ABSTRACT
The Hindu Kush Himalayan region (HKH) is an important biodiversity repository with more than 488 protected areas covering 39% of the region’s geographical coverage. However, a majority of them are small and isolated and are not large enough to address conservation challenges. About 20% of the protected areas are transboundary in nature. Conservation landscape planning based on habitat suitability is an essential step for landscape management, but there are limited data available from the Landscape Initiative for Far Eastern Himalayas (HI-LIFE). To rationalize the need for regional cooperation, this study used remote sensing (RS) data and a geographic information system (GIS) to estimate the habitat suitability for four globally significant speciesconsidering available but limited secondary information. The results showed variation in habitat suitability at an individual species level, but the combined map showed about 43% of the total area as a suitable habitat. Substantial amounts of suitable habitat also recorded from outside the existing protected areas. The results also highlighted the fact that 75.40% of the existing forest within the landscape is intact, the majority of which is outside the existing protected areas. Thus, there is a strong rationale and opportunity to strengthen regional cooperation to safeguard irreplaceable and unique biodiversity resources of this wilderness landscape.
1. Introduction
The HKH, stretched over four million km2s across the Himalayas and adjoining ranges, is endowed with a rich variety of gene pools, species and ecosystems of global importance (Mittermeier et al., Citation2005; Pei, Citation1995). The region has been in the spotlight as a part of “Crisis Ecoregions”, “Endemic Bird Areas”, “Mega Diversity Countries” and “Global 200 Ecoregions” (Brooks et al., Citation2006) and also hosts parts of four of the 36 Global Biodiversity Hotspots – Himalaya, Indo-Burma, Mountains of South-West China, and Mountains of Central Asia (Mittermeier, Turner, Larsen, Brooks, & Gascon, Citation2011; Noss et al., Citation2015) – and a number of the Global 200 Ecoregions of the world (Dinerstein et al., Citation2017; Wikramanayake et al., Citation2002). Also, the region provides ecosystem services that sustain the lives and livelihoods of over 240 million people in the HKH and support over 1.7 billion people living downstream in the 10 river basins which emanate from these mountainous regions (Molden et al., Citation2017; Schild, Citation2008).
The region has a significant conservation history beginning from the 19th century (see Sharma, Chettri, & Oli, Citation2010) along with notable explorations by botanists, zoologists and nature explorers from across the world (e.g., Blandford, Citation1872; Hooker, Citation1849). In recent decades, the HKH has witnessed significant conceptual development in regional approaches to biodiversity conservation. It evolved from “people exclusionary” and “species focused” to “people-centred community-based” and “ecosystem/landscape approach,“ as reflected by conservation policies and practices within the region (Chettri & Sharma, Citation2016; Molden et al., Citation2017; Sharma et al., Citation2010). The classical approach to biodiversity conservation, which started with an emphasis on the conservation of flagship species (e.g., Wikramanayake et al., Citation1998; Yonzon, Citation1989), evolved to the understanding that “conservation and management of biodiversity are impossible without people’s participation“ (Phuntsho, Chettri, & Oli, Citation2012). Since the 1980s, decentralization, and devolution of authority for biodiversity conservation were evident in governments” efforts across the HKH through landscape-level initiatives (see Chettri & Sharma, Citation2016; Phuntsho et al., Citation2012; Sharma et al., Citation2010; Zomer & Oli, Citation2011).
Since the establishment of the Pidaung Wildlife Sanctuary in Myanmar in 1918, the first protected area in the region, the countries sharing the HKH have set aside 39% of the terrestrial area by establishing 488 protected area networks till 2007 (Chettri, Shakya, Thapa, & Sharma, Citation2008). However, a majority (68%) of them are less than 500 km2s and are scattered, isolated, and do not cover the entirety of biodiversity-rich areas with high conservation significance (Chettri et al., Citation2008; Sarkar, Mayfield, Cameron, Fuller, & Garson, Citation2007; Shrestha, Shrestha, Chaudhary, & Chaudary, Citation2010). Also, only about 25% of the Global Biodiversity Hotspots in the HKH are within the existing protected area network, leaving a very significant area unprotected (Chettri et al., Citation2008). Moreover, habitat degradation as a result of land use and cover change (Bharti, Adhikari, & Rawat, Citation2012; Sharma, Areendran, Raj, Sharma, & Joshi, Citation2016; Brandt, Allendorf, Radeloff, & Brooks, Citation2017) and challenges from prevailing climate change (Kraaijenbrink, Bierkens, Lutz, & Immerzeel, Citation2017; Shrestha, Wake, Mayewski, & Dibb, Citation1999) are bringing additional pressure on the existing protected areas system and biodiversity of the region (Chettri & Sharma, Citation2016; Chettri et al., Citation2010; Xu et al., Citation2009).
Interestingly, about 20% of the protected areas found in the region are transboundary in nature, with contiguous habitats across boundaries. They also have greater conservation significance due to their location in areas with higher biodiversity (Chettri et al., Citation2008). Though there has been significant progress in the number and coverage of protected areas, conservation plans developed for protected areas usually encompass only one country because of logistical, institutional and political challenges (Chettri et al., Citation2010; Molden et al., Citation2017; Sharma et al., Citation2010). Thus, there was an urgent need to strengthen transboundary conservation planning strategies as many protected areas are interconnected across boundaries through processes that form, utilize and maintain interfaces or connectivity essential for the survival of some species (Forrest et al., Citation2012; Palomo, Citation2017; Pauchard et al., Citation2009; Tang et al., Citation2018; Wikramanayake et al., Citation2011).
Based on the regional biodiversity significance and the need for regional cooperation, the International Centre for Integrated Mountain Development (ICIMOD) has identified six transboundary landscapes (Kailash, Kangchenjunga, Far Eastern Himalaya, Hindu Kush Karakoram Pamir, Everest, and Cherrapunjee-Chittagong) across the HKH region for the development of integrated conservation and development initiatives (Chettri, Sharma, & Thapa, Citation2009). The objective of these landscape initiatives is to improve conservation and community development beyond the political boundaries through “Ecosystem Approach” as advocated by the Convention of Biological Diversity (Secretariat of the CBD, Citation2004; Sharma, Chettri, Gurung, & Shakya, Citation2007). The initiatives focus on conservation and development planning considering biological and environmental issues (species, drivers of change and land classes) and processes (e.g., migration, adaptation, and speciation) through a participatory approach and long-term monitoring mechanisms as being advocated elsewhere (Kremen & Merenlender, Citation2018). It was realized that these processes could be best accommodated by designing large-scale conservation landscapes that capture the environmental gradients and facilitate biota movement and dispersal at spatial and temporal scales (Beger et al., Citation2010; Leonard. Baldwin, & Hanks, Citation2017; Rouget, Cowling, Lombard, Knight, & Kerley, Citation2006).
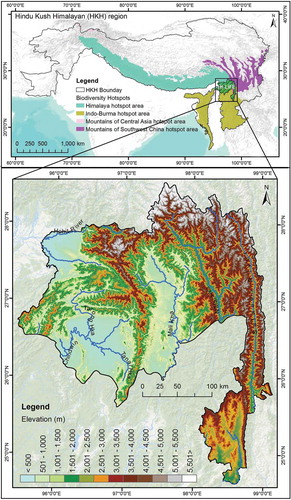
The Landscape Initiative for Far Eastern Himalayas (HI-LIFE) is one of the six proposed landscapes (). Located between the Brahmaputra (Yarlung Tsangpo in China) and Salween (Nujiang in China, and Thanlwin in Myanmar) river systems, along the easternmost extensions of the Himalayas and the westernmost extent of the Hengduan Mountains, the landscape spans with an area of 71,452 km2 across China (22%), India (12%), and Myanmar (66%) (ICIMOD, Citation2018). The landscape has global significance due to its converging location of the three Global Biodiversity Hotspots – namely Himalaya, Indo-Burma and Mountain of Southwest China (Brunner, Talbott, & Elkin, Citation1998; Mittermier et al., Citation2011; Wikramanayeke et al., Citation2002). Interestingly, out of seven protected areas found in the area, four are transboundary in nature with natural connectivity with adjacent protected areas across the political boundaries (Areendran et al., Citation2017; Li et al., Citation2017; Lodhi, Samal, Chaudhry, Palni, & Dhyani, Citation2014). Moreover, in some areas, the areas outside protected areas are equally or more significant in terms of biodiversity (Naniwadekar et al., Citation2015).
The complex with congruence of three of the 36 Global Biodiversity Hotspot (Mittermeier et al., Citation2011; Noss et al., Citation2015), it is an important area for plant diversity (Zhang, Slik, & Ma, Citation2017) and the wilderness areas have been used by many species as connectivity corridors (Ren et al., Citation2017). However, this natural connectivity across the boundaries and the resulting contiguous habitats across the landscape were never considered in the past conservation practices. Thus, the HI-LIFE has a high potential, as a transboundary landscape, considering the existing protected areas and their contiguous habitats. The concept, challenges, and opportunities have been discussed with conservation communities, who agreed in principle to explore the further development of the HI-LIFE as a transboundary landscape and to enhance regional cooperation (ICIMOD, Citation2018). To support the conservation paradigm, we came up with the following three objectives for this study and the outputs from these objectives can be used to ensure the integration of regional-scale processes into conservation assessment, management, and implementation of the ecosystem approach.
To understand the state of land use and land cover types within the delineated landscape to understand the extent of potential habitats for species that are of global significance.
To identify potential habitat contiguity of some selected species common across the transboundary landscape and understand the extent of the available habitat necessary for their conservation.
To address the knowledge gap on the state of existing habitat conditions in relation to forest fragmentation.
2. Materials and methods
2.1. Study area
The HI-LIFE is the eastern-most landscape among the six identified landscapes (). The geographic extent of the landscape comprises 71,452 km2 located between coordinates 95.51 and 99.31 E longitude and 25.01 and 28.99 N latitude. In terms of biodiversity, the proposed HI-LIFE is one of the most intact and enriched transboundary biodiversity complexes within the HKH and has been recognized as a Centre of Plant Biodiversity and Eastern Asiatic Regional Centre for Endemism (Takhtajan, Citation1969; Wikramanayake et al., Citation2002). A study of the world’s frontier forests by the World Resources Institute shows that the complex contains the last remaining tracts of intact natural forest ecosystems in mainland Southeast Asia that are relatively undisturbed and large enough to maintain biodiversity (Brunner et al., Citation1998). The protected areas in the Hi-LIFE provide habitat for many reported species of global importance such as the Himalayan black bear (Ursus thibetanus), Leaf deer (Muntiacus putaoensis); Red panda (Ailurus fulgens); Takin (Budorcas taxicolor); Black muntjac (Muntiacus crinifrons); and Stump-tailed macaque (Macaca arctoides) among others (ICIMOD, Citation2018). These globally important species inhabit wider areas across national boundaries and are reported from more than one protected area in the landscape (Datta, Pansa, Madhusudan, & Mishra, Citation2003; Naniwadekar, Shukla, Isvaran, & Datta, Citation2015; Rabinowitz, Amato, & Saw Tun, Citation1998; Ren et al., Citation2017). Thus, their potential habitat extends far beyond the existing protected area network and national boundaries.
2.2. Research methodology
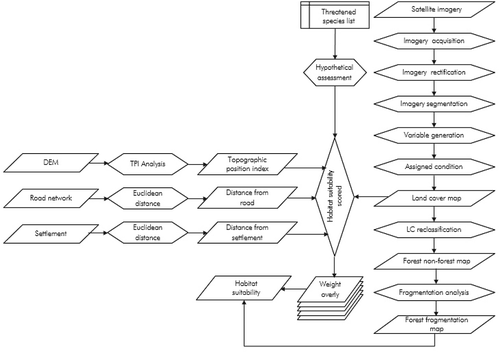
To meet the objective mentioned above, we followed the schematic analytical steps shown in . As per the objectives, three broad methodologies were used as follows:
2.2.1. Land cover mapping
Landsat data (see ) were used for land cover mapping as it has the potential to significantly improve the characterization of the Earth’s land surface (Macauley, Citation2009). Landsat Enhanced Thematic Mapper Plus (ETM+) images were accessed from USGS Global Visualization Viewer (GLOVIS, Citation2012) whereas a Shuttle Radar Topography Mission (SRTM) Digital Elevation Model was accessed from Consultative Group on International Agricultural Research (CGIAR)-Consortium for Spatial Information (CSI) GeoPortal (see SRTM, Citation2012). A hierarchical classification scheme with 10 classes was adopted using the Land Cover Classification System (LCCS) following Di Gregorio (Citation2005) (). The acquired Landsat images were atmospheric corrected and re-projected into Universal Transverse Mercator (UTM), Zone 47. After processing the images, eCognition Developer software used for object-based image analysis (OBIA). This method of land use and land cover mapping has been tested elsewhere and could be referred to for further detail (Chettri, Uddin, Chaudhary, & Sharma, Citation2013; Uddin et al. Citation2015).
Table 1. List of landsat imagery used for land use and land cover analysis.
Table 2. Description of land cover classes used to classify the study area.
2.2.2. Species habitat suitability modeling
We used secondary information of four species (Himalayan black bear, Leaf deer, Red panda, Takin) that were reported from more than one protected areas of the landscape (see Rabinowitz, Garshelis & Steinmetz, Citation2008; Harris, Citation2008; Rabinowitz et al., Citation1998; Rabinowitz, Myint, Khaing, & Rabinowitz, Citation1999; Song, Smith, & MacKinnon, Citation2008; Stotz, Harris, Moskovits, Yi, & Adlemann, Citation2003; Timmins, Duckworth, & Zaw, Citation2008a; Timmins, Long, Duckworth, Ying-Xiang, & Zaw, Citation2008b; Wang, Choudhury, Yonzon, Wozencraft, & Zaw, Citation2008; Yang, Citation2009;).These species are ecologically significant within the landscape and are representative of the majority of the key habitat types and altitudes (). Habitat suitability modeling was conducted for each of these four species considering a host of factors, such as presence/absence, habits, habitat, and potential range reported for each (IUCN, Citation2018). During mapping, an emphasis was given to major habitat factors such as vegetation type, altitude, and topographic structure, and the distance from settlements and roads following Beier et al., (Citation2009).
Table 3. Criteria for assessment of habitat suitability.
In delineating suitable habitat areas, all thematic layers and topographic layers – including digital elevation model (DEM), slope and topographic position data, settlement and road data – were analysed () as they were found to have a direct correlation to the suitability of wildlife habitat (Nandy Kushwaha, & Gaur, Citation2012; Singh, Velmurugan, & Dakhate, Citation2009). After the habitat factors had been chosen, suitability scores assigned to each of the factors (e.g., land cover types, topographic position classes) paying particular attention to the suitability threshold required to support breeding habitat for each of the species. All thematic layers and GIS factors were then converted to raster files and reclassified as per the scores obtained from individual species. Habitat suitability analysis was performed in ArcGIS using Corridor Designer to identify contiguous habitat with a user-friendly, three-step process following Beier et al. (2009).
During the habitat suitability analysis, a numerical weighting factor was assigned to each thematic layer according to the relative importance of habitat suitability. To determine the weight overlay factor procedure, an expert knowledge was used to generate the commensurable scores, where the appropriate score maps are weighted according to the habitat function prepared for the decision following Bashari and Hemami (Citation2013); Store and Jokimäki (Citation2003). Developing such kind of ranked by experts from the region is a most suitable relative influence on the suitability of habitat for the selected animal (Buruso, Citation2018). While designing, we assigned each factor a percentage weight so that the sum of the weights is 100%. To combine multiple habitat factors into one aggregate habitat suitability model for species in the landscape, we first assigned weights to each factor reflecting their relative importance as indicated in . For example, the weighted value for elevation was 10%, the topographic position was 5%, the land cover was 75%, distance to the road was 5%, and distance to the settlement was 5%. Thus, these weighted suitability values were then incorporated in the raster file for each focal species. We considered the following interpretation of habitat suitability scores: 80 to 100% = best habitat, highest survival and reproductive success; 60 to 80% = associated with successful breeding; 30 to 60% = associated with consistent use and breeding; 20 to 30% = associated with occasional use for non-breeding activities; and values less than 20% = avoided; 0 = absolute non-habitat. Based on the above criteria, habitat suitability (and unsuitability) maps for each of the four species were prepared independently to identify their potential extended habitat areas. After this, the individual four suitability maps were combined to a single suitability map to see the extended habitats within the landscape and to show their potential distribution.
2.2.3. Habitat condition concerning forest fragmentation
To understand the state of habitat condition, the forested area was used for fragmentation analysis following Vogt et al. (Citation2007). We considered four classes, namely, “core forest,“ “patch forest,“ “perforated forest” and “edge forest” (see ) as defined by Vogt et al. (Citation2007). The Landscape Fragmentation Tool (Parent & Hurd, Citation2012) used in the analysis following the four-step processes used by Vogt et al. (Citation2007). Each of the four classes mentioned were mapped by assigning patch size class following Stokes and Morrison (Citation2003). Forest fragmentation areas were generated based on a specified edge width (100 m) and the fragmentation classes and tools mentioned above.
The geospatial methods discussed above were used extensively because of the specific limitations of ground-based methods through which the entire targeted area could not be traversed and the collection of information for each species is resource intensive. While ground surveys such as counting animals, trapping, collection of droppings, and investigations of feeding sites as well as ground mapping of habitats are useful, considering the objectives of this study and the goal of transboundary landscape conservation and management initiatives, geospatial technology can supplement or circumvent ground survey methods (see Alamgir, Mukul, & Turton, Citation2015; Boitani et al., Citation2008; Xu et al., Citation2017). In addition, the study focused on making use of existing information and applying cost-effective tools before entering into extensive systematic research as envisaged by the countries during numerous regional consultations (ICIMOD, Citation2009, Citation2012, Citation2018).
3. Results
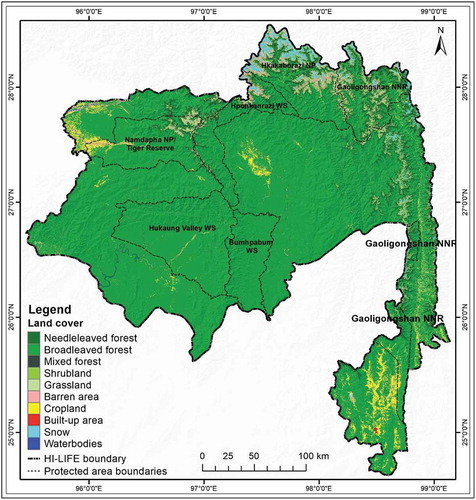
The result of the land cover analysis from the proposed landscape presented in . The results () showed that the landscape was dominated by broadleaved forest (80.02%), followed by Needleleaved Forest (8.01%), mixed forest (0.54%), and shrubland (0.60%). Agricultural land only accounted for 4% of the total area of the HI-LIFE landscape. It was observed that the majority of forested areas found in the central part of the landscape ().
Table 4. Table showing land cover classes, their areas and percentage coverage in the HiLife.
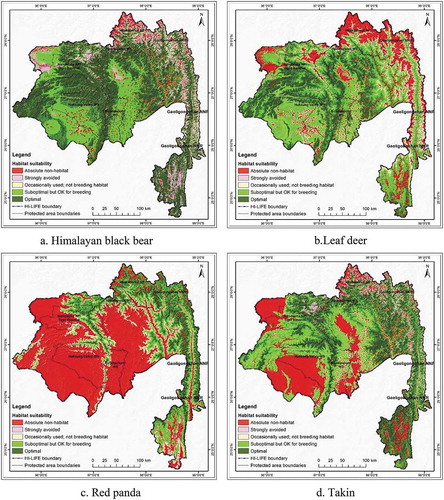
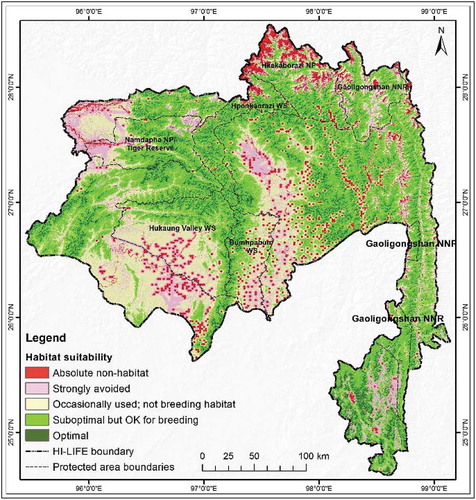
Individual species habitat maps showed differences in distribution (). The habitat map of the Himalayan black bear extended 35,666 km2 (49.92%) of the total 71,452 km2 area, which is the largest optimal habitat observed among the four species. Likewise, the suboptimal habitat was 18,517km2 or 25.92% of the total area (). The most suitable breeding area for the Himalayan black bear found in the middle and southwestern part of the HI-LIFE ()). Among the selected species the Red panda showed the smallest (12.36%) optimal area within the landscape. For the Red panda, an area of 8 830 km2 in the middle and southwestern areas of the HI-LIFE was found as a suboptimal habitat with breeding potential ()). Recognized optimal stability for Leaf deer was just 12,779 km2 and was primarily located in the north-eastern part of the HI-LIFE ()). The optimal areas for Takin ()) were 28.86% of the total landscape, respectively (see ). For all of the species considered, the suboptimal zone, which is characterized as having breeding potential, is important as it is associated with successful breeding. In the Hi-LIFE, the potential suboptimal breeding zones for Himalayan black bear, Leaf dear, Red panda and Takin are 25.92, 43.60, 18.44, and 29.93, respectively ().
Table 5. Table showing habitats suitability results for the four species considered in the analysis for the HI-LIFE.
The combined and overlaid model of the four species showing the potential habitat for all the covered species presented in . The analyzed, combined average habitat suitability of the four species shows 7% of the total area to be optimal for these species with the best habitat and highest survival possibility (). About 43% of the area is suboptimal habitat with breeding potential, 20% is occasionally used but not for breeding, and 28% area is absolute non-habitat. To summarize, the results revealed that 50% of the area is a potential habitat for these species.
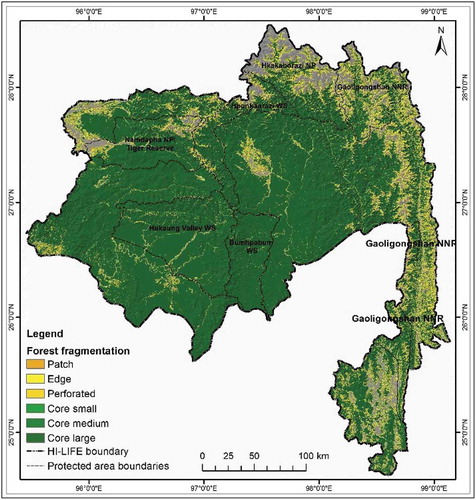
Our analysis on forest conditions within the extended habitats, including edge and perforated areas, revealed some interesting results (). It was observed that 75.4% of the total HI-LIFE consists of core large forest patches that are larger than one km2. The analysis also revealed that about 7.69% of the total forested area is denuded perforated (see ). Notably, the results also showed that most of the larger forest patches are outside the protected areas ().
Table 6. Matrix showing habitats conditions in relation to habitat suitability for species in the forested areas of the HI-LIFE
4. Discussion
The analyzed land cover results from the landscape showed a mosaic of land cover types with a high proportion (73%) of forested area. This is inconsistent with some recent mappings showing intact forest across the region (Arrendran et al., Citation2017; Leimgruber et al., Citation2005; Lodhi et al., Citation2014). Human habitation, as indicated by built-up areas and agricultural land, is comparatively low. This very strongly justifies that this particular landscape is still in a wilderness state (Brunner et al., Citation1998; Datta et al., Citation2003). The contiguous forested areas across national boundaries reflect the fact that the proposed landscape is still a good habitat for many species, especially large mammals that need larger, contiguous and compatible forested areas to ensure sufficient availability of food, places to hide and thermal cover as well as limited human disturbance (Alagador et al., Citation2012; Gupta, Mondal, Sankar, & Qureshi, Citation2012). It is also an important factor in determining the potential habitats of many species and can play an important role in predicting suitable habitats (Adhikari et al., Citation2012; Alamgir et al., Citation2015; Zhang et al., Citation2012).
Globally significant species have been widely used in the design of both conventional and contemporary conservation plans (Graves, Farley, Goldstein, & Servheen, Citation2007; Vina et al., Citation2007; Xu et al., Citation2017) and they have indicated different habitat needs and use patterns as reflected in our analysis (). This is obvious as each of the species prefers and uses different habitats and altitudinal ranges (Nandy et al., Citation2012; Zhang et al., Citation2012). Notably, the distribution ranges of these species are within the reported review works done by experts (see Garshelis & Steinmetz, Citation2008; Harris, Citation2008; Song et al., Citation2008; Timmins et al., Citation2008a; Wang et al., Citation2008). In many instances, the conservation planning could be done with data deficient species if the objectives are long term and broader (Bland et al., Citation2017). What is interesting is that the potential habitats of most of the species stretched far beyond the existing protected areas and across national boundaries (see ).
Analysis of the combined average habitat suitability of four species shows that about 60% of the total area is optimal for all of the species (). The results also support the home ranges of these species (IUCN, Citation2018). It is important to note that planning for multiple species representing different ecological zones and habitats brings better results. Therefore, holistic conservation requires an evaluation of multiple species with a wide range of habitat use patterns, which is fundamentally a description of what animals use, where animals are found, and also, in the eyes of most ecologists, what animals need to survive and reproduce (Cushmana & Landguthb, Citation2012; Wang, Yang, Bridgman, & Lin, Citation2008). This is particularly true for large mammals as they operate at broader spatial scales and, consequently, their populations are more likely to be fragmented if confined to isolated habitats within small protected areas (Noss, Citation1983; Roever, van Aarde, & Leggett, Citation2013). The concept also supports the adaptation strategy needed for species recovery considering the prevailing threats from various drivers of change including the climatic change (Rao et al., Citation2013). As the trend in conservation biology has widened from a species-centered approach to the ecosystem and landscape approach, it is important to consider multiple conservation values when designing protected area networks (Attorre et al., Citation2012; Huck et al., Citation2010).
Our habitat condition analysis based on forest fragmentation revealed that 82% of the existing forested area is in good condition; however, most of it is outside of the protected area network where human intervention happens to be fairly limited. For effective conservation of species that prefer forested areas, large patches of intact forest are necessary (De & Tiwari, Citation2008; Theobald, Crooks, & Norman, Citation2011). In order to have a functional conservation network, it is important to plan and promote dispersal and migration of species between the existing protected areas, particularly through the establishment of connectivity corridors (Fuller, Munguıa, Mayfield, Sanchez-Cordero, & Sarkar, Citation2006; Fung et al., Citation2017; Zipkin, DeWan, & Royle, Citation2009). The contiguity of the forested area also provides the altitudinal connectivity necessary for species to move toward higher altitudes in the event of climate change as an adaptation provision (Lenoir & Svenning, Citation2015; Nunez et al., Citation2013; Worboys & Pulsford, Citation2011; Zomer et al., Citation2014).
The future of biodiversity conservation depends on efforts applied across large landscapes, the scale at which many key ecological and evolutionary processes take place (Baldwin, Trombuluk, Leonard, Noss, & Hilty, Citation2018) with an integrated approach (Leonard et al., Citation2017). The transboundary landscape approach is not a new concept in the HKH region. ICIMOD’s past experiences in Mount Everest Ecosystem (Sherpa, Peniston, Lama, & Richard, Citation2003), Kangchenjunga Landscape (Chettri, Sharma, Shakya, & Bajracharya. Citation2007; Sharma et al., Citation2007), and the Kailash Sacred Landscape (Zomer & Oli, Citation2011; Zomer, Sharma, Oli, & Chettri, Citation2010) have highlighted the need for and significance of regional cooperation to facilitate transboundary landscape and ecosystem management approaches for improved biodiversity conservation in the HKH. Within the HI-LIFE, there are unique geopolitical issues related to insurgencies, border disputes, the illegal transboundary trade of wildlife, etc. A strategy to address these issues has also been discussed (ICIMOD, Citation2009). Moreover, learning could also be drawn from numerous examples available with unique approaches and learnings (see Beger et al., Citation2010; Kark et al., Citation2015; Roga, Ferguson, & Bagoora, Citation2017; Vasilijević & Pezold, Citation2011; Worboys, Francis, & Lockwood, Citation2010). The overall analysis from this preliminary assessment revealed that the initiative taken by ICIMOD and partners to promote the landscape approach in the Hi-LIFE is heading in the right direction. It was revealed that there is a high potential for connectivity development among the existing protected areas and other forests patches due to the presence of contiguous habitats for some flagship and globally significant species present within the landscape. Though there is a less human intervention (4% agriculture and 0.003% built-up area) relative to other transboundary landscapes in the region, keeping this pristine wilderness intact will require a strong regional commitment, meticulous planning, and cooperation among the conservation communities.
5. Conclusion
The idea of managing multiple parks, reserves, and conservation areas collectively as a part of a conservation network is recent, yet a growing trend in biodiversity conservation and management. For these networks to be ecologically viable, the focus must be on wider habitats and on ensuring the functional connectivity of the landscape as a whole. It is necessary to concentrate efforts at the regional scale, giving due consideration to the potential connectivity that exists between the existing protected areas across the boundaries of individual nations as well as along both the east-west and north-south axis. Without effective biodiversity management planning at the landscape level, it is likely that human development and haphazard encroachment could further fragment key wildlife habitats or key biodiversity areas of evolutionary significance and isolate threatened species in the proposed HI-LIFE.
Our preliminary assessment of land cover and land use patterns and habitat suitability modeling considering four species (with limited data) and their varied ecological and habitats needs and habitat conditions indicates that the landscape has a high level of conservation potential. There is an opportunity for regional cooperation among the countries sharing this landscape, not just for research and knowledge development at the national level, but also for adopting an integrated approach at the regional level to safeguard irreplaceable and unique biodiversity resources in the entire landscape. Our assessment, although based on limited information for only a few species from the landscape, points to the fact that geospatial analysis using species-based information can serve as a powerful tool for landscape conservation and development planning. For the HI-LIFE, such analysis has proven useful in identifying important areas outside protected areas. It also suggests for ecologically contiguous connectivity between the existing protected areas that require regional management consideration in the long run. The study also indicates a need for more rigorous and systematic efforts in research and to raise awareness on the conservation significance of this important landscape and generation of relevant scientific evidences to support informed decision-making. ICIMOD has initiated the process of promoting regional cooperation among China, India, and Myanmar through the landscape approach in the HI-LIFE. Cooperation is expected to improve, especially given new developments in Myanmar’s political scenario; however, this could bring both opportunity and challenges.
Acknowledgments
We express our gratitude to Dr. David Molden, Director General of ICIMOD, for his inspiration and for providing the required facilities. We are also thankful the Governments of China, India, and Myanmar for their continuous support for this initiative. We express our special thanks to Dr. Ranbeer Singh Rawal, Director of the GB Pant National Institute of Himalayan Environment and Sustainable Development, India and Mr. Win Naing Thaw, Ministry of Natural Resources and Environmental Conservation, Myanmar for their guidance and support. The financial support received from the Austrian Development Agency, and GIZ for conducting this analysis is highly appreciated.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adhikari, D., Barik, S. K., & Upadhaya, K. (2012). Habitat distribution modelling for reintroduction of Ilex khasiana Purk., a critically endangered tree species of Northeastern India. Ecological Engineering,40, 37-43. doi:10.1016/j.ecoleng.2011.12.004
- Alagador, D., Trivino, M., Cerdeira, J. O., Brás, R., Cabeza, M., & Araújo, M. B. (2012). Linking like with like: Optimising connectivity between environmentally-similar habitats. Landscape Ecology, 27, 291–301.
- Alamgir, M., Mukul, S. A., & Turton, S. M. (2015). Modelling spatial distribution of critically endangered Asian elephant and Hoolock gibbon in Bangladesh forest ecosystems under a changing climate. Applied Geography, 60, 10–19.
- Ali, A., Zhou, Z., Waseem, M., Khan, M. F., Ali, I., Asad, M., & Qashqaei, A. T. (2017). An assessment of food habits and altitudinal distribution of the Asiatic black bear (Ursus thibetanus) in the Western Himalayas, Pakistan. Journal of Natural History, 51(11–12), 689–701.
- Areendran, G., Puri, K., Raj, K., Mazumdar, S., Razali, S. M., Suk-Ueng, K., & Nuruddin, A. A. (2017). Baseline mapping for Namdapha National Park in Arunachal Pradesh, India using geospatial tools. Malaysian Journal of Remote Sensing & GIS, 6(2), 1–9.
- Attorre, F., De Sanctisa, M., Farcomeni, A., Guillet, A., Scepia, E., Vitale, M., … Fasola, M. (2012). The use of spatial ecological modelling as a tool for improving the assessment of geographic range size of threatened species. Journal for Nature Conservation, 21(1), 48–55.
- Baldwin, R. F., Trombuluk, S. C., Leonard, P. B., Noss, R. F., & Hilty, J. A. (2018). The future of landscape conservation. BioScience, 68(2), 60–63.
- Bashari, H., & Hemami, M.-R. (2013). A predictive diagnostic model for wild sheep (Ovis orientalis) habitat suitability in Iran. Journal for Nature Conservation, 21(5), 319–325.
- Beger, M., Grantham, H. S., Pressey, R. L., Wilson, K. A., Peterson, E. L., Dorfman, D., … Possingham, H. P. (2010). Conservation planning for connectivity across marine, freshwater, and terrestrial realms. Biological Conservation, 143, 565–575.
- Beier, P., Majka, D. R., & Newell, S. L. (2009). Uncertainty analysis of least‐cost modeling for designing wildlife linkages. Ecological Applications, 19(8), 2067-2077. doi:10.1890/08-1898.1
- Bharti, R. R., Adhikari, B. S., & Rawat, G. S. (2012). Assessing vegetation changes in timberline ecotone of Nanda Devi National Park, Uttarakhand. International Journal of Applied Earth Observation and Geoinformation, 18, 472–479.
- Bland, L. M., Bielby, J., Kearney, S., Orme, C. D. L., Watson, J. E., & Collen, B. (2017). Toward reassessing data‐deficient species were prepared independently species. Conservation Biology, 31(3), 531–539.
- Blandford, W. T. (1872). Account of the visit to the eastern and northern frontiers of independent Sikkim, with notes on zoology of the alpine and sub-alpine region, Part II, Zoology. Journal of Asiatic Society of Bengal, 40(2), 367–420.
- Boitani, L., Sinibaldi, I., Corsi, F., De Biase, A., d’Inzillo Carranza, I., Ravagli, M., … Trapanese, P. (2008). Distribution of medium to large-sized African mammals based on habitat suitability models. Biodiversity and Conservation, 17, 605–621.
- Brandt, J. S., Allendorf, T., Radeloff, V., & Brooks, J. (2017). Effects of national forest‐management regimes on unprotected forests of the Himalaya. Conservation Biology, 31(6), 1271–1282.
- Brooks, T. M., Mittermeier, R. A., Da Fonseca, G. A. B., Gerlach, J., Hoffmann, M., Lamoreux, J. F., … Rodrigues, A. S. L. (2006). Global biodiversity conservation priorities. Science, 313, 58–61.
- Brunner, J., Talbott, K., & Elkin, C. (1998) World resources institute, forest frontiers initiative. Logging Burma‘s frontier forests: Resources and the regime. Retrieved from http://www.wri.org/ffi/burma/index.html
- Buruso, F. H. (2018). Habitat suitability analysis for hippopotamus (H. amphibious) using GIS and remote sensing in Lake Tana and its environs, Ethiopia. Environmental Systems Research, 6(1), 1–15p6.
- Chettri, N., Sharma, E., & Thapa, R. (2009). Long term monitoring using transect and landscape approaches within Hindu Kush Himalayas. I, in: E. Sharma (ed.). Proceedings on International Mountain Biodiversity Conference. 16–18 November 2008. pp 201–208. (ICIMOD, Kathmandu, Nepal).
- Chettri, N., Shakya, B., Thapa, R., & Sharma, E. (2008). Status of protected area system in the Hindu Kush-Himalaya: An analysis of PA coverage. International Journal Biodiversity Science and Management, 4(3), 164–178.
- Chettri, N., & Sharma, E. (2016). Reconciling the mountain biodiversity conservation and human wellbeing: Drivers of biodiversity loss and new approaches in the Hindu-Kush Himalayas. Proceedings of the Indian National Science Academy, 82, 53–73.
- Chettri, N., Sharma, E., Shakya, B., & Bajracharya, B. (2007). Developing forested conservation corridors in the Kangchenjunga Landscape, Eastern Himalaya. Mountain Research and Development, 27, 211–214.
- Chettri, N., Sharma, E., Shakya, B., Thapa, R., Bajracharya, B., Uddin, K., … Choudhury, D. (2010). Biodiversity in the Eastern Himalayas: Status, trends and vulnerability to climate change: Climate change impact and vulnerability in the Eastern Himalayas – Technical report 2. Kathmandu, Nepal: ICIMOD.
- Chettri, N., Uddin, K., Chaudhary, S., & Sharma, E. (2013). Linking spatio-temporal land cover change to biodiversity conservation in Koshi Tappu Wildlife Reserve, Nepal. Diversity, 5, 335–351.
- Cushmana, S. A., & Landguth, E. L. (2012). Multi-taxa population connectivity in the Northern rocky mountains. Ecological Modelling, 231, 101–112.
- Datta, A., Pansa, J., Madhusudan, M. D., & Mishra, C. (2003). Discovery of the leaf deer Muntiacus putaoensis in Arunachal Pradesh: An addition to the large mammals of India. Current Science, 84(3), 454–458.
- De, A., & Tiwari, A. K. (2008). Estimation of Patchiness: A measure of fragmentation in the Rajaji- Corbett National Parks and adjoining areas, Uttarakhand, India. International Journal of Ecology and Environmental Sciences, 34(4), 345–349.
- Di Gregorio, A. (2005). Land Cover Classification System (LCCS), version 2: Classification concepts and user manual. FAO environment and natural resources service series, No. 8. Rome: FAO.
- Dinerstein, E., Olson, D., Joshi, A., Vynne, C., Burgess, N. D., Wikramanayake, E., … Saleem, M. (2017). An ecoregion-based approach to protecting half the terrestrial realm. BioScience, 67(6), 534–545.
- Forrest, J. L., Wikramanayake, E., Shrestha, R., Areendran, G., Gyeltshen, K., Maheshwari, A., … Thapa, K. (2012). Conservation and climate change: Assessing the vulnerability of snow leopard habitat to tree line shift in the Himalaya. Biological Conservation, 150, 129–135.
- Fuller, T., Munguıa, M., Mayfield, M., Sanchez-Cordero, V., & Sarkar, S. (2006). Incorporating connectivity into conservation planning: A multi-criteria case study from central Mexico. Biological Conservation, 133, 131–142.
- Fung, E., Pablo, I., Lenin, C., Sergio, V., Nelson, Z., Freddy, A., … Zayra, R. (2017). Mapping conservation priorities and connectivity pathways under climate change for tropical ecosystems. Climatic Change, 141(1), 77–92.
- Garshelis, D. L., & Steinmetz, R. (IUCN SSC Bear Specialist Group) (2008). Ursus thibetanus. In: IUCN 2012. IUCN red list of threatened species. Version 2012.2. www.iucnredlist.org. Downloaded on 26 June 2018.
- GLOVIS (2012). United States Geological Survey, Global Visualization Viewer (GLOVIS) Earth Resources Observation and Science Center (EROS). Retrieved from: http://glovis.usgs.gov/
- Graves, T. A., Farley, S., Goldstein, M. I., & Servheen, C. (2007). Identification of functional corridors with movement characteristics of brown bears on the Kenai Peninsula, Alaska. Landscape Ecology, 22, 765–772.
- Gupta, S., Mondal, K., Sankar, K., & Qureshi, Q. (2012). Abundance and habitat suitability model for Ratel (Mellivora capensis) in Sariska Tiger Reserve, Western India. Wildlife Biology in Practice, 8(1), 13–22.
- Harris, R. B. (2008). Muntiacus crinifrons, in: IUCN 2012. IUCN red list of threatened species. Version 2012.2. www.iucnredlist.org. Downloaded on 26 June 2018.
- Hooker, J. D. (1849). Notes of naturalist.Himalayan Journal, 1, 123-124.
- Huck, M., Jędrzejewski, W., Borowik, T., Miłosz-Cielma, M., Schmidt, K., Jędrzejewska, B., … Mysłajek, R. W. (2010). Habitat suitability, corridors and dispersal barriers for large carnivores in Poland. Acta Theriologica, 55(2), 177–192.
- ICIMOD. (2009). Consultation report on ‘Regional experience sharing consultation on the landscape approach to biodiversity conservation and management in the Eastern Himalayas: Towards developing the Brahmaputra-Salween Landscape’. 24–28 May 2009, Tengchong Country, Yunnan Province, China. Kathmandu, Nepal: Author.
- ICIMOD (2012). Towards developing the Brahmaputra-Salween Landscape – Report on the experts regional consultation for transboundary biodiversity management and climate change adaptation. ICIMOD Working Paper 2012/4. (Author, Kathmandu, Nepal).
- ICIMOD. (2018). Landscape initiative for Far-eastern Himalaya regional feasibility assessment, A synthesis of the country feasibility reports from China, India, and Myanmar. Kathmandu, Nepal: Author.
- IUCN (2018). The IUCN red list of threatened species. Version 2017–3. www.iucnredlist.org. Downloaded on 26 June 2018
- Kark, S., Tulloch, A., Gordon, A., Mazor, T., Bunnefeld, N., & Levin, N. (2015). Cross-boundary collaboration: Key to the conservation puzzle. Current Opinion in Environmental Sustainability, 12, 12–24.
- Kraaijenbrink, P. D. A., Bierkens, M. F. P., Lutz, A. F., & Immerzeel, W. W. (2017). Impact of a global temperature rise of 1.5 degrees Celsius on Asia’s glaciers. Nature, 549(7671), 257.
- Kremen, C., & Merenlender, A. M. (2018). Landscapes that work for biodiversity and people. Science, 362(6412), eaau6020.
- Leimgruber, P., Kelly, D. S., Steininger, M. K., Brunner, J., Müller, T., & Songer, M. (2005). Forest cover change patterns in Myanmar (Burma) 1990–2000. Environmental Conservation, 32(4), 356–364.
- Lenoir, J., & Svenning, J. C. (2015). Climate‐related range shifts–A global multidimensional synthesis and new research directions. Ecography, 38(1), 15–28.
- Leonard, P. B., Baldwin, R. F., & Hanks, R. D. (2017). Landscape-scale conservation design across biotic realms: Sequential integration of aquatic and terrestrial landscapes. Scientific Reports, 7(1), 14556.
- Li, W., Clauzel, C., Dai, Y., Wu, G., Giraudoux, P., & Li, L. (2017). Improving landscape connectivity for the Yunnan snub-nosed monkey through cropland reforestation using graph theory. Journal for Nature Conservation, 38, 46–55.
- Lodhi, M. S., Samal, P. K., Chaudhry, S., Palni, L. M. S., & Dhyani, P. P. (2014). Land cover mapping for Namdapha National Park (Arunachal Pradesh), India using harmonized land cover legends. Journal of the Indian Society of Remote Sensing, 42(2), 461–467.
- Macauley, M. K. (2009). Earth observations in social science research for management of natural resources and the environment: Identifying the Landsat contribution. The Journal of Terrestrial Observation, 1(2), 31–51.
- Mittermeier, R. A., Turner, W. R., Larsen, F. W., Brooks, T. M., & Gascon, C. (2011). Global biodiversity 1451 conservation: The critical role of hotspots. In F. E. Zachos & J. C. Habel (Eds.), Biodiversity hotspots, biodiversity hotspots (pp. 3–22). Berlin Heidelberg: Springer.
- Mittermeier, R. A., Gils, P. R., Hoffman, M., Pilgrim, J., Brooks, T., Mittermeier, C. G., … Da Fonseca, G. A. B. (2005). Hotspots revisited. Earth’s biologically richest and most endangered terrestrial ecoregions. USA: CEMEX.
- Molden, D., Sharma, E., Shrestha, A. B., Chettri, N., Shrestha, N. P., & Kotru, R. (2017). Advancing regional and tTransboundary cCooperation in the cConflict-pProne Hindu Kush–Himalaya. Mountain Research and Development, 37(4), 502–508.
- Nandy, S., Kushwaha, S. P. S., & Gaur, P. (2012). Identification of Swamp Deer (Cervus duvauceli duvauceli Cuvier) potential habitat in Jhilmil Jheel conservation reserve, Uttarakhand, India using multi-criteria analysis. Environmental Management, 49, 902–914.
- Naniwadekar, R., Mishra, C., Isvaran, K., Madhusudan, M. D., & Datta, A. (2015). Looking beyond parks: The conservation value of unprotected areas for hornbills in Arunachal Pradesh, Eastern Himalaya. Oryx, 49(2), 303–311.
- Naniwadekar, R., Shukla, U., Isvaran, K., & Datta, A. (2015). Reduced hornbill abundance associated with low seed arrival and altered recruitment in a hunted and logged tropical forest. PLoS One, 10(3), e0120062.
- Noss, R. F. (1983). A regional landscape approach to maintain diversity. BioScience, 33, 700–706.
- Noss, R. F., Platt, W. J., Sorrie, B. A., Weakley, A. S., Means, D. B., Costanza, J., & Peet, R. K. (2015). How global biodiversity hotspots may go unrecognized: Lessons from the North American Coastal Plain. Diversity and Distributions, 21(2), 236–244.
- Nunez, T. A., Lawler, J. J., Mcrae, B. H., Pierce, D. J., Krosby, M. B., Kavanagh, D. M., … Tewksbury, J. J. (2013). Connectivity planning to address climate change. Conservation Biology, 27(2), 407–416.
- Palomo, I. (2017). Climate change impacts on ecosystem services in high mountain areas: A literature review. Mountain Research and Development, 37(2), 179–187.
- Parent, R. J., & Hurd, J. D. (2012) Landscape fragmentation tool V2. Laboratory for Earth resources information systems, University of Conneticut. Retrieved form: http://clear.uconn.edu/tools/lft/lft2/accessed .
- Pauchard, A., Kueffer, C., Dietz, H., Daehler, C. C., Alexander, J., Edwards, P. J., … Jakobs, G. (2009). Ain‘t no mountain high enough: Plant invasions reaching new elevations. Frontiers in Ecology and the Environment, 7(9), 479–486.
- Pei, S. (1995). Banking on biodiversity: Report on the regional consultations on biodiversity assessment in the Hindu Kush - Himalaya. Kathmandu, Nepal: ICIMOD.
- Phuntsho, K., Chettri, N., & Oli, K. P. (2012). Mainstreaming community-based conservation in a transboundary mountain landscape – Lessons from Kangchenjunga. Kathmandu, Nepal: ICIMOD.
- Pradhan, S., Saha, G. K., & Khan, J. A. (2001). Ecology of the red panda Ailurus fulgens in the Singhalila National Park, Darjeeling, India. Biological Conservation, 98(1), 11–18.
- Rabinowitz, A., Amato, G., & Saw Tun, K. (1998). Discovery of the black muntjac, Muntiacus crinifrons (Artiodactyla, Cervidae), in northern Myanmar. Mammalia, 62(1), 105–108.
- Rabinowitz, A. R., Myint, T., Khaing, S. T., & Rabinowitz, S. (1999). Description of the leaf deer (Muntiacus putaoensis), a new species of muntjac from northern Myanmar. Journal of Zoology (London), 249, 427–435.
- Rao, M., Htun, S., Platt, S. G., Tizard, R., Poole, C., Myint, T., & Watson, J. E. (2013). Biodiversity conservation in a changing climate: A review of threats and implications for conservation planning in Myanmar. Ambio, 42(7), 789–804.
- Ren, G. P., Yang, Y., He, X., Li, G., Gao, Y., Huang, Z., … Xiao, W. (2017). Habitat evaluation and conservation framework of the newly discovered and critically endangered black snub-nosed monkey. Biological Conservation, 209, 273–279.
- Roever, C. L., van Aarde, R. J., & Leggett, K. (2013). Functional connectivity within conservation networks: Delineating corridors for African elephants. Biological Conservation, 157, 128–135.
- Roga, N. B., Ferguson, W., & Bagoora, F. (2017). Transboundary conservation areas in African mountains: Opportunities and challenges for addressing global change. Earth Sciences, 6(6), 117.
- Rouget, M., Cowling, R. M., Lombard, A. T., Knight, A. T., & Kerley, G. H. (2006). Designing large-scale conservation corridors for pattern and process. Conservation Biology, 20(2), 549–561.
- Sarkar, S., Mayfield, M., Cameron, S., Fuller, T., & Garson, J. (2007). Conservation area networks for the Indian region: Systematic methods and future prospects. Himalayan Journal of Sciences, 4(6), 27–40.
- Schild, A. (2008). The case of the Hindu Kush-Himalayas: ICIMOD’s position on climate change and mountain systems. Mountain Research and Development, 28, 328–331.
- Secretariat of the Convention of Biological Diversity (CBD). (2004). Programme of work on protected areas (CBD programme of work). Montreal, Canada: Secretariat of the Convention of Biological Diversity.
- Sharma, E., Chettri, N., Gurung, J., & Shakya, B. (2007). Landscape approach in biodiversity conservation: A regional cooperation framework for implementation of the convention on biological diversity in Kangchenjunga landscape. Kathmandu, Nepal: ICIMOD.
- Sharma, E., Chettri, N., & Oli, K. P. (2010). Mountain biodiversity conservation and management: A paradigm shift in policies and practices in the Hindu Kush-Himalayas. Ecological Research, 25, 905–923.
- Sharma, M., Areendran, G., Raj, K., Sharma, A., & Joshi, P. K. (2016). Multitemporal analysis of forest fragmentation in Hindu Kush Himalaya—A case study from Khangchendzonga Biosphere Reserve, Sikkim, India. Environmental Monitoring and Assessment, 188(10), 596.
- Sherpa, L. N., Peniston, B., Lama, W., & Richard, C. (2003). Hands around everest: Transboundary cooperation for conservation and sustainable livelihoods. Kathmandu, Nepal: International Centre for Integrated Mountain DevelopmentI CIMOD.
- Shrestha, A. B., Wake, C. P., Mayewski, P. A., & Dibb, J. E. (1999). Maximum temperature trend in the Himalaya and its vicinity: An analysis based on temperature records from Nepal for the period 1971-`94. Journal of Climate, 12, 2775–2786.
- Shrestha, U. B., Shrestha, S., Chaudhary, P., & Chaudhary, R. P. (2010). How rRepresentative is the pProtected aAreas sSystem of Nepal? A gGap aAnalysis bBased on gGeophysical and bBiological fFeatures. Mountain Research and Development, 30(3), 282–294.
- Singh, G., Velmurugan, A., & Dakhate, M. P. (2009). Geospatial approach for tiger habitat evaluation and distribution in Corbett Tiger Reserve, India. Journal of the Indian Society of Remote Sensing, 37(4), 573–585.
- Song, Y. L., Smith, A. T., & MacKinnon, J. (2008). Budorcas taxicolor, in: IUCN 2012. IUCN red list of threatened species. Version 2012.2. www.iucnredlist.org. Downloaded on 26 June 2018.
- SRTM. (2012). Hole-filled SRTM for the globe Version 4. Retrieved from: http://srtm.csi.cgiar.org/
- Stokes, D., & Morrison, P. (2003). GIS‐based conservation planning: A powerful tool to be used with caution. Conservation in Practice, 4(1), 38–42.
- Store, R., & Jokimäki, J. (2003). A GIS-based multi-scale approach to habitat suitability modeling. Ecological Modelling, 169(1), 1–15.
- Stotz, D. F., Harris, E. J., Moskovits, H. K., Yi, S., & Adlemann, G. W. (eds) (2003) Rapid biological inventories report no. 4. (Chicago: The Field Museum).
- Takhtajan, A. (1969). Flowering plants - Origin and dispersal. Edinburgh: Oliver and Boyd.
- Tang, Y., Winkler, J. A., Viña, A., Liu, J., Zhang, Y., Zhang, X., … Zhao, Z. (2018). Uncertainty of future projections of species distributions in mountainous regions. PloS one, 13(1), e0189496.
- Theobald, D. M., Crooks, K. R., & Norman, J. B. (2011). Assessing effects of land use on landscape connectivity: Loss and fragmentation of western US forests. Ecological Applications, 21(7), 2445–2458.
- Timmins, R. J., Duckworth, J. W., & Zaw, T. (2008a). Muntiacus putaoensis, in: IUCN 2012. IUCN red list of threatened species. Version 2012.2. www.iucnredlist.org. Downloaded on 26 June 2018
- Timmins, R. J., Long, B., Duckworth, J. W., Ying-Xiang, W., & Zaw, T. (2008b). Arctonyx collaris, in: IUCN 2012. IUCN red list of threatened species. Version 2012.2. www.iucnredlist.org. Downloaded on 26 June 2018.
- Uddin, K., Chaudhary, S., Chettri, N., Kotru, R., Murthy, M., Chaudhary, R. P., Ning, W., Shrestha, S. M., & Gautam, S. K. (2015). The changing land cover and fragmenting forest on the roof of the world: A case study in nepal's kailash sacred landscape. Landscape and Urban Planning, 141, 1-10. doi:10.1016/j.landurbplan.2015.04.003
- Vasilijević, M., & Pezold, T. (2011). Crossing borders for nature. European examples of transboundary conservation (pp. viii + 72). Gland, Switzerland and Belgrade, Serbia: IUCN Programme Office for South-Eastern Europe.
- Vina, A., Bearer, S., Chen, X., He, G., Linderman, M., An, L., … Liu, J. (2007). Temporal changes In Giant Panda habitat connectivity across boundaries of Wolong Nature Reserve, China. Ecological Applications, 17(4), 1019–1030.
- Vogt, P., Riitters, K. H., Estreguil, C., Kozak, J., Wade, T. G., & Wickham, J. D. (2007). Mapping spatial patterns with morphological image processing. Landscape Ecology, 22(2), 171–177.
- Wang, X., Choudhury, A., Yonzon, P., Wozencraft, C., & Zaw, T. (2008). Ailurus fulgens, in: IUCN 2012. iucn red list of threatened species. Version 2012.2. www.iucnredlist.org. Downloaded on 26 June 2018.
- Wang, Y. H., Yang, K. C., Bridgman, C. L., & Lin, L. K. (2008). Habitat suitability modelling to correlate gene flow with landscape connectivity. Landscape Ecology, 23, 989–1000.
- Wangchuk, T. R., Wegge, P., & Sangay, T. (2016). Habitat and diet of Bhutan takin Budorcas taxicolor whitei during summer in Jigme Dorji National Park, Bhutan. Journal of Natural History, 50(11–12), 759–770.
- Wikramanayake, E., Dinerstein, E., Loucks, C. J., Olson, D. M., Morrison, J., Lamoreux, J., … Hedao, P. (2002). Terrestrial Ecoregions of the Indo-Pacific: A conservation assessment. Washington: Island Press.
- Wikramanayake, E., Dinerstein, E., Seidensticker, J., Lumpkin, S., Pandav, B., Shrestha, M., … Than, U. (2011). A landscape-based conservation strategy to double the wild tiger population. Conservation Letters, 4, 219–227.
- Wikramanayake, E. P., Dinerstein, E., Robinson, J. G., Karanth, U., Rabinowitz, A., Olson, D., … Bolze, D. (1998). An ecology-based method for defining priorities for large mammal conservation: The tiger as a case study. Conservation Biology, 12, 864–878.
- Worboys, G. L., Francis, W., & Lockwood, M. (Eds). (2010). Connectivity conservation management: A global guide. London, UK: Earthscan.
- Worboys, G. L., & Pulsford, I. (2011). Connectivity conservation in Australian landscapes. Report prepared for the Australian Government Department of Sustainability, Environment, Water, Population and Communities on behalf of the State of the Environment 2011 Committee. Canberra: DSEWPaC.
- Xu, J., Grumbine, E. R., Shrestha, A., Eriksson, M., Yang, X., Wang, Y., & Wilkes, A. (2009). The melting Himalayas: Cascading effects of climate change on water, biodiversity, and livelihoods. Conservation Biology, 23(3), 520–530.
- Xu, W., Viña, A., Kong, L., Pimm, S. L., Zhang, J., Yang, W., … Ouyang, Z. (2017). Reassessing the conservation status of the giant panda using remote sensing. Nature Ecology & Evolution, 1(11), 1635.
- Yan, W. B., Zeng, Z. G., Gong, H. S., He, X. B., Liu, X. Y., Si, K. C., & Song, Y. L. (2017). Habitat use and selection by takin in the Qinling Mountains, China. Wildlife Research, 43(8), 671–680.
- Yang, Y. (2009). Biodiversity Conservation in Transboundary Landscapes (BCTL). A final technical report submitted to ICIMOD (unpublished).
- Yonzon, P. B. (1989). Ecology and conservation of the red panda in the Nepal-Himalayas. Unpub. Ph.D. thesis, University of Maine, Orono.
- Zhang, M. G., Slik, J. F., & Ma, K. P. (2017). Priority areas for the conservation of perennial plants in China. Biological Conservation, 210, 56–63.
- Zhang, M. G., Zhou, J. K., Chen, W. Y., Slik, F. J. W., Cannon, C. H., & Raes, N. (2012). Using species distribution modeling to improve conservation and land use planning of Yunnan, China. Biological Conservation, 153, 257–264.
- Zhang, Z., Hu, J., Yang, J., Li, M., & Wei, F. (2009). Food habits and space-use of red pandas Ailurus fulgens in the Fengtongzhai nature reserve, China: Food effects and behavioural responses. Acta Theriologica, 54(3), 225–234.
- Zipkin, E. F., DeWan, A., & Royle, A. J. (2009). Impacts of forest fragmentation on species richness: A hierarchical approach to community modeling. Journal of Applied Ecology, 46(4), 815–822.
- Zomer, R., Sharma, E., Oli, K. P., & Chettri, N. (2010). Linking biodiversity conservation and climate change perspectives in bio-culturally rich transboundary areas in the Kailash Sacred Landscape Region of China, India, and Nepal. In Biodiversity and Climate Change: Achieving the 2020 targets. Abstracts of Posters Presented at the 14th Meeting of the Subsidiary Body on Scientific, Technical and Technological Advice of the Convention on Biological Diversity, 10–21 May 2010, Nairobi, Kenya, Technical Series No. 51. Montreal, SCBD, 142–144.
- Zomer, R. J., & Oli, K. P. (2011). Kailash sacred landscape conservation initiative: Feasibility assessment report. Kathmandu, Nepal: ICIMOD.
- Zomer, R. J., Trabucco, A., Metzger, M. J., Wang, M., Oli, K. P., & Xu, J. (2014). Projected climate change impacts on spatial distribution of bioclimatic zones and ecoregions within the Kailash Sacred Landscape of China, India, Nepal. Climatic Change, 125(3–4), 445–460.