarid environments—an emerging remediation technology. Environment Health Perspective, 116, 278–283.
- Monni, S., Salemaa, M., & Millar, N. (2000). The tolerance of Empetrum nigrum to copper and nickel. Environmental Pollution, 109, 221–229.10.1016/S0269-7491(99)00264-X
- Nagajyoti, P.C., Lee, K.D., & Sreekanth, T.V.M. (2010). Heavy metals, occurrence and toxicity for plants: A review. Environmental Chemistry Letters, 8, 199–216.10.1007/s10311-010-0297-8
- Oh, K., Li, T., Cheng, H.Y., Xie, Y., & Yonemochi, S. (2013). Development of profitable phytoremediation of contaminated soils with biofuel crops. Journal of Environmental Protection, 4, 58–64.10.4236/jep.2013.44A008
- Olaniran, A.O., Balgobind, A., & Pillay, B. (2013). Bioavailability of heavy metals in soil: Impact on microbial biodegradation of organic compounds and possible improvement strategies. International Journal of Molecular Sciences, 14, 10197–10228.10.3390/ijms140510197
- Pais, I., & Jones, J.B. (2000). The handbook of trace elements (p. 223). Boca Raton, FL: Saint Lucie Press.
- Pourakbar, L., Khayami, M., Khara, J., & Farbidina, T. (2007). Physiological effects of copper on some biochemical parameters in Zea mays L. seedlings. Pakistan Journal of Biological Sciences, 10, 4092–4096.
- Punamiya, P., Datta, R., Sarkar, D., Barber, S., Patel, M., & Das, P. (2010). Symbiotic role of Glomus mosseae in phytoextraction of lead in vetiver grass [Chrysopogon zizanioides (L.)]. Journal of Hazardous Materials, 177(1-3), 465–474.10.1016/j.jhazmat.2009.12.056
- Rachit, K., Verma, K.S., Meena, T., Yashveer, V., & Shreya, H. (2016). Phytoextraction and bioconcentration of heavy metals by Spinacia oleracea grown in paper mill effluent irrigated soil. Nature Environment and Pollution Technology, 15, 817–824.
- Rosselli, W., Keller, C., & Boschi, K. (2003). Phytoextraction capacity of trees growing on metal contaminated soil. Plant Soil, 256, 265–272.10.1023/A:1026100707797
- Saydut, A., Duz, M.Z., Kaya, C., Kafadar, A.B., & Hamamci, C. (2008). Transesterified sesame (Sesamum indicum L.) seed oil as a biodiesel fuel. Bioresource Technology, 99, 6656–6660.10.1016/j.biortech.2007.11.063
- Sengar, R.S., Gautam, M., Sengar, R.S., Sengar, R.S., Garg, S.K., Sengar, K., & Chaudhary, R. (2009). Lead stress effects on physiobiochemical activities of higher plants. Reviews of Environmental Contamination and Toxicology, 196, 1–21.
- Shahid, M., Pinelli, E., Pourrut, B., Silvestre, J., & Dumat, C. (2011). Lead-induced genotoxicity to Vicia faba L. roots in relation with metal cell uptake and initial speciation. Ecotoxicology and Environmental Safety, 74(1), 78–84.10.1016/j.ecoenv.2010.08.037
- Sharma, S., Singh, B., & Manchanda, V.K. (2014). Phytoremediation: Role of terrestrial plants and aquatic macrophytes in the remediation of radionuclides and heavy metal contaminated soil and water. Environmental Science and Pollution Research, 22, 946–962.
- Srinivasan, M., Sahi, S.V., Paulo, J.C.F., & Venkatachalam P. (2014). Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Botanical Studies, 55, 54.
- Stanisław W., & Gawronski, H.G. (2007). Plant taxonomy for phytoremediation. Advanced Science and Technology for Biological Decontamination of Sites Affected by Chemical and Radiological Nuclear Agents, 79–88.
- Talebi, S., Nabavi, K.S.M., & Sohani, D.A.L. (2014). The study effects of heavy metals on germination characteristics and proline content of Triticale (Triticoseale Wittmack). International Journal of Farming and Allied Sciences, 3, 1080–1087.
- Tian, S., Lu, L., Yang, X., Webb, S. M., Du, Y., & Brown, P.H. (2010). Spatial imaging and speciation of lead in the accumulator plant Sedum alfredii by microscopically focused synchrotron X-ray investigation. Environmental Science & Technology, 44, 5920–5926.10.1021/es903921t
- Uzu, G., Sobanska, S., Aliouane, Y., Pradere, P., & Dumat, C. (2009). Study of lead phytoavailability for atmospheric industrial micronic and sub-micronic particles in relation with lead speciation. Environmental Pollution, 157(4), 1178–1185.10.1016/j.envpol.2008.09.053
- Vega, F., Andrade, M., & Covelo, E. (2010). Influence of soil properties on the sorption and retention of cadmium, copper and lead, separately and together, by 20 soil horizons: Comparison of linear regression and tree regression analyses. Journal of Hazardous Materials, 174(1-3), 522–533.10.1016/j.jhazmat.2009.09.083
- Hao, X., Taghavi, S., Xie, P., Orbach, M.J., Alwathnani, H.A., Rensing, C., & Wei, G. (2014). Phytoremediation of heavy and transition metals aided by legume-rhizobia symbiosis. International Journal of Phytoremediation, 16(2), 179–202.doi:10.1080/15226514.2013.773273
- Zheng, L.J., Liu, X.M., Lütz-Meindl, U., & Peer, T. (2011). Effects of lead and EDTA-assisted lead on biomass, lead uptake and mineral nutrients in Lespedeza chinensis and Lespedeza davidii. Water, Air, & Soil Pollution, 220, 57–68.
