Abstract
The present study is to understand the intrinsic ecological trait of invasive plant Senna alata relating to soil characteristics and seasonality distributed in the Puducherry region located under tropical climate. Plant biomass, soil parameters linked with soil enzyme activities are examined in six sites where S. alata is found growing. From the study, it is demonstrated that invasive species S. alata exhibits heterogeneity in invaded soil characteristics but variation in aboveground biomass (AGB) of S. aalata is not significant. Soil moisture and soil enzymes activities and AGB are significantly positively correlated whereas pH and EC negatively correlated. Seasonal variation in total nitrogen, phosphorus, and elements viz. Mg, Ca, and Na is inconsistent in their seasonality. Based on observations made and results obtained in the study, it is stated soil characteristics and their seasonality in the S. alata invaded soil is, site-specific resulting in heterogeneity; but such heterogeneity is not exhibited in AGB in six sites and it is, therefore, reported that such an idiosyncratic trait of S. alata is one of the potential traits influencing successful invasion by S. alata as mono-species population. The statistical analyses also confirmed the observation made. The outcome of the study would help to prepare management programmes to check spreading of invasive species so as to restore native plant diversity in and around Puducherry region.
1. Introduction
Biological invasions by alien species are widely recognized as a significant component of global environmental change, often resulting in irrevocable loss of native plant biodiversity and even livelihood of those who sustain on locally available native plants. Consequently, in the recent years, it is becoming more important to understand the ecology of invasive plant species as they are capable of not only altering biodiversity but also functioning of the ecosystem (Marcelino & Verbruggen, Citation2015; Powell, Chase, & Knight, Citation2011; Tylianakis, Didham, Bascompte, & Wardle, Citation2008). It is widely reported that invasive plants can cause alternation in abundance or species composition, diversity and also soil microbes (Quist et al., Citation2014; Rusterholz, Salamon, Ruckli, & Baur, Citation2014; Stefanowicz, Stanek, Nobis, & Zubek, Citation2016; Uddin & Robinson, Citation2017). Recently, Gibbons et al. (Citation2017) have found invasive plants with their unique species-specific trait, changes invaded soil environment from surrounding native soil characters. Khadka (Citation2017) has emphasized the need for such study in Nepal as the invasion of Mikania micrantha, directly and indirectly, modifies natural resources which are the rural household livelihoods. As pointed by Marcelino and Verbruggen (Citation2015) eco-biological aspects to understand the mode and success of invasion and spreading are very limited and such gap would weaken the speed management programmes targeting invasive plants under such circumstances, it is learned from our intensive field survey covering almost all parts of the Puducherry region, that Senna alata one of the invasive species under family Fabaceae, is coming up both in urban and rural areas of Puducherry region. To chalk out management programme targeting spread of invasive population in the Puducherry region, basic inputs relating to the ecology of the invasive plant and its habitat structure pertaining to the study region are very much required. Therefore, keeping these aspects as objectives, presently an attempt has been made to study and examine aboveground biomass (AGB) of the plant species linked with soil characteristics and soil enzyme activities by fixing six sites where S. alata are profusely growing. Moreover, as of now a comprehensive study on ecological characteristics of S. alata and its habitat, are not available and hence present investigation has been undertaken. The outcome of the study would bring out status and influence of invasive species on soil characteristics linked with enzyme activity in an alien-invaded region. It would be a substantial contribution to the annals of the ecology of invasives, particularly S. alata (L.) Robx.
2. Materials and methods
2.1. Study region
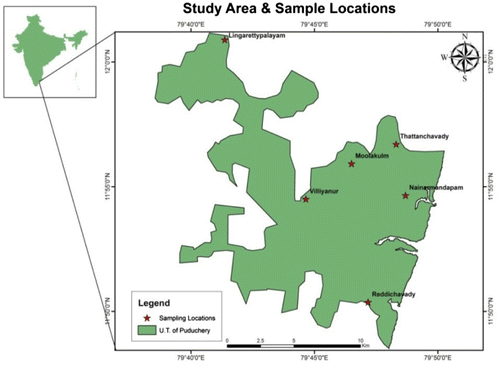
Puducherry is located along the Coromandel Coast of peninsular India with the geographical coordinates 11°52′N, 79°45′E, and 11°59′N and 79°52′ E covering an area of 480 km (Figure ). The total rainfall received by the Pondicherry region is 1463.5 mm; during monsoon 710 mm and pre-monsoon 334 mm. The monthly mean temperature ranges between 26.0 and 34.2 °C. Weather data relating to temperature, rainfall and relative humidity for the study period (2015) is presented in Tables and . The study region experiences four seasons viz. Post-monsoon (January–March) summer (April–June), pre-monsoon (July–September), and monsoon (October–December). Because of its geographical position (along Coromandel Coast of India) the region receives rainfall during two seasons i.e. during north-east monsoon (October–December) and a short spell of rain during south-east monsoon (from late summer till early pre-monsoon). As the relative humidity ranges between 64 and 88% the region is moderately wet throughout the year.
Table 1. Different sites in geographical coordination and soil physical parameters of six study sampling sites in Puducherry, India.
Table 2. Weather report of Puducherry, India.
2.2. Study species and sampling sites
S. alata (L.) Roxb. Syn. Cassia alata (L), a medicinally important member of the genus Senna, is an invasive species from Mexico, South America (Figure ). The study is conducted in six sites where S. alata is growing profusely, located in and around the Puducherry region Figures and and the geographical coordinates of study sites are also recorded using GPS (Table ). The study is undertaken during January–December 2015 that covers four seasons’ viz. post-monsoon, summer, pre-monsoon, and monsoon. AGB of S. alata is estimated by quadrant method (harvest method) using 50 cm × 50cm quadrat. Since destructive method is adopted for estimation of AGB, harvesting of plant is done from six quadrants in each site during each season. The total area covered by study plant varies from site to site i.e. from 10 × 15 m to 14 × 23 m (in the beginning of the study) and further season to season. The AGB per metre square is found out using digital hand weighing scale in the field itself and later dry weight is found out. Values are converted to AGB per square metre area (dry wt.). The same procedure is followed for AGB assessment in all sites for all seasons. Soil samples are collected every season from six study sites to analyze soil parameters and soil enzymes activities. About 500 g of soil sample is collected from 10 to 15 cm depth in 6 points in each site where ever plant samples are taken for AGB estimation. Six soil subsamples are pooled together; about 500 g of from pooled soil is taken for analysis. The same sampling procedure is followed in other sites for soil sample collection. Enzymes analyses are done within 48–72 h from the time of collection. Tofind out variation between sites and correlation among parameters, collected data are subjected to ANOVA and Pearson Correlation. To find the relationships between different variables, principal component analysis (PCA) was performed on all the data (Figure ).
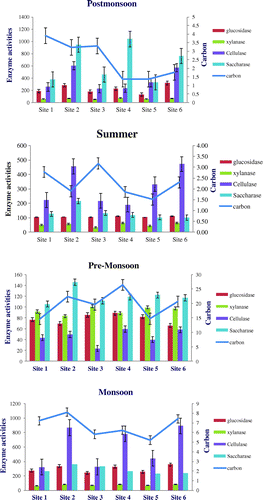
Figure 3. Relationship between soil enzyme activity and TOC during in different season post-monsoon, summer, pre-monsoon, monsoon in the unit of enzyme activity in of β-glucosidase (μg p-NP g−1dwt h−1) cellulase (glucose equivalent μg g−1dwt 24 h−1), saccharase (glucose equivalent μg g−1dwt 3 h−1), Xylanase (glucose equivalent μg g−1dwt 24 h−1), TOC (g/kg) in six sites in Puducherry, India.
2.3. Methods of soil analysis
Soil bulk density is determined by volumetric flask method (Bashour & Sayegh, Citation2007). Soil water holding capacity is determined by gravimetric method (Margesin & Schinner, Citation2005). Soil moisture content is determined by (Hesse, Citation1971). Soil texture is determined by International pipette method (Piper, Citation1966) and percentage values of sand silt and clay are calculated.Soil reaction (pH) and electrical conductivity (1:2 soil–water suspensions) is determined by potentiometry, conductometry method (Waring & Bremner, Citation1964). Total nitrogen (N), sulfur total organic carbon (TOC) was determined by Elemental CHNS Vario El cube (Liu, Charrua, Weng, Yuan, & Ding, Citation2015). Extractable phosphorus was determined by sodium bicarbonate method (Olsen, Citation1954). Extractable potassium and sodium are determined by Flame photometer (Richards, Citation1954). Nitrate – nitrogen is determined by chromotropic acid spectrophotometric method (Sims & Jackson, Citation1971). Soluble calcium and magnesium are determined by soil extract titration with EDTA (Richards, Citation1954). Soil enzymes viz. urease activity (μg NH4-N g−1 dwt 2 h−1) is by method described by Tabatabai and Bremner (Citation1972). Acid phosphatase and alkaline phosphatase by Tabatabai and Bremner (Citation1969) method; β-glucosidase activity is determined by methods described by Tabatabai (Citation1982) and Eivazi and Tabatabai (Citation1988). Cellulase activity is determined by the method given by Schinner and von Mersi (Citation1990) and Saccharase activity is determined by the method described by Schinner and von Mersi (Citation1990). In all analyses, triplicate samples are run for each parameter and mean values are used for data analysis.
3. Result
Among all studied soil physical parameters, soil water holding capacity show no variation between sites. The soil texture significantly differs in all the six sites except clay (p < 0.683). Soil moisture content is higher in monsoon (13.78%) and least in the summer (1.23%). Table indicates variation in all measured parameters between sites and seasons (except in summer (p < 0.19). pH and EC show higher values during summer; between seasons the variation is significant (p < 0.016) (Tables and ). The AGB is higher in the post-monsoon (2333.3 gm/m2) with a second peak in pre-monsoon and least in summer (1200 gm/m2). Except during summer (p < 0.046) variation in AGB is not significant between sites. TOC is higher in the pre-monsoon (12.37 g/kg) lesser in the summer (1.90 g/kg). There is no significant seasonal variation in TOC except during summer (Table ). Macronutrients viz. potassium (88.5 g/kg), sodium (23.50 g/kg), calcium (17.40 g/kg), and Magnesium (7.77 g/kg), sulphur (0.86 g/kg) are significantly higher in summer and significantly vary between sites. Total nitrogen (2.76 g/kg), nitrate nitrogen (2.14 g/kg), and phosphorus (75.40 g/kg) show inconsistent values both site-wise and season-wise. Total nitrogen showed higher values during summer in three sites (p-0.016) but during monsoon in other three sites (p-0.010). Similarly, nitrate nitrogen showed higher values during summer in four sites (p-0.5791) and higher values during pre-monsoon in two sites exhibiting inconsistency. During other two seasons viz. pre-monsoon and post-monsoon, it showed lesser variation (p-0.016 and 0.036 respectively). Enzymes activity viz. Acid and Alkaline phosphatase, urease, β-glucosidase, cellulose, xylanase, saccharase are higher in the monsoon and less in summer whereas saccharase and acid phosphates showed higher activity during post-monsoon. Site wise and season wise values are presented in tables.
Table 3. Soil physico-chemical properties in post-monsoon and summer season six study sites in Puducherry, India.
Table 4. Soil physico-chemical properties inpre-monsoon and monsoon seasons of six study sites in Puducherry, India.
Correlation analysis shows a positive correlation between soil moisture and electrical conductivity (r = 0.576*, 0.535*). Positive correlation between total nitrogen, TOC, phosphorus, magnesium, acid phosphatase, alkaline phosphatase, urease, β-glucosidase, cellulose, xylanase (r = 0.663**, 0.647**, 0.607**, 0.948**, 0.729**) (Table ). There is a strong negative correlation between total nitrogen, TOC with soil moisture (r = −0.704**, −0.599**). Table shows strong negative correlation soil moisture between total nitrogen and TOC, phosphorus, nitrate, nitrate nitrogen (r = −0.704**, −0.599**, −0.536**, −0.693*, −0.544*) in summer. Table indicates soil enzymes and physicochemical parameters in pre-monsoon there a strong positive correlation in the electrical conductivity between TOC, sodium (r = 0.698**, 0.743**); TOC and phosphorus (r = 0.782**) and others are showing no significant correlation. There is a negative correlation between soil moisture and sodium (−0.658*). Total nitrogen and nitrate nitrogen are positively correlated (r = 0.740**). Table shows a negative correlation of pH with cellulose (r = 0.600**). PCA explains 85% of the total variance in the correlation matrix. Dim 1 and 2 show 68% for post-monsoon; 62% for summer; 73% for pre-monsoon, and 66% for monsoon. Season-wise correlated explained variables are depicted in Figure (a)–(d) which demonstrate grouping of positively correlated variables and negatively correlated variables during different seasons with their level of significance (Table ).
Table 5. Soil enzyme activities in post-monsoon and summer six study sites in Puducherry, India.
Table 6 Soil enzyme activities in pre-monsoon and monsoon six study sites in Puducherry India.
Figure 4. (a,b,c,d). Principal component analysis with three sets of variables: enzymes, nutrients measured the symbols indicate the corresponding score group means ± SE and the arrows represent variable eigenvectors in the space plotted by the first two PCA axes. The projection of the lines along each axis indicates their relative importance.
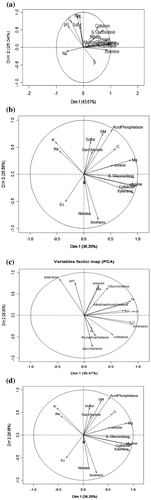
Table 7. Correlation between soil enzymes and physico-chemical properties six study sites in Puducherry, India.
4. Discussion
The enzyme levels in soil systems vary in amounts primarily due to the fact that each soil type has different amounts of organic matter content, composition, and activity of micro-organisms and intensity of biochemical changes (Caldwell, Citation2006), Moreover, soil enzyme activities are mostly vary in quantity and level of activity due to each soil type as well as above ground vegetation and such changes are more pronounced in case of invasive plants invaded soil. One such region is selected in the present study were invasive plant S. alata is profusely growing as monospecies population in the Puducherry region. The outcome and observations made during different seasons in six sites are discussed in two levels. Firstly, it is tried to find out whether there exists variation in the soil characteristics and enzyme activity among the six sites with densely growing as monospecific population of S. alata; secondly, the seasonality of enzyme activities at the face of various soil characteristics and AGB of the invasive plant, have been examined and discussed.
4.1. Heterogeneity in soil parameters enzyme activities among selected sites
Studies on various physico-chemical parameters and enzymes activity of soil in six sites revealed that variations in their soil characteristics except for soil profile viz. sand, silt, and clay, is not significant. Similarly, except soil nitrogen and activities of enzyme cellulose and xylanase, other parameters and activities of other enzyme differ from each factor significantly though the study plant is growing densely as a monospecies population in all sites. Besides, AGB of S. alata also showed varying level at any point of time during the study period in all six sites. The ANOVA results and Pearson correlation also reflected similar trend. It is presumed that such a spatial heterogeneity found in soil parameters where the invasive plant S. alata growing as nonspecific population, might be the important characteristics of an invasive plant. It has been already reported for other invasive plants that alien species establish as monospecific population and significantly change the properties of native ecosystems (Vilà et al., Citation2011). These invasive can alter the biogeochemistry of ecosystems mediated by secondary metabolites released by invasive species as well as plant–plant and plant–microbe interactions (Weidenhamer & Callaway, Citation2010). This is also again support the observation made in the present study those variations between the sites. Besides, one of the basic factors that influence heterogeneity in soil characteristics is the type and quantity of AGB presence of dense vegetation provide the soil adequate cover, thereby reducing the loss in nutrients that are necessary for plants growth and energy fluxes catalyzed by soil enzyme activities (Moraghebi, Matinizadeh, Khanjani, Teimouri, & Afdideh, Citation2012). It is also more clear from present field survey that dense foliage, continuous litter fall, shady under storey climate of the soil, and the prevailing moisture facilitate a conducive environment supported diversified micro-organisms – the producers of various enzymes and trigger various soil biochemical changes leading to spatial heterogeneity among six sites. Shao, Yang, and Wu (Citation2015) have affirmed that vegetation type is an important factor influencing the spatial-temporal variation of soil enzyme activities and labile organic carbon. These results are also found to be similar with the findings of (Krämer & Green, Citation2000; Sinsabaugh, Benfield, & Linkins, Citation1981; Sinsabaugh & Linkins, Citation1988; Sinsabaugh & Moorhead, Citation1994). To reinforce the present observation further, it is relevant to cite specific reports on heterogeneity in soil characteristics invaded by a particular alien species wherever they grow viz. the exotic plant Mikania micrantha invasion in south-east China (Li, Zhang, Jiang, Xin, & Yang,Citation2006) and B. thunbergii in New Jersey (Kourtev, Ehrenfeld, & Häggblom, Citation2003) and three sites of Eupatorium adenophorum (Sun, Gao, & Guo, Citation2013). Variation in plant inputs to soil due to the invasion, has the potential to cause variation in enzyme composition (Hernández & Hobbie, Citation2010) and might be a spatial heterogeneity effect (Sun et al., Citation2013).
To substantiate the present observation on variability in soil quality and enzyme activity, the success of invasion of S. alata that has been reported to possess potential allelopathic property by virtue of its phytochmeical constituents (Rodrigues, Souza Filho, Ferreira, & Demuner, Citation2010) and these are capable of interfering with chemical ecology of given invaded region. Though there is no report on the exact chemistry of root exudates of S. alata, it could not be ignored because plants modify the soil environment through root exudates that affect soil structure, and mobilize and/or chelate nutrients and long-term impact of litter and root exudates can modify soil nutrient pools or nutrient cycles differently from native species (Weidenhamer & Callaway, Citation2010). Moreover, plant released-compounds such as polysaccharides, aromatic compounds, and esters, together with detritus and root exudates serve as sources of substrates for enzymatic degradation and provide the energy and elements’ necessary for enzyme synthesis (Hernández & Hobbie, Citation2010). Sun et al. (Citation2013) showed that the soil microbial composition was significantly different in the three Eupatorium adenophorum invaded sites which are the principle causative bio factor in enzyme activity.
Therefore, it is concluded primarily that invasive plant S. alata could invade any site successfully and modify it by virtue of its invasive trait and exhibit heterogeneity in the soil characteristics and create an allelopathic regime in the invaded region/soil and flourish as monospecific population; besides invasive plants are capable of modifying ecosystem function and such changes can be species specific and site-specific (Dassonville et al., Citation2008). The second level of study relates to seasonality in soil characteristic, enzyme activities, and AGB of S. alata.
4.2. TOC on soil enzyme activities
The relationship between TOC and enzyme activities is not significant as the principle source of carbon i.e. litter is almost continuously added by the densely growing invasive plant almost throughout the year. During summer, the plant species showed a reduction in the biomass of aboveground parts; but large amount of litter fall was contributed to the soil during summer. Which contribute to higher amount of TOC; secondly because of high temperature and lesser soil moisture the microbial activity is low resulting in lesser utilization of carbon by the microbes. Under such conditions more of TOC is left unused in the soil (Figure ). TOC is also highly influenced by the type of vegetation and its level of litter production under shady microclimate. Vegetation type is an important factor influencing the spatial-temporal variation of soil enzyme activities and labile organic carbon (Shao et al., Citation2015). Ehrenfeld (Citation2003) stated that soil carbon (C) and nutrients pools are often modified by invasions and that the direction and magnitude of the impacts were probably determined by the composition of the invaded community and soil properties. Soil enzymes’ involvement in biogeochemical cycles of the soil will result in the transforming and cycling of soil labile organic carbon pool (Shao et al., Citation2015). Based on the carbon as of energy for the microbes involved in enzymatic activity, due to unfavourable soil microclimate, the enzyme activity is higher during post-monsoon and monsoon but lesser during summer. However, β-glucosidase, urease, and acid phosphatase showed positive correlation with AGB and nutrients viz. phosphorus and nitrogen with TOC.
4.3. Seasonality in soil characteristics and enzyme activity
Seasonality of soil characteristics showed significant relation with certain factors. Due to previous rainy season, the soil retains its wetness during post-monsoon and influences microbial activities triggering more enzyme activities resulting in more availability of nutrients. Therefore, during post-monsoon above ground biomass showed higher values. The enzyme activity is also higher as suitable environmental factors viz. moisture, ambient temperature, and pH. EC also showed higher values due to presence of more elements/ions in the soil due to higher microbial oxidation process. During summer, there is less enzyme activity influenced by higher temp and low soil moisture and these factors reduce the availability of the nutrients to the root system resulting in lesser growth and above ground of plants; on the other hand due to higher temp, leaves and other plant parts stated drying and triggers higher amount of litter fall. The effects of enzyme activities on the decay of major litter components have been found to couple with major nutrient cycles, such as nitrogen (N), phosphorus (P), and sulphur (S) cycles. Fioretto, Papa, Curcio, Sorrentino, and Fuggi (Citation2000) reported that cellulase in soils are derived mainly from plant debris incorporated into the soil (Table ). The relatively dense structure of plants and a greater accumulation of litter and fine roots in the understory of forest and plantation may favour the growth of microbial populations which in turn pave way for higher level of enzyme activity particular enzymes related to degradation of plant debris. β-glucosidase present in plant debris decomposing in the ecosystem (Ajwa & Tabatabai, Citation1994; Martinez & Tabatabai, Citation1997). Glucosidase enzyme plays an important role in soils because it is involved in catalyzing the hydrolysis and biodegradation of cellulose in plant debris and the accumulation of C in microbial biomass. The more density of plants cover provides microclimate condition resulting in more activity of micro-organisms which are the main source of these enzymes in the soil (Grierson & Adams, Citation2000; Moraghebi et al., Citation2012; Sedia & Ehrenfeld, Citation2006). The differences in the sources of substrate availability and composition may lead to the changed behaviours of the activity of hydrolytic enzymes, such as, urease, and β-glucosidase in soils. Such enzymatic disparity of enzyme activities is revealed also in other studies (Tian, Dell, & Shi, Citation2010). Enzymatic activity depends on temperature, soil moisture, and flora (Błońska, Citation2010).The moisture response function of β-glucosidase, xylanase, alkaline phosphatase, urease, cellulase activity almost stable during the year β-glucosidase were closely related to plant growth and the biomass amount (Shao et al., Citation2015). The results indicated that vegetation type is an important factor influencing the spatial-temporal variation in enzyme activities and labile carbon. Caldwell, Griffiths, and Sollins (Citation1999) found that the relationship between major C- and P-processing enzymes changed under different soil and vegetation regime.
Total nitrogen also showed no significant seasonal variation due to variation inplant biomass and litter fall. Liao et al. (Citation2008) conducted a meta-analysis and found that on average, with much higher litter decomposition rates and increases in soil nitrogen mineralization and nitrification. Seasonal variation of plant biomass was the potential driving factor for them that are associated with C, N, and P cycling (Shao et al., Citation2015). Stepwise regression analysis showed that vegetation was the best predictor of N cycling metrics the higher productivity, the higher the rates of net mineralization, nitrification, and microbial biomass-N (Corbin & D’antonio, Citation2011). However, there exists significant relation between soil nitrogen and enzyme urease. These two factors showed higher values during post-monsoon when there is high AGB production and lesser during summer. Similar observation is also made by Dormaar, Johnston, and Smoliak (Citation1984) and Błońska (Citation2010) that the activity of urease clearly increases in winter and decrease in summer.
Usually extractable P is negatively correlated with the percentage of clay, organic carbon and positively correlated with pH but here no such correlation because of a higher rate of litterfall which contains more P. Such varying levels of both soil parameter and enzyme activities in alien invaded soil have been already reported in different plants. For instance, Amaranthus viridis invasion significantly increased the concentration of soil total P in Senegal (Sanon et al., Citation2009). Soil -N content was 30% higher in invaded ecosystems than in native ecosystems based on the meta-analysis of 94 experimental studies. The reason for the differences in soil chemical property might be the effect of Eupatorium adenophorum invasion or also might be a spatial heterogeneity effect (Sun et al., Citation2013). All micronutrients viz. Extractable Ca, Mg. K, Na, and sulphur were showed no significant seasonality or site relation may be the effect of invasive because of site specific variation in biomass and litter fall. However, it has been noticed in the computed data that there is no definite pattern of variation in enzyme activity either among selected sites or between seasons. Such trend was also reported in previous studies. The differences in the sources of substrate availability and composition may lead to the changed behaviours of the activity of hydrolytic enzymes, such as urease and β-glucosidase in soils (Song et al., Citation2012).
5. Conclusion
Therefore, it is learnt from the study that as far as the densely growing invasive plant S. alata is concerned, in addition to the soil characteristics and root exudates as reported earlier, the shady environment liked with moisture content of the soil beneath the plant canopies influence much not only spatial heterogeneity in soil enzyme activity but also seasonal disparity in soil characteristics. Concluding, it is strongly stated that invasive plants particularly S. alata, is extremely successful in its invasion because of its invasive characteristics viz. continuous litter production and allelopathic chemical constituents present in the litter inked with longer period of moisture content formed by denser canopy slowly reorient original soil characteristics and nutrient recycling in the invaded soil.
Disclosure statement
No potential conflict of interest was reported by the authors.
Acknowledgement
Authors are thankful for Department of Ecology and Environmental Sciences for providing laboratory facilities to take up various soil analyses. We express our gratitude to Dr Yogamoorthi, Dr Deviprasad, and Dr S.M. Sundarapandian for their help in statistical analysis and suggestion gave while preparing the draft. We also extend our thanks to all laboratory assistant for their full cooperation extended throughout sample analysis.
References
- Ajwa, H.A., & Tabatabai, M.A. (1994). Decomposition of different organic materials in soils. Biology and Fertility of Soils, 18(3), 175–182.10.1007/BF00647664
- Bashour, I., & Sayegh, A.H. (2007). Methods of analysis for soils of arid and semi-arid regions. Rome: Published by food and agricultural organization of United Nations.
- Błońska, E. (2010). Seasonal changeability of enzymatic activity in soils of selected forest sites. Acta Scientiarum PolonorumSilvarum, Colendarum Ratio et IndistriaLignaria, 9(3–4), 5–15.
- Caldwell, B.A. (2006). Effects of invasive scotch broom on soil properties in a Pacific coastal prairie soil. Applied Soil Ecology, 32(1), 149–152.10.1016/j.apsoil.2004.11.008
- Caldwell, B.A., Griffiths, R.P., & Sollins, P. (1999). Soil enzyme response to vegetation disturbance in two lowland Costa Rican soils. Soil Biology and Biochemistry, 31(12), 1603–1608.10.1016/S0038-0717(99)00067-X
- Corbin, J.D., & D’antonio, C.M. (2011). Abundance and productivity mediate invader effects on nitrogen dynamics in a California grassland. 2(3), 1–20.
- Dassonville, N., Vanderhoeven, S., Vanparys, V., Hayez, M., Gruber, W., & Meerts, P. (2008). Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia, 157(1), 131–140.10.1007/s00442-008-1054-6
- Dormaar, J. F., Johnston, A., & Smoliak, S. (1984). Seasonal changes in carbon content, and dehydrogenase, phosphatase, and urease activities in mixed prairie and fescue grassland Ah horizons. Journal of Range Management, 31–35.10.2307/3898819
- Ehrenfeld, J.G. (2003). Effects of exotic plant invasions on soil nutrient cycling processes. Ecosystems, 6, 503–523.10.1007/s10021-002-0151-3
- Eivazi, F., & Tabatabai, M.A. (1988). Glucosidases and galactosidases in soils. Soil Biology and Biochemistry, 20(5), 601–606.10.1016/0038-0717(88)90141-1
- Fioretto, A., Papa, S., Curcio, E., Sorrentino, G., & Fuggi, A. (2000). Enzyme dynamics on decomposing leaf litter of Cistusincanus and Myrtuscommunis in a Mediterranean ecosystem. Soil Biology and Biochemistry, 32(13), 1847–1855.10.1016/S0038-0717(00)00158-9
- Gibbons, S.M., Lekberg, Y., Mummey, D.L., Sangwan, N., Ramsey, P.W., & Gilbert, J.A. (2017). Invasive plants rapidly reshape soil properties in a grassland ecosystem. mSystems, 2(2), e00178–16.
- Grierson, P.F., & Adams, M.A. (2000). Plant species affect acid phosphatase, ergosterol and microbial P in a Jarrah (Eucalyptus marginata Donn ex Sm.) forest in south-western Australia. Soil Biology and Biochemistry, 32(13), 1817–1827.10.1016/S0038-0717(00)00155-3
- Hernández, D.L., & Hobbie, S.E. (2010). The effects of substrate composition, quantity, and diversity of microbial activity. Plant and Soil, 335(1–2), 397–411.10.1007/s11104-010-0428-9
- Hesse, P.R. (1971). A textbook of soil chemical analysis. London: John Murray.
- Khadka, A. (2017). Assessment of the perceived effects and management challenges of Mikania micrantha invasion in Chitwan National Park buffer zone community forest, Nepal. Heliyon, 3(4), e00289.10.1016/j.heliyon.2017.e00289
- Kourtev, P.S., Ehrenfeld, J.G., & Häggblom, M. (2003). Experimental analysis of the effect of exotic and native plant species on the structure and function of soil microbial communities. Soil Biology and Biochemistry, 35(7), 895–905.10.1016/S0038-0717(03)00120-2
- Krämer, S., & Green, D.M. (2000). Acid and alkaline phosphatase dynamics and their relationship to soil microclimate in a semiarid woodland. Soil Biology and Biochemistry, 32(2), 179–188.10.1016/S0038-0717(99)00140-6
- Li, W.H., Zhang, C., Jiang, H.B., Xin, G.R., & Yang, Z.Y. (2006). Changes in soil microbial community associated with invasion of the exotic weed, Mikaniamicrantha HB K. Plant, and Soil, 281(1–2), 309–324.10.1007/s11104-005-9641-3
- Liao, C., Peng, R., Luo, Y., Zhou, X., Wu, X., Fang, C., … Li, B. (2008). Altered ecosystem carbon and nitrogen cycles by plant invasion: A meta-analysis. New Phytologist, 177(3), 706–714.10.1111/nph.2008.177.issue-3
- Liu, N., Charrua, A.B., Weng, C.H., Yuan, X., & Ding, F. (2015). Characterization of biochars derived from agriculture wastes and their adsorptive removal of atrazine from aqueous solution: A comparative study. Bioresource Technology, 198, 55–62.10.1016/j.biortech.2015.08.129
- Marcelino, V.R., & Verbruggen, H. (2015). Ecological niche models of invasive seaweeds. Journal of Phycology, 51(4), 606–620.10.1111/jpy.12322
- Margesin, R., & Schinner, F. (2005). Manual for soil analysis -monitoring and assessing soil bioremediation. Berlin: Springer-Verlag Publication.
- Martinez, C.E., & Tabatabai, M.A. (1997). Decomposition of biotechnology by-products in soils. Journal of Environment Quality, 26(3), 625–632.10.2134/jeq1997.00472425002600030006x
- Moraghebi, F.1, Matinizadeh, M., Khanjani, S.B., Teimouri, M., & Afdideh, F. (2012). Seasonal variation of urease and alkaline phosphatase activity in natural and artificial habitats of hazel. Journal of Medicinal Plants Research, 6(14), 2714–2720.
- Olsen, S.R. (1954). Estimation of available phosphorus in soils by extraction with sodium bicarbonate. Washington, DC: United States Department Of Agriculture.
- Piper, C.S. (1966). Soil and plant analysis; A laboratory manual of methods for the examination of soils and the determination of the inorganic constituents of plants. Bombay: Hans Publications.
- Powell, K.I., Chase, J.M., & Knight, T.M. (2011). A synthesis of plant invasion effects on biodiversity across spatial scales. American Journal of Botany, 98(3), 539–548.10.3732/ajb.1000402
- Quist, C.W., Vervoort, M.T., Van Megen, H., Gort, G., Bakker, J., Van der Putten, W. H., & Helder, J. (2014). Selective alteration of soil food web components by invasive giant goldenrod Solidagogigantea in two distinct habitat types. Oikos, 123(7), 837–845.10.1111/more.2014.123.issue-7
- Richards, L.A. (1954). Diagnosis and improvement of saline and alkali soils (p. 160). Washington, DC: United States Salinity Laboratory. USDA. Agriculture Handbook, 60.
- Rodrigues, I.M.C., Souza Filho, A.P.S., Ferreira, F.A., & Demuner, A.J. (2010). Chemical prospecting of compounds produced by Senna alata with allelopathic activity. Planta Daninha, 28(1), 1–12.
- Rusterholz, H.P., Salamon, J.A., Ruckli, R., & Baur, B. (2014). Effects of the annual invasive plant Impatiens glandulifera on the Collembola and Acari communities in a deciduous forest. Pedobiologia, 57(4–6), 285–291.10.1016/j.pedobi.2014.07.001
- Sanon, A., Beguiristain, T., Cebron, A., Berthelin, J., Ndoye, I., Leyval, C., … Duponnois, R. (2009). Changes in soil diversity and global activities following invasions of the exotic invasive plant, Amaranthus viridis L., decrease the growth of native sahelian Acacia species. FEMS Microbiology Ecology, 70(1), 118–131.10.1111/fem.2009.70.issue-1
- Schinner, F., & von Mersi, W. (1990). Xylanase-, CM-cellulase- and invertase activity in soil: An improved method. Soil Biology & Biochemistry, 22, 511–515.10.1016/0038-0717(90)90187-5
- Sedia, E.G., & Ehrenfeld, J.G. (2006). Differential effects of lichens and mosses on soil enzyme activity and litter decomposition. Biology and Fertility of Soils, 43(2), 177–189.10.1007/s00374-006-0077-6
- Shao, X., Yang, W., & Wu, M. (2015). Seasonal dynamics of soil labile organic carbon and enzyme activities in relation to vegetation types in Hangzhou Bay tidal flat wetland. PloS One, 10(11), e0142677.10.1371/journal.pone.0142677
- Sims, J.R., & Jackson, G.D. (1971). Rapid analysis of soil nitrate with chromotropic acid. Soil Science Society of America Journal, 35, 603–606.10.2136/sssaj1971.03615995003500040035x
- Sinsabaugh, R.L., & Moorhead, D.L. (1994). Resource allocation to extracellular enzyme production: A model for nitrogen and phosphorus control of litter decomposition. Soil Biology and Biochemistry, 26(10), 1305–1311.10.1016/0038-0717(94)90211-9
- Sinsabaugh, R.L., & Linkins, A.E. (1988). Adsorption of cellulase components by leaf litter. Soil Biology and Biochemistry, 20(6), 927–931.10.1016/0038-0717(88)90105-8
- Sinsabaugh, R.L., Benfield, E.F., & Linkins, A.E. (1981). Cellulase activity associated with the decomposition of leaf litter in a woodland stream [Quercusalba, Acer rubrum, Cornusflorida, Virginia (USA)]. Oikos (Denmark).
- Song, Y., Song, C., Yang, G., Miao, Y., Wang, J., & Guo, Y. (2012). Changes in labile organic carbon fractions and soil enzyme activities after marshland reclamation and restoration in the Sanjiang Plain in Northeast China. Environmental Management, 50(3), 418–426.10.1007/s00267-012-9890-x
- Stefanowicz, A.M., Stanek, M., Nobis, M., & Zubek, S. (2016). Species-specific effects of plant invasions on activity, biomass, and composition of soil microbial communities. Biology and Fertility of Soils, 52(6), 841–852.10.1007/s00374-016-1122-8
- Sun, X., Gao, C., & Guo, L. (2013). Changes in soil microbial community and enzyme activity along an exotic plant Eupatorium adenophoruminvasion in a Chinese secondary forest. Chinese Science Bulletin, 58(33), 4101–4108.10.1007/s11434-013-5955-3
- Tabatabai, M.A., & Bremner, J.M. (1969). Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biology and Biochemistry, 1, 301–307.10.1016/0038-0717(69)90012-1
- Tabatabai, M.A. (1982). Soil enzymes. In A.L. Page, R.H. Miller, & D.R. Keeney (Eds.), Methods of soil analysis. Part I (pp. 903–947). Madison, WI: Agronomy 9.
- Tabatabai, M.A., & Bremner, J.M. (1972). Assay of urease activity in soils. Soil Biology and Biochemistry, 4(4), 479–487.10.1016/0038-0717(72)90064-8
- Tian, L., Dell, E., & Shi, W. (2010). The chemical composition of dissolved organic matter in agroecosystems: Correlations with soil enzyme activity and carbon and nitrogen mineralization. Applied Soil Ecology, 46(3), 426–435.10.1016/j.apsoil.2010.09.007
- Tylianakis, J.M., Didham, R.K., Bascompte, J., & Wardle, D.A. (2008). Global change and species interactions in terrestrial ecosystems. Ecology Letters, 11(12), 1351–1363.10.1111/ele.2008.11.issue-12
- Uddin, M.N., & Robinson, R.W. (2017). Responses of plant species diversity and soil physical-chemical-microbial properties to Phragmites australis invasion along a density gradient. Scientific Reports, 7(1), 11007.10.1038/s41598-017-11205-0
- Vilà, M., Espinar, J.L., Hejda, M., Hulme, P.E., Jarošík, V., Maron, J.L., … Pyšek, P. (2011). Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecology Letters, 14(7), 702–708.10.1111/ele.2011.14.issue-7
- Waring, S. A., & Bremner, J. M. (1964). Ammonium production in soil under waterlogged conditions as an index of nitrogen availability. Nature, 201, 951–952.10.1038/201951a0
- Weidenhamer, J.D., & Callaway, R.M. (2010). Direct and indirect effects of invasive plants on soil chemistry and ecosystem function. Journal of Chemical Ecology, 36(1), 59–69.10.1007/s10886-009-9735-0