ABSTRACT
Landmine contamination is a vital contributor to long-lasting destructive effects on terrestrial ecosystem. Libya has experienced severe landmine impacts involving soil contamination with hazardous heavy metals. This study was conducted in Benghazi, Libya, to examine the phytotoxicity of soil contaminated with residues of a recent exploded mine, on seed germination and seedling growth of field beans (Vicia faba L.). Results revealed that soil contents of heavy metals (Pb, Cd, and Hg) showed a gradual decreasing trend from the explosion site with higher concentrations recorded near the site centre, and lower concentrations at a greater distance (6 m). At 50 cm, 1, 2, 4, and 6 m, respectively from the explosion site, mean Pb concentration was 1240, 960, 510, 180, and 28.5 mg/kg, whereas, mean Cd concentration was 44, 38, 21, 8.4, and 0.8 mg/kg and mean Hg concentration was 52, 38, 28.5, 16.2, and 0.51 mg/kg. A strong inverse correlation was obtained between seed germinationand soil content of heavy metals. Seeds sown at the closest distance to the explosion site failed to germinate, whereas, germination percentage for seeds sown at 1, 2, 4, and 6 m from the explosion site was 15%, 15%, 30%, and 60%, respectively, relative to 95% for control seeds. Above-ground vegetation variables of the surviving seedlings, Leaf number plant−1 and plant height, were negatively correlated with soil contamination level. Findings of this study demonstrated the severity of damage caused by landmine contamination to the site soil . Furthermore, the intolerance shown by V. faba to stress associated with the explosive-contaminated soil revealed that the soil was highly polluted and unsuitable for cultivating crop plants.
1. Introduction
Like a deadly disease, long absent and assumed conquered, landmine crisis is the most atrocious humanitarian disaster of our time (Boutros-Ghali, Citation1994). Landmine Monitor (Citation2019) reported that landmines are responsible for killing 3059 people and injuring 3837 people in 2018, 71% of them are civilians, and recorded mostly in countries with armed conflicts. Landmines may also pose a serious threat to terrestrial ecosystem, as soil and vegetation cover can be substantially damaged during explosion or de-mining (Troll, Citation2000). As landmines are planted in the ground, a few centimeters below soil surface, their direct detrimental impacts can mostly be observed on soil structure and function. Soil can be heavily polluted with hazardous non-biodegradable toxic chemicals as landmines are basically made of metal casing filled with 2,4,6-trinitrotoluene (TNT), hexahydro-1,3,5-trinitro-1,3,5-triazine (RDX or Cyclonite). They also contain heavy metals such as copper, iron, zinc, nickel, manganese, cadmium, chromium, lead, and mercury, in addition to depleted uranium (DU) (Berhe, Citation2007). When landmine explodes or when the casing corrodes, these highly toxic pollutants leach in the surrounding soil and can be easily absorbed by roots, and hence, may negatively affect plant growth and development (Thijs et al., Citation2014).
TNT and RDX have been proven to be carcinogenic to human beings and toxic to a wide range of living organisms, however, studies investigating their phytotoxic effects on plants are still limited (Best et al., Citation2008; Elly et al., Citation2006; Görge et al., Citation1994; Krishnan et al., Citation2000; Schott & Worthley, Citation1974; Sens et al., Citation1999; Simini et al., Citation1995; Travis et al., Citation2008; Via et al., Citation2015; Vila et al., Citation2008). A decline in germination rate and seedling growth in response to increased concentrations of TNT have been reported for several plant species; Medicago sativa (Scheidemann et al., Citation1998); Raphanus sativus L. and Triticum sativum L. (Gholamian & Gholamian, Citation2008); Lepidium sativum L. and Brassica rapa (Gong et al., Citation1999); Zea mays and Medicago sativa (Khatisashvili et al., Citation2009), and Baccharis halimifolia (Ali et al., Citation2014).
Soil contamination with heavy metals, and plant tolerance to metal toxicity have been broadly reviewed and investigated in the last few decades as it is thought that the concentration of heavy metals in plants is a reflection of metal concentration in the soil as heavy metals can be readily taken up by plants (Benavides et al., Citation2005; Clemens & Ma, Citation2016; Clemens et al., Citation2002; Das et al., Citation1997; Foy et al., Citation1978; A. Khan et al., Citation2015; Manara, Citation2012; Q.S. Li et al., Citation2012; Rahman & Singh, Citation2019; Z. Wang et al., Citation2019). Lead, Cadmium, and Mercury are among the phytotoxic heavy metals that constitute landmines and can be found in high concentrations in explosive-contaminated soils. Lead (Pb) is non-biodegradable heavy metal and extremely persistent in soil (Fahr et al., Citation2013). Lead phtyotoxicity has been well-documented as studies on the effects of Pb on plant have appeared in literature for over 80 years. Contradicting results of stimulation and reduction of plant growth have been reported; at low Pb concentrations, growth stimulation has been observed, however, at high concentrations, Pb has been found to adversely affect plant growth and development by disturbing vital processes such as photosynthesis (Ekmekci et al., Citation2009) and root cell division (Eun et al., Citation2000; Woźny & Jerczyńska, Citation1991). Numerous studies have also shown a significant reduction in seed germination and seedling growth with increasing Pb concentrations (Verma & Dubey, Citation2003; Liu et al., Citation2009; Cavusoglu et al., Citation2010; Wang et al. Citation2010; Lou et al., Citation2017)
Cadmium (Cd) is non-essential element and highly mobile in soil. It can be easily absorbed by the plant and transported to all its organs as it occurs as a Cd2+ ion in soil, therefore, even at low concentrations, it can negatively affect plant growth (Ernst, Citation1998; Wagner, Citation1993) by inhibiting root elongation and impairing several growth processes such as photosynthesis, respiration, and mineral nutrition (Benavides et al., Citation2005; Chaoui & El Ferjani, Citation2005; Chen et al., Citation2011; Jinbiao et al., Citation2010; Parrotta et al., Citation2015; Vassilev & Yordanov, Citation1997). A significant reduction in germination in response to high levels of Cd has been noticed in Vicia faba L. (Rahoui et al., Citation2008), Hordeum vulgare and Pisum sativum (Piršelová, Citation2011), Arabidopsis thaliana (W. Li et al., Citation2005), Vigna unguiculata L. (Vijayaragavan et al., Citation2011). Vigana radiata (N. Khan et al., Citation2020) and Festuca arundinacea Schreb (Lou et al., Citation2017).
Due to its hazardous impacts on the environment, mercury (Hg) is considered a highly toxic heavy metal (Bernhoft, Citation2012; Bridges & Zalups, Citation2017; Feng et al., Citation2003; Leitch et al., Citation2007; Xu et al., Citation2015). Effects of Hg-contaminated soil have been examined on several plant species (Chen & Yang, Citation2012; Munzuroglu & Geckil, Citation2002; Sheppard et al., Citation1993; Wang & Greger, Citation2004; Weaver et al., Citation1984; Zhang & Tyerman, Citation1999). Studies have also shown that mercury can interfere with normal chromosome segregation in plant cell (Yu, Citation2005) and disturb mitochondrial activities (Cargnelutti et al., Citation2006); it can also suppress seed germination, depress root elongation and shoot growth and induce visible injuries in plants (Zhou et al., Citation2007).
Landmine monitor (Citation2019) reported that an estimate of 80–120 million landmines were spread around 90 countries including 26 African countries. Libya is among countries which have experienced severe landmine impacts; it was listed as a state with antipersonnel mine contamination in 2019 (Landmine Monitor, Citation2019). Nachón (Citation2000) stated that about 9% of the arable land of Libya is mined as it is thought that 1000s of landmines were deployed during world war II. Furthermore, during an armed conflict in 2014–2015 in the city of Benghazi, Libya, 32.11°N, 20.08°E, hundreds of antipersonnel, antivehicle and improvised mines were laid in different parts of the city. The Libyan army has managed to clear and de-mine massive number of landmines across the city, however, thousands of buried undetonated landmines are still hidden. To understand their damaging effects on soil and vegetation, nature of soil contamination should be frequently assessed and the impact of explosive-contaminated soil on plant growth should be thoroughly examined. However, in view of the lack of information concerning plant responses to soil contaminated with explosive residues of landmines in Libya, the objectives of the present study were to (i) determine heavy metal concentrations for Pb, Cd, and Hg in soil contaminated with residues of a recent exploded mine in Al-Quwarsha district of Benghazi, Libya, (ii) investigate the phytotoxicity of the explosive-contaminated soil on seed germination and early seedling growth of field bean (Vicia faba L.), a widely grown crop in Benghazi, and (iii) examine the capacity of V. faba at early seedling stage to tolerate such unfavorable conditions as changes observed at this life stage can be used as an index for evaluating plant sensitivity and tolerance to pollutants such as heavy metals (Peralta-Videaa et al., Citation2002). The hypothesis was that explosive-contaminated soil would reduce seed germination rate of V. faba and adversely affect growth variables of the surviving seedlings.
2. Materials and methods
2.1. Site description
Al-Quwarsha district is located on the outskirts of Benghazi, 32.11°N, 20.08°E. It has suffered from the destructive impacts of landmines as it is believed that hundreds of mines were used during the armed conflict in 2014–2015. A site of a recent landmine explosion in Al-Quwarsha district was chosen for this study as soil in and around the explosion site was expected to be heavily contaminated with residues of the exploded mine containing highly toxic compounds such as TNT, RDX, DU, in addition to some hazardous heavy metals such as lead, cadmium, and mercury. The soil of the study site was silty clay in nature, slightly alkaline with a mean pH of 7.8 and EC of 2.50 mS/cm.
2.2. Soil sampling
Soil samples were collected at a depth of 0–20 cm, around the centre of the explosion site, at distances of 50 cm, 1 m, 2 m, 4 m, and 6 m from the site centre. Additional soil samples were collected from uncontaminated site to serve as control samples. Soil samples were sealed in plastic bags, labeled at the point of the collection and transported to the laboratories of Botany department, Faculty of Sciences, University of Benghazi. After air drying, root fragments and stones were carefully removed from soil samples before sieving using sieve no. 20 (mesh size of 0.85). Concentrations of Pb, Cd, and Hg were measured using atomic absorption spectrometry.
2.3. Plant treatment and measurements
For each distance, four 3-litre pots were filled with soil collected from the location, for a total number of 20 pots for all five distances around the site centre and additional 20 pots for the control. Seeds of a spring variety of field beans (Vicia faba L.) were chosen for this experiment as this species is known to grow on a large scale in Benghazi for consumption purposes. A total number of 200 healthy seeds were used for all pots (100 seeds sown in the contaminated soil and 100 seeds sown in the control soil). Five seeds were sown in each pot for a total number of 20 seeds per location (distance). To prevent fungal growth, seeds were sterilized with 2% sodium hypochloride for 10 min, then rinsed five times in sterile distilled water. Seeds were gently placed in 4 cm furrows and covered with soil. In each pot, seeds were placed approximately 6 cm apart. The pots were well-irrigated and seeds were left to germinate in the growth room at Botany department under controlled conditions of 12 h light/12 h dark with a temperature of 27 °C (day, 12 hrs), 17 °C (night, 12 h) and relative humidity of 60–70.
All newly emerged seedlings were counted as germinated seeds. Regular counts were made to determine the rate of seedling emergence in each pot. Germination percentage was measured for each pot using the following formula:
Germination percentage = number of germinating seeds/total number of seeds*100
During growth, plant growth parameters for each seedling were regularly measured; number of leaves per plant were counted and plant height, from the soil surface to the tip of the main stem, was measured using a ruler. Regular growth measurements began on 18 DAS (Days After Sowing) for the emerged seedlings in each pot and continued until the experiment was terminated 32 DAS.
2.4. Statistical analysis
The effect of contamination and distance from the site centre on plant growth parameters; plant height and leaf number, was statistically tested using repeated measures ANOVA. Soil contamination (treatment) was the main effect (independent factor) with two levels, contaminated and control. Distance (four distances; 1, 2, 4, and 6 m form site centre) and Date (18, 22, 26, 30, 32 DAS) were analyzed as repeated measures. Interaction effects tested were; treatment*distance, treatment*date, date*distance, treatment*date*distance. Significance was accepted at P ≤ 0.05. Statistical analyses were performed using SPSS 20 (SPSS Inc., USA, version 20).
3. Results
3.1. Seed germination percentage
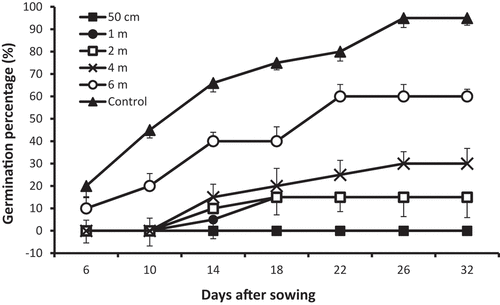
First seedling emergence was recorded 6 DAS, both, in control pots where 20% of seedling emergence was reported and for seeds sown in soil at 6 m from the site centre with germination percentage of 10%. The significant inhibitory effect of contaminated soil on seed germination was recorded early in the experiment, when seeds sown at the closest distance to the site centre failed to germinate as no seedling emergence was observed at 50 cm from the centre until the termination of the experiment, however, seeds sown in soil at distances of 1, 2, and 4 m from the centre were not capable of germination until 14 DAS when the percentage of seed germination was 05%, 10%, 15%, respectively, compared with a higher percentage of 40% reported further from the center, at 6 m from the plot centre. On the same date, seed germination percentage in control pots was 66% and substantially increased to reach 95% in 12 days (at 26 DAS) when maximum seedling emergence was recorded, whereas, the values of germination percentage for seeds sown in soil at 1, 2, 4, and 6 m were 15%, 15%, 30%, and 60%, respectively, with no further significant increase until experiment was terminated, at 30 DAS ().
3.2. Plant growth
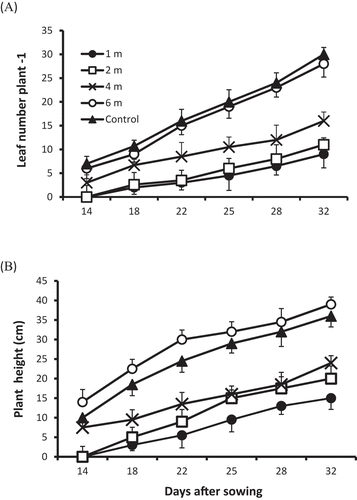
Statistical analysis showed that treatment effect and all interactions tested; treatment*distance, treatment*date, date*distance, treatment*date*distance, were significant for both plant height and leaf number plant −1 (P < 0.05–0.001; ) indicating a strong negative effect on seedling growth and development as plants located towards the centre of the site showed a considerable growth reduction compared to control plants and those located at a greater distance from the centre (at 6 m). Results revealed that values of leaf number plant −1 varied with distance as plants grown close to the site centre had fewer leaves, whereas plants grown 6 m from the centre produced more leaves.
Table 1. Summary of two-way repeated measures ANOVA to determine the effect of contaminated soil on plant height and leaf number plant −1 for five dates between 18 and 32 days after sowing (DAS) (n = 4) .
At 18 DAS, mean leaf number plant −1 for plants grown at distances of 1, 2, 4, and 6 m from the centre was 2, 2.6, 6.7, and 9, respectively, whereas, it was 10.7 in control plants (). leaf number plant −1 increased with time at all distances, however, plants grown further from the plot centre were capable of producing greater number of leaves; at 32 DAS, mean leaf number plant −1 was 9, 11, 16, and 28 for those grown at 1, 2, 4, 6 m from the site centre, whereas, it increased by three-fold for the control plants as they had the highest value; 30 (). Results showed that mean plant height increased with time in both treatments, however, plants grown at 6 m from the plot centre attained a maximum mean height of 22.5 cm, early in the experiment at 18 DAS, followed by control plants (18.5 cm; ). By the end of the experiment, mean height of plants grown further from the centre (6 m) increased by 1.7 fold (39 cm), compared to 15 cm at a distance closer to the centre (1 m). Mean height of 20 and 24 cm were recorded for plants grown in soil at a distance of 2 and 4 m from the centre, whereas, control plants continued to increase to reach a mean height of 36 cm when the experiment was terminated, at 32 DAS ().
Figure 2. (A) Leaf number plant −1, (B) plant height in control and contaminated soil for plants grown at distances of 1, 2, 4, and 6 m from the centre of the explosion site. A single standard errors of the means are shown where larger than symbols (n = 4, comprising 80 plants in contaminated soil and 80 control plants) .
3.3. Soil heavy metals
The main lead content in the uncontaminated soil (control) was 14.5 mg/kg ranging from 2.16 to 30.5 mg/kg. Mean Pb content at the closest point to the site centre (50 cm) was clearly greater than the other four distances, however, soil Pb content decreased with distance as values of Pb concentration at 50 cm, 1, 2, 4, and 6 m from the site centre were, respectively, 1240, 960, 510, 180, and 28.5 mg/kg ().
Figure 3. Mean Pb (A), Cd (B), and Hg concentrations (C) for control and contaminated soil measured at 50 cm, 1, 2, 4, and 6 m from the centre of the explosion site. A single standard errors of the means are shown where larger than symbols (n = 4) .
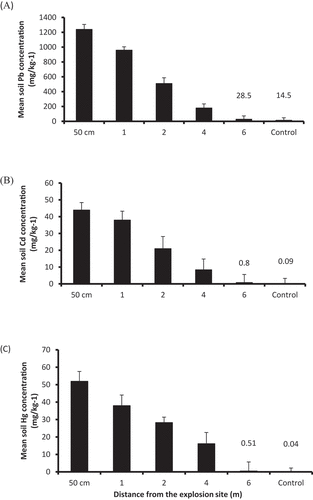
Mean soil Cd content showed a similar gradual decreasing trend from the centre of the explosive-contaminated site. Mean Cd concentration at 50 cm was the greatest (44 mg/kg), ranging from 30.5 to 50.2, whereas, at 1, 2, 4, and 6 m from the centre, it was 38, 21, 8.4, and 0.8 mg/kg, respectively. In control soil, it was 0.09 mg/kg ranging from 0.01 to 0.72 mg/kg ().
Mercury content of the uncontaminated soil was between 0.01 and 0.28 mg/kg with an average of 0.04 mg/kg. Soil Hg concentration was the lowest at a greater distance (6 m) from the site centre (0.51 mg/kg) and gradually increased towards the site centre; at 50 cm from the centre, it was increased by 100-fold (52 mg/kg) ranging from 43.4 to 59.4 ().
4. Discussion
Seedling emergence was first observed 14 DAS in both control and at 4–6 m from the explosion site centre. Low rate of seedling emergence was recorded at 18 DAS at 1–4 m from the centre, however, a strong inhibitory effect was noticeable on seeds sown in soil near the site centre, at a distance of 50 cm, as all seeds failed to germinate and emerge until the experiment was terminated (). This clearly indicates that seeds at this close distance to the contaminated site centre were exposed to stressful conditions that significantly suppressed germination. Seed germination is usually influenced by a number of factors, such as water, temperature, and oxygen (Dasberg & Mendel, Citation1971; Jensen et al., Citation2010; Odabas & Mut, Citation2007). In this study, temperature, water, and oxygen were not limiting factors as all seeds equally received sufficient water and sown in well-aerated soil under optimal temperature. This suggests that seeds were subjected to certain unfavorable conditions that may have inhibited successful germination of seeds sown closer to the site centre and delayed germination of seeds sown further from the centre. The results of the present study also revealed that soil at 50 cm from the centre was extremely contaminated with Pb, Cd, and Hg as their values were the highest at this distance, compared with other distances (). Therefore, this marked inhibition in germination of seeds at 50 cm is likely to have resulted from the presence of high concentrations of these phytotoxic heavy metals in the soil at this distance.
Average value of soil Pb at 50 cm from the centre was 1240 mg Hg/kg (); this soil Pb concentration greatly exceeds the acceptable concentration proposed for agricultural area in the UK by DEFRA (80 mg/kg; DEFRA (Department for Environment, food and rural affairs) and Environment Agency, Citation2009). This Pb level was also highly above the Canadian acceptance levels for agricultural land use (70 mg Pb/kg; CCME (Canadian Council of Ministers of the Environment), Citation1999). At the present value, soil is considered highly contaminated with lead as this value falls within the proposed limits for polluted industrial land in the UK (2300 mg/kg; DEFRA (Department for Environment, food and rural affairs) and Environment Agency, Citation2009). High concentration of Pb recorded in the present study is likely to have substantially contributed to the significant inhibition of seed germination. Our findings are consistent with those of Cavusoglu et al. (Citation2010) and Wang et al. (Citation2010) who found that high concentrations of Pb (2000 mg/kg) caused inhibition in seed germination and seedling growth of V. faba. Interference of lead with several vital enzymes, such as; protease and amylase, plays a considerable role in inhibiting seed germination (Mukherji & Maitra, Citation1976).
At 50 cm from the site centre, Cd content in the soil was 44 mg Cd/kg (), greatly exceeding the permissible values proposed for agricultural lands by DEFRA in the UK and CCME of Canada; 3.9 and 1.4 mg Cd/kg; respectively (DEFRA (Department for Environment, food and rural affairs) and Environment Agency, Citation2009; CCME (Canadian Council of Ministers of the Environment), Citation1999). Our findings substantiate those of Rahoui et al. (Citation2008) who reported that V. faba showed a delay in germination of 59% when treated with a toxic concentration of 20 mM Cd for four days indicating that Cd impaired seed germination. Previous studies have shown that Cd can inhibit germination and reduce the germination rate in several plant species such as Arabidopsis thaliana (W. Li et al., Citation2005); Hordeum vugare and Pisum sativum Piršelová (Citation2011) and negatively affect growth and photosynthetic activities in Brassica juncea and Brassica campestris (Chen et al., Citation2011) and Vigna radiata (N. Khan et al., Citation2020). It has been reported that Cd can reduce root absorption of nitrate (Hernandez et al., Citation1996) and interfere with the uptake of several essential elements such as, Ca, Mg, P, and K (Das et al., Citation1997).
Similarly, soil Hg concentration at a distance of 50 cm from the site centre was higher than those at the other distances (52 mg Hg/kg; ); this Hg soil level exceeds the guideline value for agricultural land in the UK (26 mg/ Kg−1; DEFRA (Department for Environment, food and rural affairs) and Environment Agency, Citation2009) and in Canada (6.6 mg/kg; CCME (Canadian Council of Ministers of the Environment), Citation1999). At the present high concentration, Hg is likely to have impaired germination as it known to have some detrimental impacts on plant growth including interference with normal chromosome segregation of the cell (Yu, Citation2005). Inhibition of germination and seedling emergence by high levels of Hg has been observed in several crop plants as 25% reduction in seedling emergence of Lactuca sativa and Raphanus sativus L. was reported at Hg levels ranged between 11 and 73 mg Hg/ kg−1 (CCME Environment Canada Citation1999). The lowest concentrations at which phytotoxic effects have been reported were 7 and 8 mg Hg/kg−1 which caused a growth reduction of 50% in Brassica rapa (Weaver et al., Citation1984). Munzuroglu and Geckil (Citation2002) found that Hg caused a complete inhibition in germination in Triticum aestivum and Cucumis sativus at a concentration of 1.7 mM (350 mg/kg). They also indicated that Hg was the most inhibitory heavy metal among other metals; Cd, Pb, Cu, and Co. High levels of Hg was proved to cause disruption of cellular metabolism (Cargnelutti et al., Citation2006) and induce physiological disorders (Zhou et al., Citation2007).
The results of the present study suggest that these substantial negative effects that significantly impaired seed germination sown at the closest distance to the explosion site centre may have originated from a combined inhibitory effect of several phytotoxic heavy metals, commonly detected in the residues of explosives, such as Pb, Cd, and Hg, which were found in greater concentrations at the closest point to the site. Our findings are consistent with the response of V. faba to a combined effect of a mixture of Pb, Cd, and Hg as Cavusoglu et al. (Citation2010) noted that the refinery wastewater containing high levels of Pb, in addition to Cd and Hg caused a substantial decline in germination percentage and root growth of V. faba.
Results showed that soil contents of the heavy metals examined, Pb, Cd, and Hg decreased with increasing distance from the explosion site. Soil Pb concentration measured at 1 and 2 m from the site was lower than values recorded at 50 cm (960 and 510 mg/kg, respectively), similarly, soil Cd and Pb concentrations gradually declined with distance from the explosion site with lower values obtained at 1, 2, and 4 m from the site (), however, values recorded at these distances were sufficient to increase the level of soil toxicity as they exceeded the guideline values for agricultural land proposed by the UK (DEFRA (Department for Environment, food and rural affairs) and Environment Agency, Citation2009) and Canada (CCME (Canadian Council of Ministers of the Environment), Citation1999). In contrast, at greater distance from the explosion site centre (6 m), soil content of heavy metals was highly reduced as the measured values of Pb, Cd, and Hg were respectively, 28, 0.8, and 0.51 mg/kg and all fall within the permissible limits for agricultural land in the UK (DEFRA (Department for Environment, food and rural affairs) and Environment Agency, Citation2009) and Canada (CCME (Canadian Council of Ministers of the Environment), Citation1999). The effect of stress on the growth of the surviving seedlings, apparently, was sharply reduced with distance as exposure to heavy metals decreased with distance from the explosion site. However, the levels of heavy metals recorded at 1–4 m from the explosive-contaminated site centre was sufficient to reduce all growth variables measured compared to control seedlings. Therefore, growth variables; plant length and leaf number were significantly reduced in the surviving seedlings located at this distance (P < 0.05) relative to those of control seedlings. In contrast, seed germination, seedling growth, and performance were the greatest at the distance furthest from the centre (6 m), where heavy metals concentrations were the lowest. Our results are consistent with Bonifacio and Montano (Citation1998) who indicated a consistent negative trend for seedling growth with increasing concentration of Cd and Hg when Enhalus acoroides (L.f) Royle was tested with different concentrations of both heavy metals.
Heavy metals distribution pattern that can be created from values of Pb, Cd. and Hg measured at different distances from the explosion site showed that heavy metals spread from the explosion point to create a concentric concentration gradient from a maximum concentration found close to the explosion point (at 50 cm) to the lowest concentrations recorded at 6 m from the centre. Accordingly, a clear negative correlation was obtained between soil contamination level and plant-related parameters as germination and seedling growth gradually and significantly increased with distance from the explosion site centre with maximum growth variables being achieved at locations further from the centre.
Soil contents of TNT, RDX, and DU were not measured in this study, however, visible stress symptoms such as curled leaf margins and leaf necrosis, were noticed on the surviving seedlings located at a distance of 1–4 m from the explosion site, indicating a possible negative response to RDX (Via at al. Citation2015). These highly toxic compounds are expected to be found in high concentrations close to the explosion site centre as they constitute the basic components used to manufacture explosive devices such as landmines. Talmage et al. (Citation1999) reported that explosive-contaminated soil concentrations ranges from 0.7 to 74,000 mg kg−1 for RDX and from 0.08 to 87,000 mg kg−1 for TNT. Such high concentrations are expected to highly increase the level of toxicity in the soil, particularly, at closer distances to the explosion site centre. At lower concentrations of TNT and RDX, several studies have reported strong negative effects on seed germination and seedling growth; Peterson et al. (Citation1996) observed that seed germination of Festuca arundinacea was declined with increasing TNT levels and was strongly inhibited when treated with >30 mg TNT L−1. Gholamian and Gholamian (Citation2008) found that seed germination of Raphanus sativus L. and Triticum sativum L. was largely decreased with increasing TNT and HMX concentrations in contaminated soil and inhibited when concentrations exceeded 500 mg/kg. Similarly, at concentrations of >200 mg TNT kg−1, TNT extinguished seed germination and retarded seedling growth of Lepidium sativum L. and Brassica rapa Metzg (Gong et al., Citation1999), whereas, growth of Medicago sativa was inhibited at 100 mg TNT/kg (Scheidemann et al., Citation1998). Robidoux et al. (Citation2003) indicated that seedling emergence as well as root and shoot biomass of surviving seedlings of Lactuca sativa and Hordeum vugare were significantly reduced when exposed to high concentrations (1040 mg/kg) of TNT.
It has been shown that clay soil adsorbs more explosive compounds than sandy soil (Larson et al., Citation2008). In the present study, physiochemical measurements revealed that soil of the study site was silty clay with high percentage of clay (65%), which may have increased the ability of soil to adsorb and retain toxic components as it is strongly related to soil type. The results showed that V. faba clearly could not survive the stress associated with increased soil heavy metals. A combined effect of high concentration of soil heavy metals and presumably, high levels of soil TNT, RDX, and DU may have caused mortality of the most severely stressed seeds sown at the closest point to the site centre, and retarded growth of the surviving seedlings sown further from the site centre as the effect of leaf number and plant height was more pronounced in the surviving seedlings located at a distance of 1–4 m from the explosion site centre than in those located at 4–6 m from the centre as the former seedlings, apparently, experienced higher concentration of heavy metals; Pb, Cd, and Hg in addition to the other toxic components that constitute landmines.
5. Conclusion
Based on the findings of the present study, it can be concluded that (1) soil content of heavy metals was the greatest near the explosion site and decreased with increasing distance from the site centre, (2) a strong inverse correlation was found between seed germination of V. faba and soil content of heavy metals, (3) seed germination was significantly inhibited at the closest distance to the explosion site, (4) increased seedling emergence, survival and growth were noticed further from the explosion site with a gradual decreasing trend towards the site centre, (5) As it was hypothesized, V. faba has proved to be intolerant to the unfavorable conditions associated with the explosive-contaminated soil, therefore, further studies involving a wide range of plant species are needed to improve our knowledge of the damaging phytotoxic effects of landmine contamination on native plant species and natural vegetative communities. Due to the magnitude of damage caused by landmine contamination, immediate measures should be taken by authorities and land users to clean up the contaminated soil and improve its quality.
Acknowledgments
Authors would like to thank Mr. Adel Ali for his assistance in collecting and transporting soil samples.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ali, A., Zinnert, J. C., Muthukumar, B., Peng, Y., Chung, S. M., & Stewart, C. N. (2014). Physiological and transcriptional responses of Baccharis halimifolia to the explosive Composition B (RDX/TNT) in amended soil. Environmental Science and Pollution Research, 21(13), 8261–8270. https://doi.org/10.1007/s11356-014-2764-4
- Benavides, M. P., Gallego, S. M., & Tomaro, M. L. (2005). Cadmium toxicity in plants. Brazilian Journal of Plant Physiology, 17(1), 21–34. https://doi.org/10.1590/S1677-04202005000100003
- Berhe, A. A. (2007). The contribution of landmines to land degradation. Land Degradation & Development, 18(1), 1–15. https://doi.org/10.1002/ldr.754
- Bernhoft, R. A. (2012). Mercury toxicity and treatment: A review of the literature. Journal of Environmental Public Health, 460-508. 460508, 10. http://dx.doi.org/10.1155/2012/460508
- Best, E. P. H., Tatem, H. E., Geter, K. N., Wells, M. L., & Lane, B. K. (2008). Effects, uptake, and fate of 2,4,6-trinitrotoluene aged in soil in plants and worms. Environmental Toxicology and Chemistry, 27(12), 2539–2547. https://doi.org/10.1897/08-017.1
- Bonifacio, R. S., & Montano, M. N. (1998). Inhibitory effects of mercury and cadmium on seed germination of Enhalus acoroides (L.f.) Royle. Bulletin of Environmental Contamination and Toxicology, 60(1), 45–51. https://doi.org/10.1007/s001289900589
- Boutros-Ghali, B. (1994). The land mine crisis: A humanitarian disaster. Foreign Affairs, 73(5), 8. https://doi.org/10.2307/20046826
- Bridges, C. C., & Zalups, R. K. (2017). Mechanisms involved in the transport of mercuric ions in target tissues. Archives of Toxicology, 91(1), 63–81.
- Cargnelutti, D., Tabaldi, L. A., Spanevello, R. M., Jucoski, G. O., Battisti, V., Redin, M., Linares, C. E. B., Dressler, V. L., Flores, M. M., Nicoloso, F. T., Morsch, V. M., & Schetinger, M. R. C. (2006). Mercury toxicity induces oxidative stress in growing cucumber seedlings. Chemosphere, 65(6), 999–1106. https://doi.org/10.1016/j.chemosphere.2006.03.037
- Cavusoglu, K., Yalcin, E., & Ergene, A. (2010). The investigation of cytotoxic effects of refinery wastewater on root tip cells of Vicia faba L. Journal of Environmental Biology, 31, 465–470.
- CCME (Canadian Council of Ministers of the Environment). (1999). Canadian soil quality guidelines for the protection of environmental and human health. Winnipeg
- Chaoui, A., & El Ferjani, E. (2005). Effects of cadmium and copper on antioxidant capacities, lignification and auxin degradation in leaves of pea (Pisum sativum L.) seedlings. Comptes Rendus Biologies, 328(1), 23–31. https://doi.org/10.1016/j.crvi.2004.10.001
- Chen, J., & Yang, Z. M. (2012). Mercury toxicity, molecular response and tolerance in higher plants. Biological Metals, 25 (5), 847–857. http://doi.org/10.1007/s10534-012-9560–8
- Chen, X., Wang, J., Shi, Y., Zhao, M. Q., & Chi, G. Y. (2011). Effects of cadmium on growth and photosynthetic activities in pakchoi and mustard. Botanica Studies, 52, 41–46.
- Clemens, S., & Ma, J. F. (2016). Toxic heavy metal and metalloid accumulation in crop plants and foods. Annual Review of Plant , Biology 67(1), 489–512. https://doi.org/10.1146/annurev-arplant-043015-112301
- Clemens, S., Palmgreen, M. G., & Kramer, U. (2002). A long way ahead: Understanding and engineering plant metal accumulation. Trends in Plant Science, 7(7), 309–315. https://doi.org/10.1016/S1360-1385(02)02295-1
- Das, P., Samantaray, S., & Rout, G. R. (1997). Studies on cadmium toxicity in plants: A review. Environmental Pollution, 98(1), 29–36. https://doi.org/10.1016/S0269-7491(97)00110-3
- Dasberg, S., & Mendel, K. (1971). The effect of soil water and aeration on seed germination. Journal of Experimental Botany, 22(4), 992–998. https://doi.org/10.1093/jxb/22.4.992
- DEFRA (Department for Environment, food and rural affairs) and Environment Agency. (2009). Soil guideline values for heavy metals. Almondsbury.
- Ekmekci, Y., Tanyolac, D., & Aythan, B. (2009). A crop tolerating oxidative stress induced by excess Pb: Maize. Acta Physiologiae Plantarum, 31(2), 319–330. https://doi.org/10.1007/s11738-008-0238-3
- Elly, P. H., Best, E. T., Kaaren, N. G., Melissa, L. W., & Bryan, K. L. (2006). Toxicity and metabolites of 2,4,6-trinitrotoluene (TNT) in plants and worm from exposure to aged soil. ERDC/EL TR-, 04–18.
- Ernst, W. H. O. (1998). Effects of heavy metals in plants at the cellular and organismic level ecotoxicology. In S. Gerrit & M. Bernd (Eds.), III, Bioaccumulation and biological effects of chemicals (pp. 587–620). Akademischer Verlag.
- Eun, S. O., Youn, H. S., & Lee, Y. (2000). Lead disturbs microtubule organization in the root meristem of Zea mays. Physiologia Plantarum, 110(3), 357–365. https://doi.org/10.1034/j.1399-3054.2000.1100310.x
- Fahr, M., Laplaze, L., Bendaou, N., Hocher, V., El Mzibri, M., Bogusz, D., & Smouni, A. (2013). Effect of lead on root growth. Frontiers in Plant Science, 4, 175. https://doi.org/10.3389/fpls.2013.00175
- Feng, X., Tang, S., Shang, L., Yan, H., Sommar, J., & Lindqvist, O. (2003). Total gaseous mercury in the atmosphere of Guiyang, PR China. Science of the Total Environment, 304(1–3), 61–72. https://doi.org/10.1016/S0048-9697(02)00557-0
- Foy, C. D., Chaney, R. L., & White, M. C. (1978). The physiology of metal toxicity in plants. Annual Review of Plant Physiology, 29(1), 511–566. https://doi.org/10.1146/annurev.pp.29.060178.002455
- Gholamian, F., & Gholamian, F. (2008). Effects of HMX and TNT contaminations on biochemical constitutes in Triticum Sativum L. and Raphanus Sativus L. plants. Indian Journal of Plant Physiology, 13(3), 211–216.
- Gong, P., Wilke, B., & Fleischmann, S. (1999). Soil-based phytotoxicity of 2,4,6-Trinitrotoluene (TNT) to terrestrial higher plants. Archives of Environmental Contamination and Toxicology, 36(2), 152–157. https://doi.org/10.1007/s002449900455
- Görge, E., Brandt, S., & Werner, D. (1994). Uptake and metabolism of 2,4,6-trinitrotoluene in higher plants. Environmental Science and Pollution Research, 1(4), 229–233. https://doi.org/10.1007/BF02986535
- Hernandez, L. E., Carpena-Ruiz, R., & Garate, A. (1996). Alterations in the mineral nutrition of pea seedlings exposed to cadmium. Journal of Plant Nutrition, 19(12), 1581–1598. https://doi.org/10.1080/01904169609365223
- Jensen, E., Peoples, M., & Hauggaard-Nielsen, H. (2010). Faba bean in cropping systems. Field Crops Research, 115(3), 203–216. https://doi.org/10.1016/j.fcr.2009.10.008
- Jinbiao, Z., Hanwen, W., Xiangping, W., & Weinan, H. (2010). Subcellular distribution and chemical forms of cadmium in the cells of strawberry (Fragaria ananassa Duch.). The Journal of Horticultural Science and Biotechnology, 85(6), 563–569. https://doi.org/10.1080/14620316.2010.11512715
- Khan, A., Khan, S., Khan, A., Qamar, Z., & Waqas, M. (2015). The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: A review. Environmental Science and Pollution Research, 22, 13772–13799. http://doi.org/10.1007/s11356-015-4881-0
- Khan, N., Siddiqui, M., AlSolami, M., Alamri, S., Hu, Y., Ali, H., Al-Amri, A. A., Alsubaie, Q. D., Al-Munqedhi, B. M. A., & Al-Ghamdi, A. (2020). Crosstalk of hydrogen sulfide and nitric oxide requires calcium to mitigate impaired photosynthesis under cadmium stress by activating defense mechanisms in Vigna radiata. Plant Physiology and Biochemistry, 156, 278–290. https://doi.org/10.1016/j.plaphy.2020.09.017
- Khatisashvili, G., Gordeziani, M., Adamia, G., Kvesitadze, E., Sadunishvili, T., & Kvesitadze, G. (2009). Higher plants ability to assimilate explosives. World Academy of Science and Engineering Technology, 33, 256–270.
- Krishnan, G., Horst, G. L., Darnell, S., & Powers, W. L. (2000). Growth and development of smooth bromegrass and tall fescue in TNT-contaminated soil. Environmental Pollution, 107(1), 109–116. https://doi.org/10.1016/S0269-7491(99)00126-8
- Landmine Monitor. (2019). Executive Summary. International Campaign to Ban Landmines. Human Rights Watch.
- Larson, S. L., Martin, W. A., Escalon, B. L., & Thompson, M. (2008). Dissolution, sorption, and kinetics involved in systems containing explosives, water, and soil. Environmental Science & Technology, 42(3), 786–792. https://doi.org/10.1021/es0717360
- Leitch, D. R., Carrie, J., Lean, D., Macdonald, R. W., Stern, G. A., & Wang, F. Y. (2007). The delivery of mercury to the Beaufort Sea of the Arctic Ocean by the Mackenzie River. Science of the Total Environment, 373(1), 178–195. https://doi.org/10.1016/j.scitotenv.2006.10.041
- Li, Q. S., Chen, Y., Fu, H. B., Cui, Z. H., Shi, L., Wang, L. L., & Liu, Z. F. (2012). Health risk of heavy metals in food crops grown on reclaimed tidal flat soil in the Pearl River Estuary, China. Journal of Hazardous Materials, 227-228, 148–154. https://doi.org/10.1016/j.jhazmat.2012.05.023
- Li, W., Khan, M. A., Yamaguchi, S., & Kamiya, Y. (2005). Effects of heavy metals on seed germination and early seedling growth of Arabidopsis thaliana. Plant Growth Regulation, 46(1), 45–50. https://doi.org/10.1007/s10725-005-6324-2
- Liu, D., Zou, J., Meng, Q., Zou, J., & Jiang, W. (2009). Uptake and accumulation and oxidative stress in garlic (Allium sativum L.) under lead phytotoxicity. Ecotoxicology, 18(1), 134–143. https://doi.org/10.1007/s10646-008-0266-1
- Lou, Y., Zhao, P., Wang, D., Amombo, E., Sun, X., Wang, H., Zhuge, Y., & Rouached, H. (2017). Germination, physiological responses and gene expression of tall fescue (Festuca arundinacea Schreb.) growing under Pb and Cd. PLoS One, 12(1), e0169495. https://doi.org/10.1371/journal.pone.0169495
- Manara, A. (2012). Plant responses to heavy metal toxicity. In: Furini, A., (Ed), Plants and heavy metals (pp. 27–53). Springer Briefs in Biometals.
- Mukherji, S., & Maitra, P. (1976). Toxic effects of lead growth and metabolism of germinating rice (Oryza sativa L.) seeds mitosis of onion (Allium cepa) root tip cells. Industrial Journal of Experimental Biology, 14, 519–521.
- Munzuroglu, O., & Geckil, H. (2002). Effects of metals on seed germination, root elongation, and coleoptile and hypocotyl growth in Triticum aestivum and Cucumis sativus. Archives of Environmental Contamination and Toxicology, 43(2), 203–213. https://doi.org/10.1007/s00244-002-1116-4
- Nachón, C. T., 2000. Environmental aspects of the international crisis of antipersonnel landmines and the implementation of the 1997 Mine Ban Treaty: Thematic report. Landmine Monitor Report 2000. International Campaign to Ban Landmines.
- Odabas, M. S., & Mut, Z. (2007). Modeling the effect of temperature on percentage and duration of seed germination in grain legumes and cereals. American Journal of Plant Physiology, 2(5), 303–310. https://doi.org/10.3923/ajpp.2007.303.310
- Parrotta, L., Guerriero, G., Sergeant, K., Cai, G., & Hausman, J. F. (2015). Target or barrier? The cell wall of early-and later-diverging plants vs cadmium toxicity: Differences in the response mechanisms. Frontiers in Plant Science, 6, 133. https://doi.org/10.3389/fpls.2015.00133
- Peralta-Videaa, J. R., Gardea-Torresdey, J. L., Gomezc, E., Tiemanna, K. J., Parsonsa, J. G., & Carrillod, G. (2002). Effect of mixed cadmium, copper, nickel and zinc at different pHs upon alfalfa growth and heavy metal uptake. Environmental Pollution, 119(3), 291–301. https://doi.org/10.1016/S0269-7491(02)00105-7
- Peterson, M. M., Horst, G. L., Shea, P. J., Comfort, S. D., & Peterson, R. K. D. (1996). TNT and 4-amino-2,6-dinitrotoluene influence on germination and early seedling development of tall fescue. Environmental Pollution, 93(1), 57–62. https://doi.org/10.1016/0269-7491(96)00016-4
- Piršelová, B. (2011). Monitoring the sensitivity of selected crops to lead, cadmium and arsenic. Journal of Stress Physiology and Biochemistry, 7(4), 31–38.
- Rahman, Z., & Singh, V. P. (2019). The relative impact of toxic heavy metals (THMs) (arsenic (As), cadmium (Cd), chromium (Cr)(VI), mercury (Hg), and lead (Pb)) on the total environment: An overview. Environmental Monitoring and Assessment, 191(7), 419. https://doi.org/10.1007/s10661-019-7528-7
- Rahoui, S., Chaoui, A., & Ferjani, E. E. (2008). Differential sensitivity to cadmium in germinating seeds of three cultivars of faba bean (Vicia faba L.). Acta Physiologiae Plantarum, 30(4), 451–456. https://doi.org/10.1007/s11738-008-0142-x
- Robidoux, P., Bardai, G., Paquet, L., Ampleman, G., Thiboutot, S., Hawari, J., & Sunahara, G. I. (2003). Phytotoxicity of 2,4,6-Trinitrotoluene (TNT) and Octahydro-1,3,5,7-Tetranitro-1,3,5,7-Tetrazocine (HMX) in Spiked Artificial and Natural Forest Soils. Archives of Environmental Contamination and Toxicology, 44(2), 0198–0209. https://doi.org/10.1007/s00244-002-2018-1
- Scheidemann, P., Klunk, A., Sens, C., & Werner, D. (1998). Species dependent uptake and tolerance of nitro aromatic compounds by higher plants. Journal of Plant Physiology, 152(2–3), 242–247. https://doi.org/10.1016/S0176-1617(98)80139-9
- Schott, C. D., & Worthley, E. G. 1974. The toxicity of TNT and related wastes to an aquatic flowering plant: Lemna perpusilla Torr. Edgewood Arsenal Technical Report, No. EB-TR–74016.
- Sens, C., Scheidemann, P., & Werner, D. (1999). The distribution of 14C-TNT in different biochemical compartments of the monocotyledonous Triticum aestivum. Environmental Pollution, 104(1), 113–119. https://doi.org/10.1016/S0269-7491(98)00142-0
- Sheppard, S. C., Evenden, W. G., Abboud, S. A., & Stephenson, M. (1993). A plant life-cycle bioassay for contaminated soil, with comparison to other bioassays: Mercury and zinc. Archives of Environmental Contamination and Toxicology, 25(1), 27–35. https://doi.org/10.1007/BF00230707
- Simini, M., Wentsel, R. S., Checkai, R., Phillips, C., Chester, N. A., Major, M. A. (1995). Evaluation of soil toxicity at Joliet Army Ammunition Plant. Environmental Toxicology and Chemistry, 14(4), 623–630. https://doi.org/10.1002/etc.5620140410
- Talmage, S. S., Opresko, D. M., Maxwell, C. J., Welsh, C. J. E., Cretella, F. M., Reno, P. H., & Daniel, F. B. (1999). Nitroaromatic munition compounds: Environmental effects and screening values, In: Ware, G.W. (eds) Reviews ofEnvironment Contamination and Toxicology, vol 161. New York, NY: Springer. https://doi.org/10.1007/978-1-4757-6427-7_1
- Thijs, S., Weyens, N., Sillen, W., Gkorezis, P., Carleer, R., & Vangronsveld, J. (2014). Potential for plant growth promotion by a consortium of stress-tolerant 2,4- dinitrotoluene-degrading bacteria: Isolation and characterization of a military soil. Microbial Biotechnology, 7(4), 294–306. https://doi.org/10.1111/1751-7915.12111
- Travis, E. R., Bruce, N. C., & Rosser, S. J. (2008). Microbial and plant ecology of a long-term TNT-contaminated site. Environmental Pollution, 153(1), 119–126. https://doi.org/10.1016/j.envpol.2007.07.015
- Troll, K. (2000). The impact of anti-personnel landmines on the environment. Available from United Nations Institute for Disarmament Research: Geneva, CH. .
- Vassilev, A., & Yordanov, I. (1997). Reductive analysis of factors limiting growth of Cd-exposed plants: A review. Bulgarian Journal of Plant Physiology, 23, 114–133.
- Verma, S., & Dubey, R. S. (2003). Lead toxicity induces lipid peroxidation and alters the activities of antioxidant enzymes in growing rice plants. Plant Science, 164(4), 645–655. https://doi.org/10.1016/S0168-9452(03)00022-0
- Via, S. M., Zinnert, J. C., & Young, D. R. (2015). Differential effects of two explosive compounds on seed germination and seedling morphology of a woody shrub, Morella cerifera. Ecotoxicology, 24(1), 194–201. https://doi.org/10.1007/s10646-014-1372-x
- Vijayaragavan, M., Prabhahar, C., Sureshkumar, J., Natarajan, A., Vijayarengan, P., & Sharavanan, S. (2011). Toxic Effect of Cadmium on Seed Germination, Growth and Biochemical Contents of Cowpea (Vigna Unguiculata L.) Plants. International Multidisciplinary Research Journal, 1, 5.
- Vila, M., Lorber-Pascal, S., & Laurent, F. (2008). Phytotoxicity to and uptake of TNT by rice. Environmental Geochemistry and Health, 30(2), 199–203. https://doi.org/10.1007/s10653-008-9145-1
- Wagner, G. J. (1993). Accumulation of cadmium in crop plants and its consequences to human health. Advances in Agronomy, 51, 173–212.
- Wang, C., Tian, Y., Wang, X., Geng, J., Jiang, J., Yu, H., & Wang, C. (2010). Lead-contaminated soil induced oxidative stress, defense response and its indicative biomarkers in roots of Vicia faba seedlings. Ecotoxicology, 19(6), 1130–1139. https://doi.org/10.1007/s10646-010-0496-x
- Wang, Y., & Greger, M. (2004). Clonal differences in mercury tolerance, accumulation, and distribution in willow. Journal of Environmental Quality, 33(5), 1779–1785. https://doi.org/10.2134/jeq2004.1779
- Wang, Z., Liu, X., & Qin, H. (2019). Bioconcentration and translocation of heavy metals in the soil-plants system in Machangqing copper mine, Yunnan Province, China. Journal of Geochemical Exploration, 200, 159–166. https://doi.org/10.1016/j.gexplo.2019.02.005
- Weaver, R., Melton, W., Wang, D., & Duble, R.L., J. R. (1984). Uptake of arsenic and mercury from soil by bermudagrass Cynodon dactylon. Environmental Pollution Series A, Ecological and Biological, 33(2), 133–142. https://doi.org/10.1016/0143-1471(84)90173-9
- Woźny, A., & Jerczyńska, E. (1991). The effect of lead on early stages of Phaseolus vulgaris L. growth in vitro conditions. Biologia Plantarum, 33(1), 32–39. https://doi.org/10.1007/BF02873785
- Xu, J., Bravo, A. G., Lagerkvist, A., Bertilsson, S., Sjöblom, R., & Kumpiene, J. (2015). Sources and remediation techniques for mercury contaminated soil. Environment International, 74, 42–53. https://doi.org/10.1016/j.envint.2014.09.007
- Yu, M. (2005). Environmental Toxicology (Second ed.). CRC Press.
- Zhang, W. H., & Tyerman, S. D. (1999). Inhibition of water channels by HgCl2 in intact wheat root cells. Plant Physiology, 120(3), 849–857. https://doi.org/10.1104/pp.120.3.849
- Zhou, Z. S., Huang, S. Q., Guo, K., Mehta, S. K., Zhang, P. C., & Yang, Z. M. (2007). Metabolic adaptations to mercury-induced oxidative stress in roots of Medicago sativa L. Journal of Inorganic Biochemistry, 101(1), 1–9. https://doi.org/10.1016/j.jinorgbio.2006.05.011