Abstract
The concentration of trace elements Ba, Sr, V, Cr, Ni, Pb, Cu, Zn, As, Mn, and Co were assed in the surface soil of agricultural lands, Puducherry region. The ranking order of occurrence of the heavy metals in 10 farms soils was Ba > Sr > Mn > Cr > V > Zn > Ni > Cu > Pb > Co > As indicating that Ba concentration was high. Trace elements such as, Cu, Cr, V, Zn, Pb, Ni, Co, and As enrichment levels were high in current years. Most of the farming soil showed metals Ba and Sr were higher than maximum permissible limit. Organic farming (farm 3, 6, and 9) soils were containing higher amount of plant available nutrients, sol microbial population and β-glucosidase activity. Cr, Zn, Cu, Pb, Co, and As trace elements were stronger inhibitory effects on soil biological properties. The enrichment of these trace elements in agriculture soil samples conforms their higher input of trace element contaminated organic manures, synthetic fertilizer, and fungicides by the farmers.
Introduction
Trace elements are very essential for growth of plants, enzyme production, hormone regulation, and protein synthesis. There are nine elements such as Fe, Mn, Cl, Zn, Ni, Cu, B, Co, and Mo that are required in very small quantities. However, when the concentration of such trace elements goes higher which leads to negative effects on soil physico-chemical and biological properties (Venkatesan & Senthurpandian, Citation2006). The concentrations of trace elements in soils are associated with biological and geochemical cycles. They are influenced by anthropogenic activities, such as, transport, waste disposal, industrialization, social, and agricultural activities have an effect on environmental pollution and the global ecosystem (Wong, Li, Zhang, Qi, & Min, Citation2002). These functions lead to a negative effect on human health and on all living organisms. Although trace elements are naturally present in soil, contamination and comes from local sources: mostly industry waste incineration, combustion of fossil fuels, irrigation with polluted waters, syntactic fertilizer, contaminated manure, and pesticide containing heavy metals (Fytianos, Katsianis, Triantafyllou, & Zachariadis, Citation2001).
Additional use of fertilizers and pesticides in agricultural activities to increase productivity due to the rapid population increase. However, excessive usage threatens to the groundwater quality and soil health on a large scale. In most of the countries, soils and waters have been contaminated by fertilizers and pesticides used during agricultural activities (Rapheal & Adebayo, Citation2011). Organic materials, such as farm manures, bio-solids, or composts contain higher concentration of trace elements than most agricultural soils. The use of bio-solids and compost increases the total amount of Cu, Zn, Pb, Cd, Fe, Ba, and Mn in soils (Keskin, Citation2010).
The agricultural system in India is typically a monsoon-driven low-input farming with limited use of organic amendments. The inorganic chemical fertilizers with inadequate organic amendments are used primarily to meet the gap between the soil reserve and crop requirement. However, such farming practices affects the physical, chemical, mineral, biological processes, and biochemical properties of soil. In Pondicherry region, most of the farmers follow indiscriminate usage of inorganic fertilizer and synthetic pesticides which leads to enhance ecological imbalance, soil salinity, and health hazards (Reddy, Satpathy, & Dhiviya, Citation2013). Hence, the present study was undertaken to assess the trace elements and its influence on physico-chemical and biological properties in agricultural farming soils, Puducherry region.
Materials and methods
Study area and soil sampling
Pondicherry is located along the Coramandel coast of peninsular India with the geographical coordinates 11° 52′N, 79° 45′E, 11° 59′N and 79° 52′ E covering an area of 480 km. The mean annual rainfall of the study area is about 1311–1172 mm. The mean number of annual rainy days is 55; the mean monthly temperature ranges between 21 and 30 °C in the study area. This region gets more rainfall during north-east monsoon. Humidity is also high in this region as the study area is located near the coast. The study sites, Sorriyankuppam, Bahour, and Kuruvinatham is located 25 km away from the Puducherry city towards to Cuddalore district, Nallavadu site is 10 km away from Puducherry city and the other site is Kalapet, located 11 km from the city. In this study region, comes under Eastern Coastal plains, Agro-climatic zone of India, predominant soil type is coastal alluvium and the texture of soils is sandy loam. Rice, groundnut, and sugarcane are the predominant crops cultivated in the study area (Figure ).
Figure 1 Study area map. Image modified from: Pondicherry/Puducherry Road Map (available at https://www.mapsofindia.com/maps/pondicherry/pondicherry_road.htm).
Soil from 10 agricultural farms were sampled from June 2013 to December 2014. They were located in Kalapet (farm 1&2), Kuruvinatham (farm 3&4), Sorriyankuppam (farm 5&6), Bahour (7&8), and Nallavadu (farm 9&10). Among 10 farms, farm 3, 6, and 9 were adopted organic farming and any synthetic fertilizer, pesticides and herbicides were not used. Three composite soil samples were collected from each of the 10 farms. Composite samples were done by sampling approximately 15 kg of soil from each of the three farming system using augur at 0–15 cm depth. Bulked samples were kept separately according to the location within each field for replication maintenance. Composite soil samples were stored in deep freezer to control microbial and enzyme activities for soil dilution, plating and biological analysis. The soil was transferred to the storage room and were stored at 40 °C until the time of analysis. Microbial and enzyme analysis were done within 48–72 h.
Analyses of soil trace elements and physico-chemical properties
The soil trace elements Ba (barium), Sr (strontium), V (vanadium), Cr (chromium), Ni (nickel), Pb (lead), Cu (copper), Zn (zinc), As (arsenic), Mn (manganese), and Co (cobalt) were analyzed by WD-XRF method (Beckhoff, Kanngießer, Langhoff, Wedell, & Wolff, Citation2007). Soil reaction (pH) (1:2 soil water suspension) was determined by potentiometry method (Jackson, Citation1973). Soil salinity (E C) (1:2 soil water suspension) was determined by conductometry method (Jackson, Citation1973). Total nitrogen (N) was determined by Macro-Kjeldahl digestion (Piper, Citation1966). Total phosphorus, total potassium, were analyzed by WD XRF instrument (Beckhoff et al., Citation2007).Organic carbon (OC) was determined by chromic acid wet digestion (Walkley & Black, Citation1934). Ammonium nitrogen (NH4-N) was determined by nitroprusside catalyst method (Bashour & Sayegh, Citation2007). Nitrate nitrogen (NO3-N) was determined by chromotrophic acid spectrophotometric method (Sims & Jackson, Citation1971). Extractable phosphorus was determined by modified Olsen method (Olson & Somers, Citation1982). Exchangeable potassium (K) and calcium (Ca) were determined by flame photometry (Stanford & English, Citation1949). Sulphate (SO4) amount was determined by turbidimetric method (Tendon, Citation1991).
Analyses of soil biological properties
Soil respiration was determined by CO2 during the incubation of soil in closed system, CO2 is trapped in NaOH (0.05 M) solution, then the trapped solution was titrated with HCl solution (0.05 M) which is expressed as CO2(mg). 100 g of soil−1 day−1(Monaco, Hatch, Sacco, Bertora, & Grignani, Citation2008).β-glucosidase activity was determined by para nitrophenol release after the incubation of soil with para nitrophenylglucoside solution for 1 h at 37 °C (Tabatabai, Citation1982). The numbers of bacteria, fungi, and actinomycetes were determined by serial dilution plate count method (Germida, Citation1993). The colony forming units were expressed as CFU 10n g of soil on the moisture basis.
Data analysis
All the experimental data were analyzed with SPSS/16. The relationship among heavy metals, soil chemical, and biological properties was analyzed by person correlation.
Result and discussion
Trace elements in coastal agroecosystem soils
The individual results obtained for each trace elements concentration in soil and maximal permitted threshold of potentially toxic metals prescribed by WHO guidelines were also given in Table . Among 10 farming soil chromium concentration was varied between 27 and 66 mg/kg, followed by Ni 9–17 mg/kg, Cu 6–16 mg/kg, Zn 9–43 mg/kg, As 2–7 mg/kg, Pb 7–16 mg/kg, Mn 52–152 mg/kg, V 27–58 mg/kg, Co 5–9 mg/kg, Sr 21–336 mg/kg, and Ba 78–654 mg/kg. The ranking order of occurrence of the heavy metals in 10 farms soils was Ba > Sr > Mn > Cr > V > Zn > Ni > Cu > Pb > Co >As indicating that Ba concentration was high. Cr, Ni, Cu, and V concentration were higher in farm 3 soil. Pb, Sr, and Ba concentration were higher in farm 9 soil. Mn and Co concentration were higher in farm 6, followed by Zn and As concentration were higher in farm 2 and farm 10. These findings agree with previous research study and also concentration of heavy metal such as Mn, Zn, Pb, Cr, and Cu were increased in recent times (Reddy et al., Citation2013).
Table 1. Concentration of trace elements in soils.
During the study period Cu, Cr, V, Zn, Pb, Ni, Co, and As levels in soil were shows low as compare to permissible limit. The levels of Cu and V in soil normally reflect the concentration in parent and pedogenic process, like Cu in igneous basaltic rocks (90 mg/kg). Composition of the parent material has less bearing on V content of mature, developed soils. Zinc is readily adsorbed by clay minerals, carbonates. Moreover, the Zn, Cu, and V level in soil are within permissible levels, which indicate its normal concentration and reflect the background value in soil. The geogenic contribution and fertilizer applications are the main sources of trace elements occurrence in faming soils. Our findings showed level of Zn and Cu were increased compare with previous study (Reddy et al., Citation2013). Most of the farming soil showed higher concentration of Ba compare with maximum permissible limit. Barium waste may be released to air, soil, and water during nearest industrial operations (Kabata-Pendias, Citation2010).
Soil physico-chemical and biological properties
The results revealed that pH range was higher in farm 8 (8) lowest in farm 10 (6). EC was higher in farm 5 (0.43 mS/cm) lower was in farm 1 (0.069 mS/cm) (Table ). The total amount of nitrogen (3.57 g/kg), total phosphorus (2.38 g/kg) and NO3-N (2.17) and SO4 (1.14 mg/kg) were present higher amount in farm 3 soil. Total potassium (62.2 g/kg), NH4-N (1.1 g/kg) and extractable phosphorus (0.62 g/kg) were present higher amount in farm 9 soil. Farm 2 soil was containing higher amount of exchangeable calcium (2.04 mg/kg) (Table ). Amount of organic carbon was higher in farm 6(9.6 g/kg) (Tables and ). Among 10 farms, farm 3, 6, and 9 were practicing organic agriculture and application of biofertilizers, farmyard manure, and vermicompost were also huge. These factors were enhanced the amount total N, total P, NO3-N, SO4-S, and microbial population in soil (Padmavathy & Poyyamoli, Citation2011).
Table 2. Physico-chemical characterization of soil samples.
Table 3. Physico-chemical characterization of soil samples.
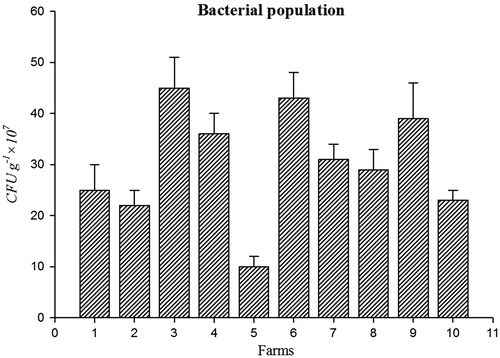
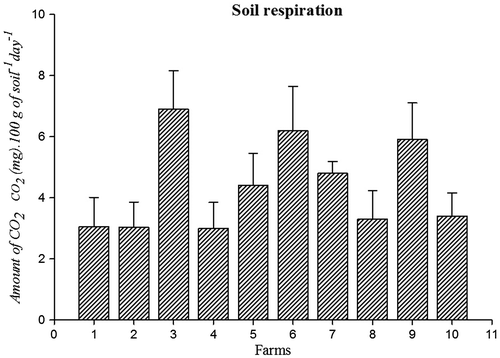
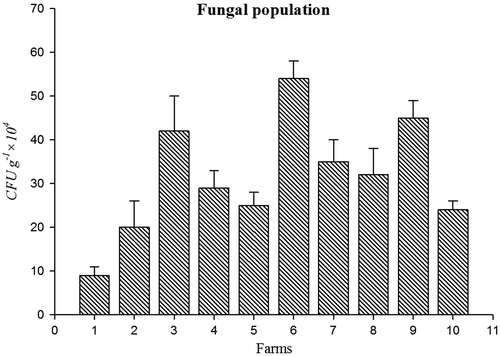
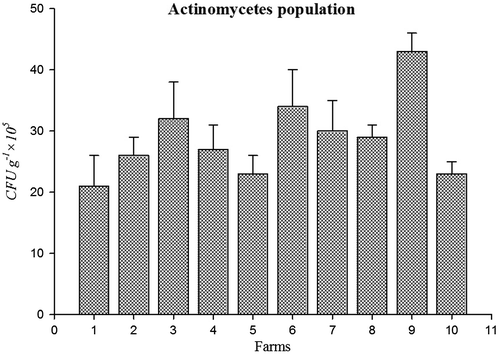
Bacterial population (45 CFU g−1 × 107) (Figure ) and amount of soil respiration (Figure ) (6.9 CO2 (mg).100 g of soil−1 day- 1) were higher in farm 3 soil. Population of fungi (54 CFU g−1 × 104) was high in farm 6 soil (Figure ) and actinomycetes (CFU g−1 × 105) Population was high in farm 9 (Figure ). Soil respiration was significantly higher in organic farms 3, 6, and 9. This indicates a higher soil microbial activity due to the addition of liable organic matter to the soil because of the stimulation of heterotrophic micro-organisms (Araújo, Leite, Santos, & Carneiro, Citation2009). Soil organisms contributed to wide range of functions are essential for all ecosystem. This function includes C, P, N, and S cycling and turns over soil organic matter by enhancing the efficiency of plant available nutrients and utilized by crops (Chinnadurai, Gopalaswamy, & Balachandar, Citation2014).
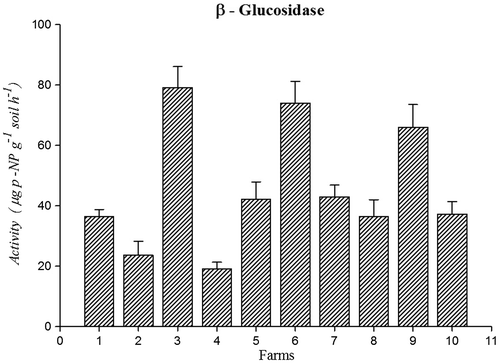
Activity of β-glucosidase (79.18 mg p-NP g−1 soil h−1) (Figure ) was higher in farm 3 soil. β-glucosidase was common and major enzyme in agriculture soils. Compare to 10 farms soils organically managed farming soils showed higher activity of β-glucosidase enzyme. Microbial degradation and organic matter deposition were improved the β-glucosidase activities in soil (Sudhakaran, Ramamoorthy, & Kumar Rajesh, Citation2013). These enzyme properties can be used as a good biochemical indicator for measuring ecological changes resulting from soil acidification (Acosta-Martínez, Zobeck, Gill, & Kennedy,Citation2003). β-glucosidase enzyme is produced by a wide range of soil organism and their activity mainly links to the amount of soil organic matter. This enzyme was characteristically used as a soil quality indicator and may give reflection of past biological activity and soil organic matter (Ndiaye, Sandeno, McGrath, & Dick, Citation2000).
Influence of trace elements on soil physico-chemical and biological properties
Correlation among soil physico-chemical and biological properties showed that Ni was significantly positive correlated with total N, total P, organic carbon, SO4-S, β-glucosidase and soil respiration. Cu was significantly positive correlated with electrical conductivity, total P, and organic carbon. Pb was significantly positive correlated with total and SO4-S. Water holding capacity and total P were significantly positive correlated with Mn. total N, total P, NO3-N, bacterial population, and fungal population were significantly correlated with V. Cobalt was significantly positive correlated with total N and total P. Sr was significantly positive correlated with total K, exchangeable K and soil respiration (Table ). However, trace elements Cr, Zn, Cu, Pb, Co, and As were not show any positive correlation with biological properties. It indicates that Cr, Zn, Cu, Pb, Co, and As trace elements were stronger inhibitory effects on soil biological properties (Wiatrowska, Komisarek, & Dluzewski, Citation2015).
Table 4. Correlation among trace elements, soil physico-chemical, and biological properties.
Ba trace elements was positively correlated with more number of soil parameters Viz, total K, organic carbon, SO4-S, exchangeable K, β-glucosidase activity, soil respiration, fugal population, and actinomycetes population. Cr and Zn metals were not showed significant positive correlation with any soil parameters (Table ). Micro-organisms can adapt to high concentrations of trace elements. These findings showed that microbial populations are highly tolerated to excess level of Ba (Kabata-Pendias, Citation2010). Application of excess amount of nitrate and DAP fertilizers, Cu-based fungicides and pesticides main reason to enhance the level of heavy metal in agricultural soils. In other hand organic materials, such as farm manures, composts contain higher concentration of trace elements than most agricultural soils (Irshad, Malik, Shaukat, Mushtaq, & Ashraf, Citation2013). These factors also lead to increasing level of trace elements in organic farming soils. The use of bio-solids and composts increases total amount of Cu, Zn, Pb, Cd, Fe, and Mn in soils (Hariprasad & Dayananda, Citation2013).
Conclusion
The present study showed that organic farming (farm 3, 6, and 9) soils were containing higher amount of plant available nutrients, soil microbial population, and β-glucosidase activity. Microbial activities was the main factor for enhance the plant available nutrients in agriculture soil thorough the process of organic matter decomposition and mineralization. Heavy metals such as, Cu, Cr, V, Zn, Pb, Ni, Co, and As concentration were less than permissible limits. However, enrichment level of these metals was higher in current years. Most of the farming soil showed metals Ba and Sr were higher than maximum permissible limit. It can be concluded that all farming soils effected by Ba and Sr metals toxicity. The enrichment of these metals in agriculture soil samples conforms their higher input of trace element contaminated organic manures, synthetic fertilizer, and fungicides by the farmers.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The present research was funded through a Research project ID. No. 10/DSTE/RP/JSA-1/2013/184, dated 12.03.2013, funded by Department of Science, Technology and Environment, Government of Puducherry.
Acknowledgement
The authors are grateful towards the department of Ecology and Environmental Science, Pondicherry University for providing laboratory facilities in order to conduct the experiment. They extend their gratitude to Central Instrumentation Facility, Pondicherry University for soil trace element analysis.
References
- Acosta-Martínez, V., Zobeck, T.M., Gill, T.E., & Kennedy, A.C. (2003). Enzyme activities and microbial community structure in semiarid agricultural soils. Biology and Fertility of Soils, 38(4), 216–227.10.1007/s00374-003-0626-1
- Araújo, A.S., Leite, L.F., Santos, V.B., & Carneiro, R.F. (2009). Soil microbial activity in conventional and organic agricultural systems. Sustainability, 1(2), 268–276.10.3390/su1020268
- Bashour, I.I., & Sayegh, A.H. (2007). Methods of analysis for soils of arid and semi-arid regions. Rome: FAO.
- Beckhoff, B., Kanngießer, B., Langhoff, N., Wedell, R., & Wolff, H. (2007). Handbook of practical X-ray fluorescence analysis. Berlin: Springer Science & Business Media.
- Chinnadurai, C., Gopalaswamy, G., & Balachandar, D. (2014). Impact of long-term organic and inorganic nutrient managements on the biological properties and eubacterial community diversity of the Indian semi-arid Alfisol. Archives of Agronomy and Soil Science, 60(4), 531–548.10.1080/03650340.2013.803072
- Fytianos, K., Katsianis, G., Triantafyllou, P., & Zachariadis, G. (2001). Accumulation of heavy metals in vegetables grown in an industrial area in relation to soil. Bulletin of Environmental Contamination and Toxicology, 67(3), 423–430.10.1007/s001280141
- Germida, J.J. (1993). Cultural methods for soil microorganisms. In M.R. Carter (Ed.), Soil sampling and methods of analysis. A special publication of the canadian society of soil science (pp. 263–275). Boca Raton, FL: Lewis.
- Hariprasad, N.V., & Dayananda, H.S. (2013). Environmental impact due to agricultural runoff containing heavy metals – A review. International Journal of Scientific and Research Publications, 3(5), 224–280.
- Irshad, M., Malik, A.H., Shaukat, S., Mushtaq, S., & Ashraf, M. (2013). Characterization of heavy metals in livestock manures. Polish Journal of Environmental Studies, 22(4), 1257–1262.
- Jackson, M.L. (1973). Soil chemical analysis. New Delhi: Prentice Hall of India.
- Kabata-Pendias, A. (2010). Trace elements in soils and plants. London: CRC Press.10.1201/b10158
- Keskin, T.E. (2010). Nitrate and heavy metal pollution resulting from agricultural activity: A case study from Eskipazar (Karabuk, Turkey). Environmental Earth Sciences, 61(4), 703–721.10.1007/s12665-009-0385-x
- Monaco, S., Hatch, D.J., Sacco, D., Bertora, C., & Grignani, C. (2008). Changes in chemical and biochemical soil properties induced by 11-yr repeated additions of different organic materials in maize-based forage systems. Soil Biology and Biochemistry, 40(3), 608–615.10.1016/j.soilbio.2007.09.015
- Ndiaye, E.L., Sandeno, J.M., McGrath, D., & Dick, R.P. (2000). Integrative biological indicators for detecting change in soil quality. American Journal of Alternative Agriculture, 15(1), 26–36.10.1017/S0889189300008432
- Olson, S.R., & Somers, L.E. (1982). Phosphorus. In A.L. Page (Ed.), Methods of soil analysis, Part 2 Agron. No. 9, part 2, Chemical and microbiological properties, 2nd ed., Am. Soc. (pp. 403–430). Madison, WI: Agronomy USA.
- Padmavathy, A., & Poyyamoli, G. (2011). Effects of conventional and organic management strategies on soil quality and biodiversity in agricultural fields of Bahour, Puducherry, India. American-Eurasian Journal of Agriculture and Environmental Science, 10(4), 644–652.
- Piper, C.S. (1966). Soil and plant analysis; A laboratory manual of methods for the examination of soils and the determination of the inorganic constitutents of plants. Bombay: Hans Publications.
- Rapheal, O., & Adebayo, K.S. (2011). Assessment of trace heavy metal contaminations of some selected vegetables irrigated with water from River Benue within Makurdi Metropolis, Benue State Nigeria. Advances in applied science research, 2(5), 590–601.
- Reddy, M.V., Satpathy, D., & Dhiviya, K.S. (2013). Assessment of heavy metals (Cd and Pb) and micronutrients (Cu, Mn, and Zn) of paddy (Oryzasativa L.) field surface soil and water in a predominantly paddy-cultivated area at Puducherry (Pondicherry, India), and effects of the agricultural runoff on the elemental concentrations of a receiving rivulet. Environmental Monitoring and Assessment, 185(8), 6693–6704.10.1007/s10661-012-3057-3
- Sims, J.R., & Jackson, G.D. (1971). Rapid analysis of soil nitrate with chromotropic acid. Soil Science Society of America Journal, 35(4), 603–606.10.2136/sssaj1971.03615995003500040035x
- Stanford, S., & English, L. (1949). Use of flame photometer in a rapid soil test for K and Ca. Agronomy Journal, 41, 446–447.10.2134/agronj1949.00021962004100090012x
- Sudhakaran, M., Ramamoorthy, D., & Kumar Rajesh, S. (2013). Impacts of conventional, sustainable and organic farming system on soil microbial population and soil biochemical properties, Puducherry. India. International Journal of Environmental Sciences, 4(1), 28.
- Tabatabai, M.A. (1982). Soil enzymes. In A.L. Page, R.H. Miller, & D.R. Keeney (Eds.), Methods of soil analysis. Parr I (pp. 903–947). Madison, WI: Agronomy 9.
- Tendon, H.L.S. (1991). Sulphur research and agricultural production in India (3rd ed.). Washington, DC: The Sulphur Institute.
- Venkatesan, S., & Senthurpandian, V.K. (2006). Enzyme activities of laterite soils as influenced by trace element contamination. International Journal of Soil Science, 1, 20–26.
- Walkley, A., & Black, I.A. (1934). An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Science, 37(1), 29–38.10.1097/00010694-193401000-00003
- Wiatrowska, K., Komisarek, J., & Dluzewski, P. (2015). Effects of heavy metals on the activity of dehydrogenases, phosphatases and urease in naturally and artificially contaminated soils. Journal of Elementology, 20(3), 743–756.
- Wong, S.C., Li, X.D., Zhang, G., Qi, S.H., & Min, Y.S. (2002). Heavy metals in agricultural soils of the Pearl River Delta, South China. Environmental Pollution, 119(1), 33–44.10.1016/S0269-7491(01)00325-6