?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Sewage, waste organic matter from domestic and municipal wastewater, causes increased secondary productivity, eutrophication and trace metal contamination, reduced oxygen levels, and biodiversity which can lead to ecological disturbances in the natural aquatic ecosystem. The impact of sewage-derived organic matter (SDOM) on the nearshore marine ecosystem of the Otago Coast was assessed before, and 15 years after upgrade of the Dunedin sewage treatment plant. Carbon and nitrogen isotopic ratios in the tissues of sentinel organisms were used as bioindicators to elucidate the primary sources of nutrition the coastal environment. Mytilus galloprovincialis, a marine bivalve, exhibited a strong influence of SDOM from two sites in 2001. In 2015, M. galloprovincialis had a trophic enrichment factor of 3‰ (δ15N) and 1‰ (δ13C) when compared to the marine particulate organic matter (POM), suggestive of a dietary change away from the SDOM. Suspended POM collected from riverine and estuarine sources revealed other possible nitrogen sources from human-driven activities such as pastoral farming, application of organic manure and inorganic fertilisers, nitrification of ammonium from semi-urban septic tanks, and animal organic waste residues.
1. Introduction
Sewage, a major organic component of domestic and municipal wastewater, can cause increased secondary productivity (Hillebrand & Sommer, Citation2000), eutrophication (Jarvie, Neal, & Withers, Citation2006), heavy metal contamination (Chary, Kamala, & Raj, Citation2008; Cheevaporn & Menasveta, Citation2003; Morillo, Usero, & Gracia, Citation2004), reduced oxygen levels, and biodiversity which can lead to ecological disturbances (Browne et al., Citation2011; Diaz, Rhoads, Blake, Kropp, & Keay, Citation2008) in the natural aquatic ecosystems (Deegan & Buchsbaum, Citation2005; Hargrave, Holmer, & Newcombe, Citation2008). Thus, it is imperative we ensure that the quality of treated wastewater effluent from municipal treatment plants meets the stipulated safe levels approved by the statutory and regulatory authorities before discharged into the receiving waterbodies (Ellis, Citation2004; Teklehaimanot, Coetzee, & Momba, Citation2014).
Inadequately treated effluent discharged into the marine area poses environmental and health hazards to the resident biota in the adjacent nearshore waters. Between 1908 and 1950s, raw sewage was discharged directly into the Pacific Ocean at Lawyers Head (Council, Citation2001) by the Dunedin Water Pollution Control Plant (now called Tahuna Wastewater Treatment Plant). Presently, the Green Island Wastewater Plant and Tahanu Wastewater Treatment Plant (TWWTP) serving over 120,000 people in Dunedin, New Zealand, discharge adequately treated wastewater effluent from Waldronville and Lawyers Head, respectively (see ), into the Pacific Ocean from two ocean outfalls pipes that were extended from 550 to 1100 m in 2009. Between 2010 and 2013, the wastewater treatment plant facilities were improved to handle both primary and secondary wastewater treatment processes. The advancement in the wastewater management involved the construction of a new pump station to the increased flow rate of the wastewater treatment plants. This was done to improve the quality of effluent and shoreline water quality, reduce organic matter content, and ensure public health protection which was a major concern at that time (Bouman & Archer, Citation2014).
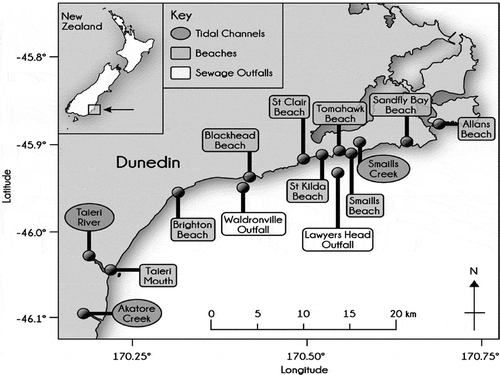
Figure 1. Schematic representation study sites along Otago Peninsula with adjoining tidal channels, creeks, and wastewater outfalls.
Before the extension of the ocean outfalls pipes, discharged wastewater effluent contaminated parts of the Otago coastal marine area which has more than 80 protected wildlife areas which accommodated marine mammals and birds along its landward edge (Council, Citation2001; Gormley et al., Citation2012; Rayment, Dawson, & Slooten, Citation2010). The various bacteriological studies (measurement of Escherichia coli conducted on shellfish, sediments, and seawater) had ascribed the contamination of the portions of Otago coastline to the wastewater effluent discharge from the TWWTP (Lewis, Loutit, & Austin, Citation2010; Nicholson, Lewis, & Loutit, Citation1989; RCL, Citation2000). One of such studies reported that over 50% of shellfish collected from the sites close to the sewage outfalls at Lawyers Head had elevated level of faecal coliforms and enteric viruses of >230 and <4600/100 g flesh, well above the European Union Class A standard for shellfish flesh. Five downstream sites closest to the sewage outfall were found to be heavily contaminated by faecal coliforms and enteric viruses while two upstream sites were considered uncontaminated (Greening, Lewis, & Dollimore, Citation2007). However, these studies were only spot tests (Doré, Henshilwood, & Lees, Citation2000; Ebner, McAllister, & Suter, Citation2009) and appropriate for assessing water quality standards (Abbasi & Abbasi, Citation2011; Dede, Telci, & Aral, Citation2013). They failed to account for other possible sources of contamination and provide no estimate of the amount of sewage-derived organic matter (SDOM) in the nearshore marine flora and fauna.
Horn (Citation2001) conducted an isotopic monitoring study on seawater samples, and the tissues of Mytilus galloprovincialis and Ulva lactuca across various beaches along the Otago Peninsula to trace the pattern and distribution of sewage effluent discharged from Lawyers Head. He observed that the nitrogen isotopic ratios of the digestive tissues of the end member mussels vary considerably. He reported that major sewage contamination occurred at Lawyers Head and Tomahawk while there was minor contamination at St. Kilda and Smaills. He found out that more than 60% of the mussels and seaweeds sampled at Lawyers Head and Tomahawk Beach had their isotopic ratios wedged by discharged sewage effluent. Isotopic study carried out by North, Frew, and Hale (Citation2006) on the possibility of landfill leachates as source of contamination from solid waste disposal site which involved the collection and analyses of surface water samples from Kaikorai wetland areas made up of stream and estuary waters from Green Island Landfill over an 8-month period revealed that landfill leachates could also be possible source of contamination to the Kaikorai downstream (North et al., Citation2006; North, Frew, & Peake, Citation2004) which eventually has a run-off into the Otago coastline at Waldronville. Consequently, there is the prospect of using stable isotopic ratios in the tissues of organisms to assess the impact SDOM and other terrigenous materials on the nearshore marine ecosystem because stable isotopic signatures in the tissues of organisms had been found to be connected with an organism diet over time and space (Bump et al., Citation2007; Rogers, Citation2003). Stable isotopic analyses on the tissues of marine flora and fauna have been extensively exploited to assess the impact of SDOM on the food web structure of nearshore marine ecosystems as well as to investigate the recovery of marine flora and fauna at sites disrupted by sewage effluent discharges (Barr, Dudley, Rogers, & Cornelisen, Citation2013; Michener & Kaufman, Citation2008; Savage, Citation2005). SDOM had been known to significantly alter the stable carbon and nitrogen isotopic signatures (expressed as the δ13C and δ15N) of marine flora (i.e., macro-algae) and fauna (e.g., filter-feeders) (Bedard-Haughn, Van Groenigen, & Van Kessel, Citation2003; Dudley & Shima, Citation2010) permitting them to exhibit distinct isotopic signatures in their tissues as a reflection of the integration, assimilation, and utilisation of the SDOM in their immediate environment over time. The carbon and nitrogen isotopic signatures are the comparisons of ratios of the heavy-to-light isotope of the element. They are mathematically expressed (see Equation 1) in δ notation in terms of parts per thousand (‰) or “per mil” and calculated as follows:
(1) (Coplen, Citation2011)
where n is the atomic mass of the heavy element, X can either be C or N whereas R is the isotopic ratios of X (l3C/12C or 15N/14N). The δ13C and δ15N are measured relative to international reference standards of Vienna PeeDee Belemnite and Atmospheric Nitrogen, respectively. Consumer organisms have been known to exhibit isotopic signatures which can either be similar or vary from their diets with an average fractionation trophic enrichment of 0.4–1‰ for δ13C 1987 and 3–4‰ for δ15N (Kline & Thomas, Citation1999; Post, Citation2002).
In assessing the impact of SDOM in a nearshore marine ecosystem, the disparities in the carbon and nitrogen isotopic ratios in the tissues of end members can become useful monitoring tools for providing more information on the source and magnitude of sewage contribution to the diet of resident biota (Peterson, Citation1999). Hence for the purpose of this study, stable isotopic ratios in the tissues of M. galloprovincialis (marine bivalve) and U. lactuca (sea lettuce) were specifically chosen as indicators to be used. Mussels are sedentary and long-lived (Alfaro, Jeffs, & Hooker, Citation2001; Nordsieck, Citation2006; SITO, Citation2006) characteristic features which make them suitable sentinel organisms as time-averaged integrators of sewage exposure for the sea lettuces which are short lived by nature (Cabana & Rasmussen, Citation1996; Dudley & Shima, Citation2010; Post, Citation2002).
Furthermore, filter feeders such as bivalves (i.e., mussels) can directly ingest (via the gills) and assimilate sewage particulate organic matter (POM) (containing carbon and nitrogen) along with their diet (phytoplankton and detritus) from the water column into the tissues and reassigned such higher up the food chain (biomagnification) (Ouédraogo, Chételat, & Amyot, Citation2015; Pan & Wen-Xiong, Citation2004). In choosing the most appropriate tissues for the stable isotopic study, consideration was given to the previous studies conducted by other workers on carbon and nitrogen isotopic turnover and enrichment in the different tissues of organisms. Most of the studies showed that carbon and nitrogen isotopic turnover rates and isotopic enrichment in organisms are tissue specific and influenced by lipid content of the tissue (Lorrain et al., Citation2002; Thompson, Phillips, Stewart, & Waldron, Citation2000). Thus, a preliminary stable isotopic study to investigate the variance in isotopic ratios of carbon and nitrogen in the different tissues of M. galloprovincialis was carried out to determine the appropriate tissues to be used for this study. The results obtained were compared with other isotopic studies using power analysis for determining sample size. This was done to control the number of samples to be collected to avoid unnecessary sacrifice of biological samples to be used and ensure the reliability of our results. The abductor tissue was noted to have the highest isotopic carbon and nitrogen turnover whereas the digestive tissue has the lowest isotopic tissue turnover. This observation was in accordance with other workers (Deudero, Box, Tejada, & Tintoré, Citation2009; Gaston & Suthers, Citation2004). The abductor and digestive tissues of the blue mussel were chosen as the appropriate indicators for assessing the impact of SDOM on the nearshore marine fauna in the nearshore marine waters. To the best of our knowledge, no stable isotopic studies to assess the impact of the modifications in the sewage treatment and disposal on the nearshore marine waters and resident biota along the Otago Peninsula had been carried out. Hence, our study is attempted to exploit the isotopic ratios of sentinel organisms at these sites so as to find out the current status of organic materials in the tissues of M. galloprovincialis and Ulva latuca of the nearshore marine waters. This present study will address the following research questions:
Are the variabilities in the δ13C and δ15N in the tissues of M. galloprovincialis and U. lactuca reliable indicators of assessing sewage contamination in the nearshore marine waters along the Otago Peninsula?
Has the improvement in the sewage treatment processes and disposal at the TWWTP brought significant changes in the carbon and nitrogen isotopic signatures of M. galloprovincialis and U. lactuca collected from the nearshore marine ecosystem along the Otago Peninsula?
Are there other possible terrestrial-based organic materials contributing carbon and nitrogenous materials to the nearshore waters which may sway the carbon and nitrogen isotopic ratios of M. galloprovincialis and U. lactuca?
How much of terrestrial-based organic δ13C carbon and δ15N nitrogenous materials are integrated into the tissues (i.e., digestive) of M. galloprovincialis collected from the nearshore marine waters along the Otago Peninsula?
To answer these questions, the linear mixed effects model analysis, an extension of regression analysis, was used to compare the stable carbon and nitrogen isotopic ratios (δ13C and δ15N) in the tissues of M. galloprovincialis and U. lactuca collected in 2001 (before upgrade of the sewage treatment plant) and 2015/16 (after upgrade of the sewage treatment plant) in the end members so as to determine if there had been changes in the isotopic signatures δ13C and δ15N in the tissues of M. galloprovincialis and U. lactuca collected from the study sites classified into control sites (uncontaminated sites) and previously sewage-contaminated sites comprising eight beaches and three tidal channels. The groupings of these sites were based on proximity to the sewage outfall, the written reports of the 2000 ORC Resource Consent 97530 97530 (RCL, Citation2000) and 2007 FRST Programme C03X0301 (Greening et al., Citation2007). The nature of possible sources of carbon and nitrogen into the nearshore marine waters was identified and discerned from the distinctive isotopic ratios of the POM collected from the tidal channels feeding the coastal marine waters. A mass balance linear mixing model equation was used in estimating the source contributions of carbon and nitrogen materials in the tissue of marine biota collected from the study sites along Otago Peninsula. The two main sources considered were marine POM and sewage effluent POM. This was done to quantify the contribution of sewage material in the marine biota. The findings from this study will show the potential of deploying multistable isotopic techniques and fitting mixing model to be used as a tool to elucidate the flow and fate of various sources of organic materials in the nearshore marine waters. This will enhance better understanding of the impact of anthropogenic organic carbon and nitrogenous materials on the nearshore marine waters and its consequences on the functioning of the coastal marine ecosystem. Insights into the influence of human-induced stressors on the dynamics of organic materials in the nearshore marine ecosystem will ensure proper management, conservation, and preservation of the coastal marine aquaculture resources through comprehensive ecosystem-based management with a focus on proper land-use management.
2. Material and methods
2.1. Sampling sites
M. galloprovincialis and U. lactuca were collected from the eight beaches along the Otago coastline at low tide before the upgrade of the treatment plants from 4/05/01 to 10/08/01 (weekly) and after the upgrade from 9/12/15 to 13/4/2016 (weekly). Samples were collected from the rocks in the intertidal zone. The marine bivalves were of average uniform sizes to avoid sampling bias which might ensue from in-site and site–site isotopic variability. The study sites cover about 48 km within the Otago Marine Area. Water samples were collected from the three tidal channels having a free connection to the nearshore marine waters (). At each of the sampling sites, the date and time of sampling, prevailing weather conditions, swell, wind direction, as well as possible visible sources of contamination, were noted and recorded. The location of each of the sampling sites was noted and recorded with the aid of a handheld GPS tracking device and illustrated in .
Table 1. Sampling site with assigned code, name, and coordinates.
2.2. Sample preparation and analysis
M. galloprovincialis and U. latuca were individually placed into clean ziplock plastic bags labelled with date and sample site location. They were immediately placed in a plastic cooler for onward transport to the laboratory where they were rinsed in distilled water and frozen prior to analysis. M. galloprovincialis was dissected into different tissues (the abductor and digestive tissues of interest in this study). The dissected tissues and samples of U. latuca were dried at 70°C for 24 h. Once dry samples were homogenised with the aid of an MM400 bench-top Retsch ball mill, duplicate aliquots of 0.8 mg of homogenised tissues of the biological samples were weighed into separate 5 × 3.5 mm tin cups and further dried under vacuum overnight. Water samples collected from the eight beaches and three tidal channels were filtered through 25 mm GF/F grade to collect suspended POM. Sewage effluent and seal faecal matter were collected from source and processed for isotopic analysis. The samples with internal and certified pre-calibrated standards and blanks were placed in an autosampler carousel and combusted using the Carlo Erba NA1500. The elemental analysis–isotope ratio mass spectrometry operates in a continuous flow mode for the determination of the carbon and nitrogen isotope ratios (δ13C and δ15N). Nitrogen and carbon isotopes were assayed by combustion of the samples in the chromium oxide combustion column of the elemental analyser to produce N2 and CO2, using helium carrier gas. These gases were resolved in a packed molecular sieve GC column and sent sequentially to the inlet of Europa Scientific continuous flow mode “20/20 Hydra” (Europa Scientific, UK) isotope ratio mass spectrometer (CF-IRMS) interfaced to the Sercon System Controller which runs on Sercon Callisto Software. During the programmed run tests, the samples were combusted at 1050°C, and NO2 reduced to N2 at 650°C within the elemental analyser using its normal reaction scheme. Raw isotopic ratios obtained were normalised by three-point calibration to the international scales using two International Atomic Energy Agency reference materials (USGS-40 and USGS-41) and internal laboratory standard (ethylenediaminetetra-acetic acid [EDTA-OAS], δ13C = − 38.92‰ ± 0.04; δ15N = −0.70‰ ± 0.17) of known carbon and nitrogen isotopic signatures, assayed with the unknown samples. Owing to the large number of samples, a linearity correction method was applied to ensure that the isotopic values obtained were not affected by instrumental drift. Accuracy and precision of the obtained isotopic results were assessed by employing the root mean square RMS differences between sequential duplicates of every 10th sample (IANZ, Citation2004) and random inclusion of two in-house standards (green mussel and copepod) to mimic the nature of the sample materials being analysed.
2.3. Statistical analysis
The carbon and nitrogen isotopic signature values obtained in the tissues of M. galloprovincialis and U. lactuca were subjected to statistical evaluation via linear mixed-effect models. The average values of carbon and nitrogen isotopic ratios with standard errors are represented in . Using the R Statistical Software via the linear mixed-effects 4 (lme4) (Bates, Citation2010), separate built-in linear mixed-effect models (see Equation 2) were fitted for the carbon and nitrogen isotopic signature values in the tissues of M. galloprovincialis and U. lactuca in order to perform a likelihood of ratio testing of interactions among the random and fixed effects. The random effects were the various sites while primary factors of interest such as contamination, tissue type, and year were the fixed effects. The built-in fitted model used for the linear mixed-effect modelling analysis (LMM) is expressed as follows:
Table 2. Mean isotopic carbon and nitrogen signatures (with their respective standard errors) in the tissues of Mytilus galloprovincialis and Ulva latuca collected from the uncontaminated and previously contaminated sites along Otago coastline, New Zealand in 2001 and 2015.
where
is the outcome for the ith experimental unit (sample) from site j,
is the coefficient for the intercept,
is the coefficient for the contamination effect,
is the coefficient for the year effect,
is the coefficient for the tissue effect,
is the coefficient for the interaction between contamination and year effects,
is the coefficient for the interaction between contamination and tissue effects,
is the coefficient for the interaction between year and tissue effects,
is the random effect for the ith was obtained, where
),
is the residual error term for the ith unit, where
), and
,
, and
are indicator variables for the contamination, year, and tissue effects, respectively, for the ith experimental unit where a value of 0 indicates the absence of a factor, and 1 indicates the presence.
3. Results
3.1. Variations in the carbon and nitrogen isotopic ratios in M. galloprovincialis and U. latuca
The mean carbon and nitrogen isotopic ratios in M. galloprovincialis and U. latuca sampled in 2001 and 2015 are illustrated in . The disparity values of the mean carbon and nitrogen isotopic ratios in M. galloprovincialis and U. latuca collected in 2001 and 2015 along Otago Peninsula are represented in . At the uncontaminated sites, the mean δ15N values recorded in the abductor tissues of M. galloprovincialis differed between 0.21‰ and 1.71‰ larger at BlackHead and Allans correspondingly whereas disparity values ranged between 2.52‰ and 0.40‰ lower at Brighton and Sandfly Bay accordingly than previously noted in 2001. The noticeable differences for the means of δ13C fluctuated from 0.38‰ to 1.61‰ larger at BlackHead and the Brighton accordingly. The mean δ15N values recorded in the abductor tissues of M. galloprovincialis had disparity values fluctuated between 1.12‰ and 2.35‰ larger at St. Kilda and Tomahawk correspondingly whereas disparity values varied between 0.32‰ and 0.39‰ lower at St. Clair and Smaills accordingly than previously noted in 2001. The disparity values for the means of δ13C fluctuated from 0.23‰ to 1.24‰ lower across the three previously contaminated sites except at St. Clair where it was 0.22‰ larger than noted in 2001. In the digestive tissues, the recorded disparity values in the means δ15N values in 2015 ranged between 1.37‰ and 4.01‰ larger across all the previously contaminated sites. The recorded disparity values for the means of δ13C varied between 1.06‰ and 2.06‰ lesser across all the previously contaminated sites (). In the tissues of U. latuca, the recorded disparity values for δ15N means varied between 1.93‰ and 11.59‰ larger across three sites and 1.36‰ lesser at Smaills than observed in 2001. Contrasts between the end member’s nitrogen and carbon isotopic ratios recorded in the abductor and digestive tissues of the mussels sampled in 2015 revealed that the weighted mean δ15N values differed from 0.11‰ to 1.55‰ larger in the abductor tissues than in the digestive tissues whereas the mean δ13C values varied from 0.68‰ to 2.03‰ larger at the uncontaminated sites. At the previously contaminated sites, weighted mean δ15N values fluctuated between 0.84‰ and 1.27‰ larger in the abductor tissue than digestive tissue while the weighted mean δ13C values varied between 0.82‰ and 1.82‰ larger.
Table 3. Noticeable differences (disparity values) between the mean carbon and nitrogen isotopic ratios in Mytilus galloprovincialis and Ulva latuca collected in 2001 (before the upgrade of TWWTP) and 2015 (after the upgrade of TWWTP) from the nearshore marine waters along Otago Peninsula.
3.2. POM in samples
The mean δ15N values for POM from filtered water samples ranged from 5.70 ± 0.20‰ to 10.06 ± 0.05‰ recorded at Akatore and Tomahawk Creek, respectively. The mean δ13C values for the POM ranged from −27.18 ± 0.01‰ to −21.57 ± 0.61‰ documented at Tomahawk Creek and Akatore correspondingly (). The isotopic signatures of POM from these varying aquatic systems indicated the probable sources and type of anthropogenic materials been transported into them for onward transport to the coastal marine waters. The reported higher mean carbon isotopic (δ13C) and lower nitrogen isotopic (δ15N) values of Akatore Creek and Taieri Mouth indicated marine-based organic matter while that of Tomahawk Creek seemed terrestrial due to the higher δ15N and lower δ13C isotopic ratios.
3.3. Sewage, marine, and terrigenous particulate matter in M. galloprovincialis
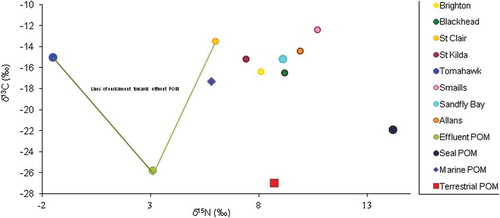
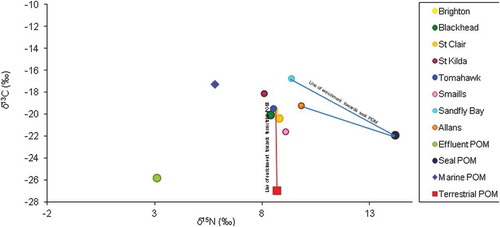
The nitrogen and carbon isotopic signatures analyses on digestive tissues of M. galloprovincialis collected in 2001 before the upgrade of the sewage treatment plant revealed that 90% of M. galloprovincialis collected from Tomahawk were heavily isotopically depleted in δ15N and δ13C with 60% of nitrogen and 40% of carbon in the digestive tissue derived from sewage particulate matter while between 30% and 60% of mussels from St. Kilda, Smaills, and St. Clair showed signs of mild sewage contamination. This is indicative that effluent seemed to provide a source of sustenance for the ingested phytoplankton biomass or direct ingestion of sewage POM by M. galloprovincialis appraised at the previously contaminated sites so much to sway the marine bivalve isotopic ratios greatly. U. latuca collected in 2001 from Tomahawk has negative mean δ15N, an indication of sewage effluent alteration, and 40% of U. latuca sampled St. Clair Beach also indicated influence by sewage effluent. In 2015, M. galloprovincialis collected and analysed showed no sign of sewage effluent contamination. The contrast between the carbon and nitrogen isotopic ratios in the digestive tissues of the mussels from all sites evidently seemed clustered close to one another () in 2015 a direct contrast to 2001 observations (). This may be attributed to these mussels feeding on a single major food source. It was observed that the mussels tend to obtain nutrition from the marine POM as most of the mussels sampled at the previously contaminated and uncontaminated sites assumed trophic shift (enrichment factor) of roughly 3‰ for δ15N and 1‰ for the δ13C cf. to the marine POM. The marine bivalve might also be deriving sustenance from other terrestrial organic materials incursions to the nearshore waters. The contrasts of carbon and nitrogen ratios in the tissues of U. latuca in the 2015 sampling were unpredictable as opposed to the trend observed in 2001. Of particular note are the results from at Tomahawk and St. Clair when 2001 nutrient enrichment was predominantly SDOM () whereas all sites exhibit the marine source predominantly in 2015 (). In 2015, no detectable sewage effluent influence was observed, rather the isotopic values plotted towards the seal faecal matter were at Allans and Sandfly Bay and terrigenous-based sources at all other sites sampled ( and ). The outline of the isotopic ratios of analysis on tissues of U. latuca seemed to assume orientation towards enrichment from characterised filtered water samples (POM) a pointer to possible nutrient enrichment from terrestrial sources.
Figure 2. Comparison of carbon and nitrogen-stable isotopic ratios in the digestive tissues of Mytilus galloprovincialis and particulate materials collected in 2001 along the Otago coastline, New Zealand.
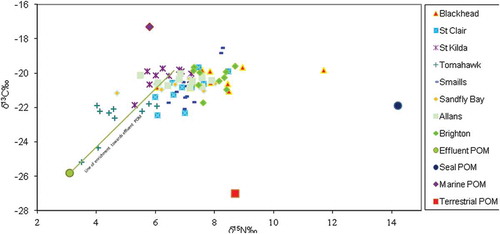
Figure 3. Comparison of carbon and nitrogen-stable isotopic ratios in the digestive tissues of Mytilus galloprovincialis and particulate materials collected in 2015 along the Otago coastline, New Zealand.
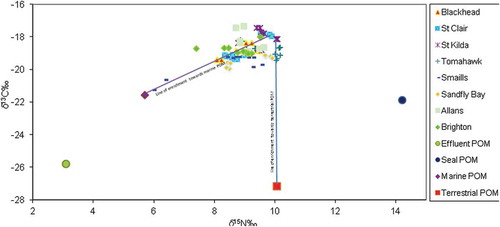
3.4. Linear isotopic mixing model
Estimating the relative contributions of carbon and nitrogen organic materials from terrestrial sources in the digestive tissues of M. galloprovincialis sampled in 2015 so as to quantify the magnitude of suspended POM (i.e., terrigenous materials) in the biological samples, a simple modified two-end member linear isotopic mixing model (Waldron, Tatner, Jack, & Arnott, Citation2001) as expressed in Equations 3 and 4 below was used:
X is the contribution from marine/phytoplankton source and Y = (1 − X) which is the terrigenous materials (multiplied by 100).
Using the observed mean isotopic ratios of the POM from filtered water samples collected from Tomahawk Creek (δ15N = 10.06‰ and δ13C = − 27.18‰) as the terrestrial source and mean isotopic ratios of mussels collected from BlackHead (uncontaminated site) (i.e., δ15N = 7.86‰ and δ13C = − 19.98‰) as the control end member while δ15Nmussel and δ13Cmussel were for the carbon and nitrogen isotopic ratios of mussels sampled from the previously sewage contaminated sites. We assumed a two-source nutrient-enrichment isotopic mixing model for the mussels coming from marine and terrigenous sources. Based on this assumption, the obtained δ15N – percentage contribution of the marine to terrestrial contribution in the digestive tissues of the mussels collected from each of the previously contaminated sites – was as follows: St. Clair (37%:63%), St. Kilda (49%:51%), Smaills (87%:13%), and Tomahawk (52%:48%). The δ13C – percentage contribution was principally from marine particulate matter for most of the sites. Smaills and Tomahawk had 4% and 1% of δ13C terrestrial particulate matter contribution accordingly. No influence of terrestrial particulate matter was detected at St. Kilda and St. Clair could not be estimated. We tried to estimate the contribution of the sewage effluent in the digestive tissue of M. galloprovincialis we obtained negative values for both δ13C and δ15N.
3.5. Analysis of variance between carbon and nitrogen isotopic signatures in M. galloprovincialis and U. lactuca
The mean carbon and nitrogen isotopic ratio values in the tissues of M. galloprovincialis and U. lactuca for 2001 and 2015 were subjected to statistical analysis using the LMM to determine if there were significant interactions between the carbon and nitrogen isotopic ratios reported in 2001 and 2015 within and across the study sites. The LMM determined the ratio testing values for the crisscross in the variability of carbon and nitrogen isotopic ratios recorded in M. galloprovincialis and U. lactuca across the end member sites (uncontaminated and previously contaminated). The outcomes of the interactions are represented in . Highly significant differences (p = < 0.0001, n = 169 [abductor]; p = 0.0022, n = 169 [digestive]) were recorded between 2001 and 2015 nitrogen isotopic ratios in the digestive tissues of M. galloprovincialis and tissues of U. lactuca (p = < 0.0001, n = 30) collected at the previously contaminated sites. There were no significant differences in the 2015 nitrogen isotopic ratios in the digestive and abductor tissues of M. galloprovincialis (p = 0.3831, n = 8 [abductor]; p = 0.8587, n = 8 [digestive]). No significant differences were observed in the 2015 carbon isotopic ratios in the digestive and abductor tissues of M. galloprovincialis (p = 0.2476, n = 6 [abductor]; p = 0.8357, n = 6 [digestive]). No significant differences were observed in δ15N (p = 0.8469, n = 6) and δ13C (p = 0.0022, n = 6) in the tissues of U. lactuca sampled at the uncontaminated and previously contaminated sites in 2015 ().
Table 4. Mean with standard error values of nitrogen and carbon isotopic signatures values from the particulate organic matter in filtered water, sewage effluent, and seal faeces samples collected from the coastal marine waters along Otago coastline in 2001(bracket) and 2015.
Table 5. Linear mixed-effects model (LMM) analysis result of the stable carbon and nitrogen isotopic signatures in the tissues of Mytilus galloprovincialis and Ulva latuca collected in 2001 and 2015 from the various study sites along Otago coastline.
4. Discussion
At the time of the first sampling event (Horn, Citation2001), two of the study sites (Smaills and Tomahawk Beaches) were known to be contaminated with sewage-derived matter and exhibited high faecal coliform counts (Greening et al., Citation2007; Lewis et al., Citation2010). In 2001, the carbon and nitrogen isotopic ratios in the tissues of M. galloprovincialis and U. latuca indicated that two sites (i.e., Tomahawk and Smaills) were heavily impacted by the discharged sewage effluent demonstrated in the lower δ13C and δ15N values. The tissues of U. latuca sampled at Tomahawk (closest site to the outfall) and St. Kilda were found to have lower δ15N than the other sites suggestive of uptake of the sewage effluent. Sewage effluent and other anthropogenic inputs had been known to modify the carbon and nitrogen isotopic ratios of macro-algae (Fry, Citation2002; Fry, Gace, & McClelland, Citation2003; Gartner, Lavery, & Smit, Citation2002; Rogers, Citation2003; Savage, Citation2005; Savage & Elmgren, Citation2004) and responsible for excessive growth of microalgae in nearshore marine waters (Baker, MacAvoy, & Kim, Citation2007; Connolly, Gorman, Hindell, Kildea, & Schlacher, Citation2013; Morand & Merceron, Citation2005; Yang, Wu, Hao, & He, Citation2008).
In 2015, the tissues of U. latuca sampled at Tomahawk and St. Kilda were found to be 15N elevated by 11.59‰ and 3.76‰, respectively, than recorded in 2001 indicating a remarkable recovery from sewage influence. There was good agreement between the 2001 and 2015 mean δ15N values in the tissues of U. latuca between the recorded values at the uncontaminated sites (i.e., reference sites) which varied between 8‰ and 9‰ (p = 0.112, n = 30) suggestive of absence of sewage organic matter as nutrient source. However, the recorded δ13C mean values between 2001 and 2015 in the tissues of U. latuca were a departure from the observed trend in δ15N at the reference sites varying from −20‰ to −10‰ (p < 0.0001, n = 30). This abnormality in the variance of δ13C might be attributed to the discrepancies in 13C discrimination during photosynthesis and respiration by the seaweeds rather than nutrient enhancement.
The modifications in the treatment and disposal of sewage effluent have had a profound positive effect on the environmental conditions at the previously contaminated sites. The extension of the outfall pipes had assisted in limiting the influence of periodic flood currents associated with the tidal and wave actions that usually cascade sewage effluent towards the previously contaminated sites. The digestive tissues of M. galloprovincialis provided a good pointer to the declined influence of the sewage-derived exhibiting a trophic enrichment (δ13C ~1‰ and δ13N ~3‰) towards the marine POM. Discriminating between the 2010 and 2015 carbon and nitrogen isotopic ratios in the tissues of M. galloprovincialis from reference and previously contaminated sites revealed highly significant differences in both abductor and digestive tissues. The contrast of the 2015carbon and nitrogen isotopic ratios in the tissues (i.e., abductor and digestive) of M. galloprovincialis from the reference and previously contaminated sites revealed no significant differences.
The two-source isotopic mixing model revealed the contribution of terrigenous organic materials in the marine bivalve to vary from 1% to 5% for δ13C and 13% to 51% for δ13N. The carbon and nitrogen isotopic values of the suspended POM from filtered water samples of the riverine and estuarine systems with open connection to the ocean reflected the diversified nature of the pool of organic materials incursions to the nearshore coastal waters. These sources range from natural to human-induced activities, e.g., atmospheric deposition, organic waste materials from the farm (manure) and grazing marine animals (sea lions and birds), fertilisers, and possibly groundwater leachates arising from the nitrification of ammonium from animal organic waste residues underground. Land-based nitrogen sources from tidal channels seemed to sway the nearshore marine waters while carbon sources were predominantly marine origin. The macro-algae communities (such as Macrocystis sp.) that are washed onshore and into the tidal channels contribute a substantial amount of carbon and nutrients to the nearshore waters and freshwater systems along the coastline (Duggins, Citation1988; Hepburn, Holborow, Wing, Frew, & Hurd, Citation2007; Michelou, Caporaso, Knight, & Palumbi, Citation2013). Other likely sources are sewage-derived matter influxes from pastoral animals, farm organic manure, detrital matter (i.e., decomposition of plant materials, seagrasses in estuaries), rural runoffs, and watershed catchment area.
5. Conclusion
Carbon and nitrogen isotopic ratios in the tissues of M. galloprovincialis and U. lactuca were found to be suitable bioindicators for investigating the impact of SDOM and possible terrigenous materials on the nearshore marine waters. M. galloprovincialis and U. lactuca were influenced by sewage at the two previously contaminated beaches in 2001. Repeat survey in 2015 showed positive changes in the isotope ratios values of the nearshore marine resident sentinel organisms in comparison with the values reported 2001, suggesting that the modifications of the sewage treatment processes and extension of the outfalls have been effective in directing sewage-derived C and N away from the previously contaminated sites. Contrast of the isotopic ratios of these sentinel organisms from previously and reference sites was indistinguishable in 2015 contrary from the 2001 survey. Mixing models reveal mussels at the two previously contaminated beaches now get most of their nutrition from marine POM with a minor subsidy from terrestrial sources.
6. Implications and directions for future study
The sentinel organisms provided an opportunity for further temporal and spatial scale isotopic studies juxtaposed with conceptual isotopic mixing models and application of chemical tracers to trace the sources, flow, cycling, and providence of nutrients and organic materials in the nearshore marine waters. Surveying the environmental conditions, biological activity and hydrodynamics of the nearshore waters will provide additional insight on the underlying mechanisms responsible for the cross-boundary (i.e., land-ocean coupling) transfer of nutrients and organic materials to the nearshore waters and their eventual sequestration to the nearshore marine food web.
Statement of Ethics
The number of shellfish collected per day from each of the study sites during the course of this study was in accordance with the New Zealand Fisheries Amended Act (Amateur Fishing) Regulations of 1986: revoked, on 1 February 2014, by regulation 161(1) (a) of the Fisheries (Amateur Fishing) Regulations 2013 (SR 2013/482) stipulated in Section 19 Sub-clause 1 and 2. Furthermore, numbers of biological samples collected and analysed did not in any way affect the species composition, diversity, and coastal ecosystem functioning of the nearshore marine waters.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abbasi, T., & Abbasi, S. (2011). Water quality indices based on bioassessment: The biotic indices. Journal of Water and Health, 9(2), 330–348.
- Alfaro, A. C., Jeffs, A. G., & Hooker, S. H. (2001). Reproductive behavior of the green-lipped mussel, Perna canaliculus, in Northern New Zealand. Bulletin of Marine Science, 69(3), 1095–1108.
- Baker, D. M., MacAvoy, S. E., & Kim, K. (2007). Relationship between water quality, δ15N, and aspergillosis of Caribbean sea fan corals. Marine Ecology Progress Series, 343, 123–130.
- Barr, N. G., Dudley, B. D., Rogers, K. M., & Cornelisen, C. D. (2013). Broad-scale patterns of tissue-δ15N and tissue-N indices in frondose Ulva spp.; Developing a national baseline indicator of nitrogen-loading for coastal New Zealand. Marine Pollution Bulletin, 67(1), 203–216.
- Bates, D. M. (2010). lme4: Mixed-effects modeling with R. Retrieved from http://lme4.r-forge.r-project.org/book.
- Bedard-Haughn, A., Van Groenigen, J., & Van Kessel, C. (2003). Tracing 15N through landscapes: Potential uses and precautions. Journal of Hydrology, 272(1–4), 175–190.
- Bouman, R., & Archer, H. (2014). Tahuna WWTP, Dunedin, New Zealand: An innovative upgrade. Proceedings of the Water Environment Federation, 2014(16), 4096–4116.
- Browne, M. A., Crump, P., Niven, S. J., Teuten, E., Tonkin, A., Galloway, T., et al. (2011). Accumulation of microplastic on shorelines worldwide: Sources and sinks. Environmental Science & Technology, 45(21), 9175–9179.
- Bump, J. K., Fox-Dobbs, K., Bada, J. L., Koch, P. L., Peterson, R. O., & Vucetich, J. A. (2007). Stable isotopes, ecological integration and environmental change: Wolves record atmospheric carbon isotope trend better than tree rings. Proceedings of the Royal Society of London B: Biological Sciences, 274(1624), 2471–2480.
- Cabana, G., & Rasmussen, J. B. (1996). Comparison of aquatic food chains using nitrogen isotopes. Proceedings of the National Academy of Sciences, 93(20), 10844–10847.
- Chary, N. S., Kamala, C., & Raj, D. S. S. (2008). Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicology and Environmental Safety, 69(3), 513–524.
- Cheevaporn, V., & Menasveta, P. (2003). Water pollution and habitat degradation in the Gulf of Thailand. Marine Pollution Bulletin, 47(1), 43–51.
- Connolly, R. M., Gorman, D., Hindell, J. S., Kildea, T. N., & Schlacher, T. A. (2013). High congruence of isotope sewage signals in multiple marine taxa. Marine Pollution Bulletin, 71(1–2), 152–158.
- Coplen, T. B. (2011). Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Communications in Mass Spectrometry, 25(17), 2538–2560.
- Council, O. R. (2001). Regional Plan: Coast. Accessed Online January, 12, 2008.
- Dede, O. T., Telci, I. T., & Aral, M. M. (2013). The use of water quality index models for the evaluation of surface water quality: A case study for Kirmir Basin, Ankara, Turkey. Water Quality, Exposure and Health, 5(1), 41–56.
- Deegan, L. A., & Buchsbaum, R. (2005). The effect of habitat loss and degradation on fisheries. The decline of fisheries resources in New England: Evaluating the impact of overfishing, contamination, and habitat degradation (pp. 67–96). MA: MIT Sea Grant College Program.
- Deudero, S., Box, A., Tejada, S., & Tintoré, J. (2009). Stable isotopes and metal contamination in caged marine mussel. Mytilus Galloprovincialis. Marine Pollution Bulletin, 58(7), 1025–1031.
- Diaz, R. J., Rhoads, D. C., Blake, J. A., Kropp, R. K., & Keay, K. E. (2008). Long-term trends of benthic habitats related to reduction in wastewater discharge to Boston Harbor. Estuaries and Coasts, 31(6), 1184–1197.
- Doré, W. J., Henshilwood, K., & Lees, D. N. (2000). Evaluation of F-specific RNA bacteriophage as a candidate human enteric virus indicator for bivalve molluscan shellfish. Applied and Environmental Microbiology, 66(4), 1280–1285.
- Dudley, B. D., & Shima, J. S. (2010). Algal and invertebrate bioindicators detect sewage effluent along the coast of Titahi Bay, Wellington, New Zealand. New Zealand Journal of Marine and Freshwater Research, 44(1), 39–51.
- Duggins, D. (1988). The effects of kelp forests on nearshore environments: Biomass, detritus, and altered flow. The Community Ecology of Sea Otters, 65(9), 192–201.
- Ebner, B. C., McAllister, R. R., & Suter, P. (2009). Effects of sample size on numerical estimates of diet prey consumption in a fish population. New Zealand Journal of Marine and Freshwater Research, 43(2), 579–590.
- Ellis, T. G. (2004). Chemistry of wastewater. Encyclopedia of Life Support System (EOLSS), 2, 1–10.
- Fry, B. (2002). Conservative mixing of stable isotopes across estuarine salinity gradients: A conceptual framework for monitoring watershed influences on downstream fisheries production. Estuaries, 25(2), 264–271.
- Fry, B., Gace, A., & McClelland, J. W. (2003). Chemical indicators of anthropogenic nitrogen-loading in four Pacific estuaries. Pacific Science, 57(1), 77–101.
- Gartner, A., Lavery, P., & Smit, A. (2002). Use of δ15N signatures of different functional forms of macroalgae and filter-feeders to reveal temporal and spatial patterns in sewage dispersal. Marine Ecology Progress Series, 235, 63–73.
- Gaston, T. F., & Suthers, I. M. (2004). Spatial variation in δ13C and δ15N of liver, muscle and bone in a rocky reef planktivorous fish: The relative contribution of sewage. Journal of Experimental Marine Biology and Ecology, 304(1), 17–33.
- Gormley, A. M., Slooten, E., Dawson, S., Barker, R. J., Rayment, W., Du Fresne, S., & Bräger, S. (2012). First evidence that marine protected areas can work for marine mammals. Journal of Applied Ecology, 49(2), 474–480.
- Greening, G. E., Lewis, G. D., & Dollimore, J. (2007). FRST programme C03X0301 safeguarding environmental health and market access for NZ foods objective 2: Virus prevalence in shellfish. 1, 1–45.
- Hargrave, B., Holmer, M., & Newcombe, C. (2008). Towards a classification of organic enrichment in marine sediments based on biogeochemical indicators. Marine Pollution Bulletin, 56(5), 810–824.
- Hepburn, C. D., Holborow, J. D., Wing, S. R., Frew, R. D., & Hurd, C. L. (2007). Exposure to waves enhances the growth rate and nitrogen status of the giant kelp Macrocystis pyrifera. Marine Ecology Progress Series, 339, 99–108.
- Hillebrand, H., & Sommer, U. (2000). Diversity of benthic microalgae in response to colonization time and eutrophication. Aquatic Botany, 67(3), 221–236.
- Horn, S. (2001). Tracking marine sewage outfall along the Otago Peninsula using stable isotope analysis of blue mussels Mytilus galloprovincialis, and sea lettuce Ulva lactuca, as biotracer species, pp33 ( BSc (Hons) Dissertation). Otago, Dunedin, New Zealand.
- IANZ. (2004). Uncertainty of measurement, precision and limits of detection chemical and microbiological testing laboratories. International Accrediation New Zealand: Auckland.
- Jarvie, H. P., Neal, C., & Withers, P. J. (2006). Sewage-effluent phosphorus: A greater risk to river eutrophication than agricultural phosphorus? Science of the Total Environment, 360(1–3), 246–253.
- Kline, J., & Thomas, C. (1999). Temporal and spatial variability of 13C/12C and 15N/14N in pelagic biota of Prince William Sound, Alaska. Canadian Journal of Fisheries and Aquatic Sciences, 56(S1), 94–117.
- Lewis, G. D., Loutit, M. W., & Austin, F. J. (2010). Human enteroviruses in marine sediments near a sewage outfall on the Otago Coast. New Zealand Journal of Marine and Freshwater Research, 19(2), 187–192.
- Lorrain, A., Paulet, Y.-M., Chauvaud, L., Savoye, N., Donval, A., & Saout, C. (2002). Differential δ13C and δ15N signatures among scallop tissues: Implications for ecology and physiology. Journal of Experimental Marine Biology and Ecology, 275(1), 47–61.
- Michelou, V. K., Caporaso, J. G., Knight, R., & Palumbi, S. R. (2013). The ecology of microbial communities associated with Macrocystis pyrifera. PLoS One, 8(6), e67480.
- Michener, R. H., & Kaufman, L. (2008). Stable isotope ratios as tracers in marine food webs: An update. Stable Isotopes in Ecology and Environmental Science, Second Edition, 2, 238–282.
- Morand, P., & Merceron, M. (2005). Macroalgal population and sustainability. Journal of Coastal Research, 215, 1009–1020.
- Morillo, J., Usero, J., & Gracia, I. (2004). Heavy metal distribution in marine sediments from the Southwest coast of Spain. Chemosphere, 55(3), 431–442.
- Nicholson, C. M., Lewis, G. D., & Loutit, M. W. (1989). Survey of human pathogenic bacteria and viruses in cockle beds at Otakou, Otago Harbour, New Zealand. New Zealand Journal of Marine and Freshwater Research, 23(4), 529–532.
- Nordsieck, R. (2006, December 12). The living world of molluscs. The Common Mussel (Mytilus edulis).
- North, J. C., Frew, R. D., & Hale, R. V. (2006). Can stable isotopes be used to monitor landfill leachate impact on surface waters? Journal of Geochemical Exploration, 88(1–3), 49–53.
- North, J. C., Frew, R. D., & Peake, B. M. (2004). The use of carbon and nitrogen isotope ratios to identify landfill leachate contamination: Green Island Landfill, Dunedin, New Zealand. Environment International, 30(5), 631–637.
- Ouédraogo, O., Chételat, J., & Amyot, M. (2015). Bioaccumulation and trophic transfer of mercury and selenium in African sub-tropical fluvial reservoirs food webs (Burkina Faso). PloS One, 10(4), e0123048.
- Pan, J.-F., & Wen-Xiong, W. (2004). Differential uptake of dissolved and particulate organic carbon by the marine mussel. Perna Viridis. Limnology and Oceanography, 49(6), 1980–1991.
- Peterson, B. J. (1999). Stable isotopes as tracers of organic matter input and transfer in benthic food webs: A review. Acta Oecologica, 20(4), 479–487.
- Post, D. M. (2002). Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology, 83(3), 703–718.
- Rayment, W., Dawson, S., & Slooten, E. (2010). Seasonal changes in distribution of Hector’s dolphin at Banks Peninsula, New Zealand: Implications for protected area design. Aquatic Conservation: Marine and Freshwater Ecosystems, 20(1), 106–116.
- RCL. (2000). Otago regional council resource consent 97530 condition 8: Rocky Shore Ecological Monitoring-January/February 2000. Dunedin, New Zealand: Ryder Consulting Ltd.
- Rogers, K. M. (2003). Stable carbon and nitrogen isotope signatures indicate recovery of marine biota from sewage pollution at Moa Point, New Zealand. Marine Pollution Bulletin, 46(7), 821–827.
- Savage, C. (2005). Tracing the influence of sewage nitrogen in a coastal ecosystem using stable nitrogen isotopes. AMBIO: A Journal of the Human Environment, 34(2), 145–150.
- Savage, C., & Elmgren, R. (2004). Macroalgal (Fucus vesiculosus) δ15N values trace decrease in sewage influence. Ecological Applications, 14(2), 517–526.
- SITO. (2006). Seafood industry training organisation- learning resource for unit standard 16340 v3 “Biology of new zealand green mussels” 1, 1–23.
- Teklehaimanot, G. Z., Coetzee, M. A., & Momba, M. N. (2014). Faecal pollution loads in the wastewater effluents and receiving water bodies: A potential threat to the health of Sedibeng and Soshanguve communities, South Africa. Environmental Science and Pollution Research, 21(16), 9589–9603.
- Thompson, D., Phillips, R., Stewart, F., & Waldron, S. (2000). Low δ13C signatures in pelagic seabirds: Lipid ingestion as a potential source of 13C-depleted carbon in the Procellariiformes. Marine Ecology Progress Series, 208, 265–271.
- Waldron, S., Tatner, P., Jack, I., & Arnott, C. (2001). The impact of sewage discharge in a marine embayment: A stable isotope reconnaissance. Estuarine, Coastal and Shelf Science, 52(1), 111–115.
- Yang, X. E., Wu, X., Hao, H.-L., & He, Z.-L. (2008). Mechanisms and assessment of water eutrophication. Journal of Zhejiang University Science B: Biomedicine and Biotechnology, 9(3), 197–209.