ABSTRACT
The natural distribution and the species ecology of the lesser-known nocturnal endemic brown palm civet in the Western Ghats are definite. We used camera trap data from the Western Ghats to predict the suitable niche, coat colour pattern, and diel activity. The environmental variables like rainfall, elevation, isothermaility, tree density, and tree height profoundly influence the brown palm civet’s habitat suitability. We determined that the most suitable habitat area in the entire Western Ghats is 21,853 km2, in four well-defined isolated blocks. The brown palm civet distribution is more towards the south, ranging from Kakkad to Anamalai. The distribution on the north side is in the Nilgiris, Bhadra, and the Sahyadri. The diel activity pattern indicates that the species is nocturnal, with activity peak in the early night and ending in the early morning, and is nearly identical in different landscapes. The species has a variety of coat patterns, which we believe is due to phenotypic plasticity. The species is not a true high altitude montane shola-dependent species, unlike the other endemic species of the Western Ghats. The destruction of rainforests resulting from excessive anthropogenic pressure results in habitat fragmentation, posing a significant threat to the brown palm civet habitats.
Introduction
The nocturnal small carnivore communities of the Indian sub-continent are less studied. Due to the size and cryptic nature, it is difficult to investigate the actual distribution and ecology. Studies on these species are limited and restricted to occurrence reports (Kalle et al., Citation2013; Kumar & Yoganand, Citation1999; Mudappa, Citation1999). They thrive in diverse habitat by operating in the available ecological niches forming an integral part of the food chain. The Western Ghats, a biodiversity hot spot in peninsular India, holds many small nocturnal carnivores having restricted distribution (Raman et al., Citation2020a). Conservation of these endemic assemblages is possible only if species-specific pertinent information on the distribution range and activity pattern are available. A species activity pattern gives information about their foraging pattern, anti-predator behaviour, resource partitioning, and competition (Shameer et al., Citation2021; Zhou et al., Citation2014). Likewise, precise distribution information assists ecologists and protected area managers in designing and developing appropriate conservation strategies. Ultimately such information can comprehend the co-existence of sympatric species and the mechanism of morphological variations, diets, and spatial preferences (Davies et al., 2007; Jennings & Veron, Citation2011; Chen et al., Citation2009), especially for the lesser-known elusive species.
Civets are small carnivores (family Viverridae) widely distributed in southeast Asia, Madagascar, and Sub-Saharan Africa. They control rodents under check and are generally seed dispersers (Colón, Citation2002; Kumara & Singh, Citation2007; Rabinowitz, Citation1991). Civets face threats due to deforestation, forest fragmentation, habitat destruction, and poaching (Balakrishnan & Sreedevi, Citation2007; Kalle et al., Citation2013). Presently in India, there are four species of civets under the subfamily Viverrinae and Paradoxurinae. Among the four species, Viverricula indica (Small Indian civet), Paradoxurus jerdoni Blanford Citation1885 (Brown palm civet), and Paradoxurus hermaphrodites (Common palm civet) are present in the Western Ghats. The brown palm civet is endemic to the Western Ghats and Srilanka, unlike the other two species with wider distribution (Blanford, Citation1885; Hutton, Citation1949; Pocock, Citation1939). Brown palm civet is of least concern as per the IUCN (3.1) and included in Appendix III of CITES and schedule II of the Indian Wildlife (Protection) Act 1972. The body has a brown or slightly grizzled pelage, darker in the neck, appendages, and tail. The tail sometimes has a white or pale tip. The hair grows in the nape in the reverse direction and weighs around ~4 kg (Blanford, Citation1888–91). Wozencraft (Citation2005) nominated two subspecies of brown palm civet from the Western Ghats landscape. The Paradoxurus jerdoni jerdoni Blanford (Citation1885) and the Paradoxurus jerdoni caniscus Pocock (Citation1933) are nominated subspecies.
The brown palm civet is predominantly frugivore and rarely depends on small vertebrates and invertebrates (Gnanaolivu & Singh, Citation2019; Mudappa et al., Citation2010). In the Western Ghats, the species reported at an altitude ranging between 500 to 1,300 m a.s.l. (Rajamani et al., Citation2002; Veron et al., Citation2015). The distribution of the species is from North Dhud Sagar in Goa to the south (Kalakkad Mundanthurai) in the Western Ghats. The habitat is primarily the rain forests, cardamom, and coffee plantations (Mudappa et al., Citation2010; Rajamani et al., Citation2002). The studies on brown palm civet in the Western Ghats is limited to occurrence reports, dietary pattern, pelage variation, and taxonomy (Ashraf et al., Citation1993; Ganesh, Citation1997; Hutton, Citation1949; Mudappa, Citation1998, Citation2001; Mudappa et al., Citation2010; Pocock, Citation1933; Rajamani et al., Citation2002; Ramachandran, Citation1990; Schreiber, Citation1989). To adopt a suitable conservation strategy for a particular species, the knowledge of natural history and biology alone is not enough. Instead, a thorough knowledge about its distribution and suitable habitat is vital (Papeş & Gaubert, Citation2007). In the current scenario of anthropogenic pressure, it is a priority to assess the possible ecological niche of the understudied animals at the landscape level. For example, fragmentation, tree density, and climate (in terms of altitude) influence the distribution of brown palm civets (Mudappa et al., Citation2007). MaxEnt (Maximum Entropy) creates species distribution models based on species occurrence records and, in response to various environmental variables (Papeş & Gaubert, Citation2007; Williams et al., Citation2009). The niche modeling advancements allow us to predict and identify the suitable habitat for the nocturnal and cryptic endemic species (Raman et al., Citation2020a, Citation2020b).
Several abiotic and biotic factors influence the activity pattern, including environmental variables, predator avoidance, and competition (Bunnell & Harestad, Citation1990; Lucherini et al., Citation2009; O’Donoghue et al., Citation1998; Rogowitz, Citation1997). The knowledge of isolated populations and regionally adapted activity patterns improve the understanding of population divergence and niche alteration in diverse landscapes. The gaps, the deep valleys, and gorges in the Western Ghats isolated the altitude-dependent species to meta-populations, and they undergo divergence for a long time (Robin et al., Citation2010, Citation2015). The consequences of fragmentation due to anthropogenic disturbances create patchy communities with limited individuals. The continuous inbreeding in the isolated patches leads to the expression of recessive alleles with visible expression in coat colour (Sanil et al., Citation2014). Chunekar et al. (Citation2017) reported coat color variations in common palm civets from the Western Ghats. The studies by different authors demonstrated such coat colour and morphometric variations in different civets from the other parts of the globe (Eaton et al., Citation2010; Vasudeva et al., Citation2021; Veron et al., Citation2004; Winaya et al., Citation2020). Coat colour variations may be influenced by geography and climate (Ajith et al., Citation1998), resulting in speciation (Vasudeva et al., Citation2021). We targeted to develop a precise species distribution model for the endemic brown palm civet ranges in the Western Ghats landscape by the present study. Further, we intended to evaluate the response of the environmental variables on brown palm civet distribution. We also targeted to understand the activity pattern and the coat colour variations of brown palm civet in the Western Ghats landscape.
Methodology
Study area
The Western Ghats in India is regarded as one of the “hottest hotspots” globally, hosting 38 endemic mammals (Myers et al., Citation2000). The continuous mountain chain of Western Ghats spread across 8º to 21º N latitudes, north to south parallel to the west coast in peninsular India. The 1,600 km stretch passes through the Indian states of Gujarat, Maharashtra, Goa, Karnataka, Kerala, and Tamil Nadu. The Western Ghats splintered at the Goa (16ºN), Palghat Gap (11ºN), and the Shencottah gap (9ºN) (Dahanukar et al., Citation2004; Jhala et al., Citation2019). The elevation of Western Ghats ranges between 600 to 2,500 MSL with higher elevations, mostly between 8º – 13º N and 18º – 19ºN (Dahanukar et al., Citation2004). The vegetation types show altitudinal gradation in the Western Ghats, with scrub jungles in the lower elevation, deciduous forest in the mid-elevation, and tropical evergreen forest in the high altitude. In the areas where the mountains rise above ~1,800 m a.s.l., a peculiar short, stunted vegetation termed shola grassland occurs. These grassland–shola ecosystem are considered as primitive and is rich in endemism due to temperate climatic conditions prevailing here (Robin et al., Citation2010). The Western Ghats receive good rainfall, which differs from place to place, and it is responsible for the formation of northeast and southwest monsoons. At present, the Western Ghats is facing severe threats due to uncontrolled anthropogenic pressure and climate change (Raman et al., Citation2020a, Citation2020b)
Occurrence data collection
We obtained the camera trap data records of brown palm civet for five years (2015–2020) from the various protected areas in Tamilnadu and Kerala state (see acknowledgments for details). The collected reserves include the Periyar tiger reserve, Munnar forest division, Parambikulam tiger reserve, Silent valley national park, Nilgiri forest division, and the Mudumalai tiger reserve. The concerned authorities collected data in 2 × 2 grid sampling patterns and provided capture details and photos for our analysis from these reserves. We also included the camera trap data collected from the private plantations as a part of lesser felid monitoring from 2011 to 2017. We placed camera traps at the height of ~30 cm (by tying to tree or poles) in the track paths and animal trails in the tea, coffee plantations, and orchards in the Nilgiris. We employed 30 camera traps and placed them randomly to monitor for 20 to 30 days (following Meek et al., Citation2014). In addition, the fourth author compiled three-year sightings (2015–2018) of brown palm civets from regions in and around the Sahyadri tiger reserve and suburbs during his tenure as a reserve biologist. We also collected occurrence details of brown palm civet reports from the Indian biodiversity portal (https://indiabiodiversity.org/) and published literature (Ganesh, Citation1997; Gnanaolivu & Singh, Citation2019; Kumara & Singh, Citation2004, Citation2007; Mudappa, Citation2006; Mudappa et al., Citation2010, Citation2007; Nikhil & Nameer, Citation2017; Pillay, Citation2009; Rajamani et al., Citation2002; Ramachandran, Citation1990; Sanghamithra & Nameer, Citation2018; Sayyed et al., Citation2019; Schreiber, Citation1989; Sreehari & Nameer, Citation2016; Sreekumar & Nameer, Citation2018).
Camera trap pictures and Coat colour variation
We obtained 972 photographs from the various cameras place across the Western Ghats landscape. We cross-checked all the photos received from various reserves for assessing the morphological differences. We considered those brown palm civets that do not have typical coat color patterns as variants and sorted the photographs separately.
Data thinning and compiling
We were able to capture multiple palm civets in a single camera trap. For habitat modeling, we used the locations of the camera traps that recorded the brown palm civet. In total, we obtained 228 records throughout the Western Ghats from the camera trap records and secondary sources like literature. In the ecological surveys from vast geographical terrains with diverse habitats and altitudes, unavoidable potential bias is common (Jennings & Veron, Citation2011). To reduce the bias and confirm the independence of records, we thinned the occurrence record from 228 to 59 using the package “spThin” (Aiello-Lammens et al., Citation2019) in the R platform. Thus, we avoided the repeated data in 4 km2, and used the species absence location as the bias file for our model. Furthermore, the thinning procedure avoids the influence of spatial autocorrelation on the species distribution model.
Camera trap records for activity pattern
The camera trapping data we obtained were systematically collected and monitored in a site for ~30 ± 5 days. We used the capture time records of the brown palm civets from Periyar tiger reserve, Munnar forest division, Parambikulam tiger reserve, Mudumalai tiger reserve, Mukkurthi National Park and the Nilgiri orest division. The data from October to January alone was considered from various reserves to maintain uniformity.
Environment variables
We downloaded 19 bioclimatic variables (Hijmans et al., Citation2005) from the WorldClim (https://www.worldclim.org/) database, elevation (https://www.earthexplorer.usgs.gov/), vegetation features like forest cover (Forest Survey of India), tree density (Crowther et al., Citation2015), roads and waterways from the Survey of India database. In addition, the human global footprint data set available with the Socioeconomic Data and Application Centre (SEDACE) managed by the NASA Earth Science Data and Information System (ESDIS) is downloaded (Van Donkelaar et al., Citation2015). We included climate variables like Bio3 (isothermality), Bio13 (precipitation of wettest month), Bioc18 (precipitation of coldest quarter), Bio19 (precipitation of the warmest quarter) for the prediction. We excluded the other climatic variables having low predictive capacity from the study based on the Pearson collinearity test. We used ≥ 0.80 as a cut-off threshold to eliminate the positively correlated climatic variables to choose the strong predictors.
Habitat suitability modeling
The MaxEnt (Phillips et al., Citation2006) or maximum Entropy modeling based on presence-only data, with various environment variables, are popular and more precise in species distribution modeling. We implemented the MaxEnt in R platform using the “dismo” package (Hijmans et al., Citation2017) using the default setting parameters. We did the model validation by k-fold partitioning with 20% training and 80% test data. We used the area under the receiver-operator curve (AUC) and kappa statistics to assess the model’s performance following the standard methods (see Duan et al., Citation2014; Fielding & Bell, Citation1997; Hanley & McNeil, Citation1983). The area under the receiver operating characteristic curve is the AUC, and kappa statistics take the best information from a mixed matrix. The evaluation criteria for kappa is excellent (0.85–1.0), very good (0.7–0.85), good (0.55–0.7), fair (0.4–0.55), and fail (<0.4). While the evaluation criteria for the AUC statistic is excellent (0.90–1.00), very good (0.8–0.9), good (0.7–0.8), fair (0.6–0.7), and poor (0.5–0.6) (Monserud and Leemans (Citation1992), Swets (Citation1988), and Duan et al. (Citation2014)). We classified the predicted suitability of habitat into very high (>0.8), high (0.6–0.8), moderate (0.4–0.6), low (0.2–0.4), and very low (<0.2) (Raman et al., Citation2020b). Each predicted habitat category is estimated by creating polygons and expressed in square kilometers (km2). We identified the isolated patches as different conservation blocks of brown palm civets. We done all the calculations and mapping in the R studio (R Development Core Team, Citation2019)
Activity pattern and coat colour variants
The activity pattern is analysed based on the recorded capture time in the camera traps. We visualised the temporal activity pattern using a nonparametric kernel density estimation model (Ridout & Linkie, Citation2009) using the R package “overlap 0.3.3” (Meredith & Ridout, Citation2014). We estimated the combined activity pattern and specific brown palm civet activity pattern from Periyar tiger reserve, Munnar forest division, Parambikulam tiger reserve, Mudumalai tiger reserve, and the Nilgiris (including Nilgiris forest division and Mukkurthi national park). Further, we estimated the activity overlap between various sites. The kernel density method provides the coefficient of overlap (D), which ranges between 0 (no overlap) and 1 (complete overlap). The overlap coefficient is D1 for a small sample size and D4 for a larger sample size (> 75) (Meredith & Ridout, Citation2014). We bootstrapped the samples 1000 times to evaluate the statistical significance at a 95% confidence interval (CI). Using a comparative gradation scale, we compared the differences in activity patterns of brown palm civets in different protected areas (see Linkie & Ridout, Citation2011; Lynam et al., Citation2013; Tian et al., Citation2020; Shankar et al., Citation2020 for more details).
Results
Coat colour variation
Colour variations exist among brown palm civets in the various study locations and appear to coexist. The typical dark brown colour pattern, golden colour pattern, whitish colour pattern, and a whitish and orange patchy colour pattern in the tail are the various colour variants observed. (). We did not follow any specific pattern from a particular locality; rather, it occurs randomly regardless of reserves or landscapes.
Model evaluation
The model analysis indicates that the AUC value of the model is 0.8816. The kappa value obtained is 0.96, and the sensitivity threshold is 0.3470 (details provided as supplementary material S1). Thus, the values evaluate a good fit model in predicting the suitable habitat of the brown palm civet in the Western Ghats.
The response of environmental models
Road and human footprint presence have no considerable influence on the distribution of brown palm civet among the ten environmental variables analysed. The variables observed to be favorably influencing the distribution of the brown palm civet are bio19 (59%), elevation (29%), tree density (8%), and bio 18 (7%). The presence of wetlands (3%), forest cover (2%), bio 13 (1%), and bio3 (0.5%) have only a minor influence on the distribution of the brown palm civet (Supplementary material S2). The variable bio 18 corresponds to the rain in the warmest quarter, and bio19 is rain in the coldest quarter. The response curves (Supplementary Material S3) indicate that the warmest quarter’s optimum rainfall is 200 mm and in the coldest quarter is around 400 mm. The precipitation of the wettest month (Bio 13) is favourable for brown palm civet and does not significantly influence the distribution. The isothermality (Bio 3) has a slightly positive impact on the brown palm civet distribution, and the observed optimum range is 60–70%. The mean diurnal range to temperature seasonality is bio 13, where the mean diurnal temperature is the monthly mean of maximum and minimum temperature. The altitude (elevation) 500–2,100 MSL is the favourable range, and the animal tends to be absent from the height above the maximum range. The forest (vegetation) cover has minimum influence in the distribution, while the tree density found influencing more positively, indicating the animal prefers densely wooded habitat like evergreen and deciduous forest. The analysis of wetlands as a variable shows it negatively impacts the brown palm civet distribution. In consolidation, the isothermality, tree density, and forest cover contribute to brown palm civets prediction of Western Ghats’ southern side. Simultaneously, the precipitation (Bio 13, 19, and 18) influences the distribution mainly in the northern region (Supplementary material S4).
Suitable habitats and subpopulations
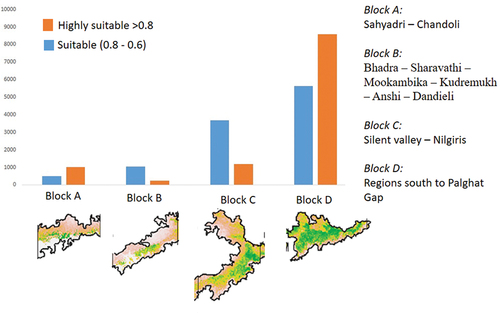
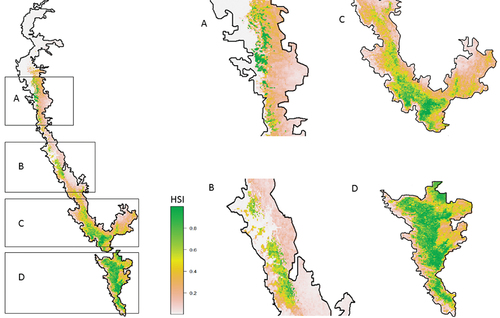
The habitat suitability analysis shows 11,241 km2 is highly suitable (>0.8), and 10,813 km2 is suitable (0.8–0.6) for the survival and reproduction of brown palm civets (). The evaluation of suitable habitats predicts four conservation clusters of blocks under the highly appropriate and suitable regions. The clusters are the “Sahyadri – Chandoli (block A),” “Bhadra – Sharavathi – Mookambika – Kudremukh – Anshi – Dandieli (block B),” “Silent valley – Nilgiri (block C)” and regions south to Palghat Gap (block D). The model predicted more than 70% of the “very high” categorised ecological niche is towards the south of the Palghat gap (8574 km2). The “high” category of habitat is more towards the south of Palghat (5266 km2) and in the Nilgiris (336 km2). The “very high” suitable areas are more towards the Sahyadri block (1022 km2) than the Bhadra block (232 km2), while “high” areas are vice versa (485 km2 & 1052 km2 respectively). The region south of the Palghat gap seems to be a single conservation block; the three other blocks towards the north are isolated and comparatively small. The primary protected area included in the conservation block D is the Anamalai tiger reserve, Periyar tiger reserve, Parambikulam tiger reserve, Kalakkad Mundanthurai tiger reserve, Meghamalai, Kodaikanal, Munnar forest division, Srivilliputhur, etc. This region accounts for a significant habitat of the brown palm civet. The second important block is the Nilgiri (block C), which includes the Silent Valley national park, Nilgiri, Mukkurthi, Mudumalai tiger reserve, Wayanad wildlife sanctuary, Nagarhole tiger reserve, Bandipur tiger reserve the Biligiri Ranganatha tiger reserve. The area of the suitable habitat in the other two regions is comparatively few indicating smaller populations ().
Figure 2. Highly suitable habitat of brown plam civet in various regions of Western Ghats. Based on the habitat suitability and connectivity, the populations are split into four blocks designated as A, B, C, and D. The figure clearly depicts that the Southern WG is the sole largest brown plam civet habitat.
Analysis of activity pattern
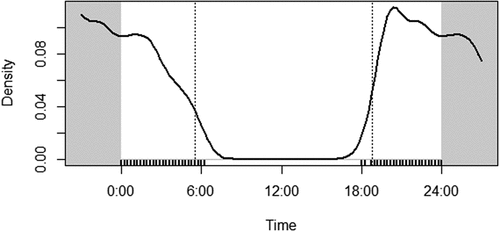
The activity pattern of the animal clearly shows the animal is strictly nocturnal (). The peak activity is in the early night hours from 18.00hr to 12.00hr and continued to cease gradually and ends at 5.00hr. The move seems to be reduced by midnight and gradually declines in the morning hours. A minor regional variation in peak activity is present in all reserves during the same season, though it is not significant. In Munnar forest division, Nilgiris, and Parambikulam, the peak reaches the maximum towards the midnight and early morning hours. In contrast, in Mudumalai tiger reserve and the Parambikulam tiger reserve, the maximal peak activity is in the early night hours (Supplementary material S5). The overlap ranges indicate a high overlap (>0.8) between various reserves except for the Periyar tiger reserve (0.70–0.80) ().
Table 1. Temporal overlap matrix in various reserves (Mean ± SD (95%CI)).
Discussion
In the Western Ghats, we predicted the suitable niche of the endemic brown palm civet. Prediction models typically interpret the target species’ fundamental niche, which is useful when assessing or creating data for a lesser-known species. Due to its nocturnal and elusive nature, the endemic brown palm civet is challenging to observe (Mudappa, Citation2006; Patou et al., Citation2010). Fundamental niche identification aids wildlife managers and researchers in developing appropriate action plans and conducting intensive monitoring. Even though we modeled the distribution with extreme caution to reduce overprediction, data interpretation also necessitates extreme caution. Inter and intra-species competition, dispersing capacity, and population dynamics influence the realised niche (Alfaya et al., Citation2019). As a result, depending on the presence of sympatric species, prey, and predators, the realised niche may be narrower or more significant than the predicted niche. A locality’s diel activity pattern assessment validates this, as peak activity corresponds to competition, prey, and predator response. We identified that the diel activity did not differ in most of the reserves included in the current prediction, indicating a typical activity pattern. Hence, we assume that the fundamental niche corresponds to the realised niche and that the projection is correct.
The distribution is patchy or fragmented, especially in the regions towards the north of the Palghat gap. The density of forests is comparatively high in the Southern Western Ghats, and the patches are more towards the northern side. The southern Western Ghats face threats like the invasion of exotic species and various kinds of anthropogenic influences. The fragmentation of forests increased in the last century in the entire Western Ghats due to increased population density, results in habitat degradation and fragmentation (Menon & Bawa, Citation1997). Habitat fragmentation significantly impacts the distribution of small carnivores, particularly endemic habitat specialists like brown palm civets (Oehler & Litvaitis, Citation1996; Ray & Sunquist, Citation2001). Fragmentation may lead the species to patchy populations that restrict the gene flow between the meta-population and lead to genetic drift and divergence. The modeling elaborates four subset populations of brown palm civet, with three towards the north of the Palghat gap, one towards the south. Many authors reported brown palm civets from southern Western Ghats, Nilgiris, Coorg, Mookambika, Sharavathy, Anshi, Haliyal, and Dhud sagar (see review consolidated in Kumara & Singh, Citation2007; Rajamani et al., Citation2002). Bhosale et al. (Citation2013), and Sayyed et al. (Citation2019), elaborated the range to the Sangli, Sindhudurg, and reserve areas of the Satara district Maharashtra. We also made multiple observations of the brown palm civet from the Sahyadri reserve. There are no previous records on the brown palm civet from the Chandoli national park and Kudremukh national park, but we also predict its existence. We also camera trapped the brown palm civet records from the tea estates in the Coonoor – Kundha regions of the Nilgiris. Ashraf (Citation1990) and Rajamani et al. (Citation2002), opined brown palm civets’ extermination from Coonoor, Ooty, and Wellington. Our records prove the species is still present in the Coonoor-Kundha regions and upper reaches of the Mudumalai in the Nilgiris. We received no trap records or direct sightings from the high altitude of the Nilgiris, including Ooty. We assume that the absence from the upper reaches may be due to the altitude preference predicted in the present study and by previous authors (Mudappa, Citation1998; Rajamani et al., Citation2002). Although Mudappa (Citation1998) and Rajamani et al. (Citation2002), suggested that the distribution is limited to 500–1,300 m a.s.l., we believe that the maximum elevation can be as high as 2,100 m a.s.l. The species can be sited or observed in the tea platations that have continuity to shola forest. The patchy population distribution within the identified blocks suggests smaller confined populations in the northern Western Ghats with no continuity.
The brown palm civet prefers densely forested or bushy evergreen areas as its preferred habitat. The preference for such a habitat demonstrates the species’ reliance on dense rain forests. The study forecasts the behaviour of brown palm civets in response to lush vegetation, monsoon, and tree height. The animal’s reliance on dense evergreen vegetation may confine it to a patchy, fragmented population. The seed dispersal and the regeneration of dependent plant species aid the animal in forming an appropriate habitat. (Gnanaolivu & Singh, Citation2019; Herrera, Citation1989; Mudappa et al., Citation2010; Rabinowitz, Citation1991). Although the endemic brown palm civet is highly dependent on high-altitude dense rain forests, we did not find it to be a valid montane shola-dependent species. The disappearance or fragmentation of the high altitude evergreen forest isolates the brown palm civet population to a patchy distribution, as seen on the Northern side of the Western Ghats. The habitat specialist species like brown palm civet may suffer from the loss of a specific habitat. (Raman et al., Citation2020b). Because the brown palm civet is strictly arboreal, removing fruiting trees from its habitat significantly impacts the species. (Chen et al., Citation2004; Gnanaolivu & Singh, Citation2019; Nowak, Citation2005; Rajamani et al., Citation2002). Endemism may be due to acclimatisation to a specific geographical range with a unique floral nature and climate. Civets are known to be primarily influenced by precipitation. (Kalle et al., Citation2013; Mathai et al., Citation2019). We also identified that bio13, bio18, and bio19 climatic variables influence species distribution, corroborating the previous findings.
The study confirmed the brown palm civets’ nocturnal behaviour, with the peak period occurring in the early evening hours and ending by midnight. This pattern varies slightly between reserves, especially during the early evening hours. The vulnerability of civets to predation influences their nocturnal behaviour. They hide during the day in hollow trees, canopy vine angles, and other natural crevices. (Joshi et al., Citation1995; Mudappa, Citation2006; Rabinowitz, Citation1991). The brown palm civet observed foraging in a larger area at night and staying in a smaller range during the day. (Mudappa, Citation2001, Citation2006). Mammalian 24-hour rhythmic activity has an endogenous basis, regulated by the nocturnal and diurnal periods. (Ashby, Citation1972). The body size and the environment are the two most essential factors in determining such an activity period (Van Schaik & Griffiths, Citation1996). The brown palm civet’s nocturnal behaviour may be an adaptation to avoid predation by felids and canids. The presence of sympatric species in the area forces the recessive species to modify their activity pattern (Schuette et al., Citation2013; Shankar et al., Citation2020). Because they do not receive nourishment during the day, nocturnal species typically exhibit an increase in activity immediately after sunset (Diete et al., Citation2017; Shankar et al., Citation2020; Vieira & Baumgarten, Citation1995).
Authors such as Pocock (Citation1933) and Hutton (Citation1949) described two brown palm civet subspecies based on morphological characteristics. Corbet and Hill (Citation1992) explained the morphological differences in the tails of various brown palm civets. We also observed different variants of coat colour patterns in brown palm civets in the camera trap captures. The home ranges of nocturnal endemic small carnivores may be limited, and fragmentation can quickly delineate meta-populations to species. Intra-species competition within populations can result in variations in coat colour patterns, which can be ecologically advantageous phenotypes (Nicoglou, Citation2015). Mammalian colour patterns are generally grey or brown, which aids in concealment and escape from predation, and some species exhibit white colour for signaling (Caro, Citation2013). Two sub-species are unlikely to coexist in the same area with reproductive isolation; we believe the previous authors mistook colour variation for sub-speciation. In Sri Lanka, the closely related golden palm civet (P. zeylonensis) has three coat colour variations ranging from golden brown to black (Groves et al., Citation2009). The ability of a genotype to produce multiple phenotypes in response to stimuli or environmental conditions is phenotypic plasticity. It is unclear whether the phenotypic expression in brown palm civet is due to phenotypic plasticity or the stabilisation of an environmentally preferred allele.
According to Patou et al. (Citation2010), the genus Paradoxurus diverged during the Pliocene. The lineages separated based on altitude in the late Pliocene or Pleistocene. (Brandon-Jones, Citation1996; Gathorne-Hardy et al., Citation2002; Haq et al., Citation1987; Heaney, Citation1986; Meijaard, Citation2004; Miller et al., Citation2005). Despite disagreements, it is widely assumed that the Palghat gap in the Western Ghats formed before the Pleistocene. The studies on amphibians (Vijayakumar et al., Citation2016), reptiles (Chaitanya et al., Citation2019), and birds (Robin et al., Citation2010, Citation2015) demonstrated that the gaps act as barriers leading to speciation. Population isolation to the north and south of the Palghat gap could have occurred during Pleistocene climate change (Hewitt, Citation2000; Klicka & Zink, Citation1997; Robin et al., Citation2010; Singhvi & Kale, Citation2010), which is enough for species divergence. The studies on the endemic Nilgiri thar (Nilgiritragus hylocrius) based on the mitochondrial cytochrome b revealed the presence of two diverged populations on either side of the Palghat gap (Joshi et al., Citation2018). A similar divergence may have occurred for the brown palm civet, which needs genetic analysis. The molecular studies on the Paradoxurus (Veron, et al., Citation2015) demonstrated that the P. jerdoni and P. zeylonensis (endemic to Srilanka) are sister species and the P. hermaphrodites have three different clades. Their studies have shown that the speciation of P. zeylonensis proposed by coat colour pattern and anatomical variation (Groves et al., Citation2009) is invalid. The fragmentation of the suitable habitat may become patchy in the last three hundred years, which may not be sufficient for speciation or sub-speciation. However, the species separation on either side of the Palghat gap (southern and northern) dates back to the Pleistocene.
Conclusion
We conclude that the brown palm civet, which once roamed the Western Ghats, is now restricted to at least four isolated blocks. The fragmentation of the brown palm civet habitat occurred by destroying dense rainforest due to excessive anthropogenic pressure. The brown palm civet exhibits a similar pattern of activity throughout the landscape, as indicated by its diel activity. The coat colour variation is not limited to a single landscape or locality but can found throughout the Western Ghats. In contrast to globally distributed fauna, endemic species in selective habitats are critical in conservation practice. Conservation biologists can address this issue through genetic analysis and validating the genetic flow between the associated populations. We need to undertake in-depth research on brown palm civet to assess the ecosystem service and co-species interaction. Because of its endemism, the extinction of a key species can impact the native flora, causing devastation across the entire landscape.
Geolocation information
The Geo-location of the study area is given below
18°25ʹ37.24”N to 8°29ʹ25.64”S
73°32ʹ42.3”E to 77°43ʹ07.6”E W
Acknowledgments
The authors sincerely acknowledge the Tamil Nadu forest department (WL5(A)/001286/2016 Permit No 09/2016) and the Kerala forest department (KFDHQ- 758/2020-CWW/WL10) for necessary permission, sharing the data, and supporting the study. The copywright of the photos used in the study lies with the Kerala and Tamilnadu forest deparments.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Aiello-Lammens, M. E., Boria, R. A., Radosavljevic, A., Vilela, B., Anderson, R. P., Bjornson, R., Weston, S., & Aiello-Lammens, M. M. E. (2019). spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography, 38(5), 541–545. doi:10.1111/ecog.01132.
- Ajith, V. P., Alenbath, M., & Francis, V. K. (1998). White bison in Chinnar. Journal of the Bombay Natural History Society, 95(3), 499–500. https://www.biodiversitylibrary.org/page/48605049#page/543/mode/1up
- Alfaya, P., Casanovas, J. G., Lobón-Rovira, J., Matallanas, B., Cruz, A., Arana, P., & Alonso, G. (2019). Using MaxEnt algorithm to assess habitat suitability of a potential Iberian Lynx population in central Iberian Peninsula. Community Ecology, 20(3), 266–276. https://doi.org/10.1556/168.2019.20.3.7
- Ashby, K. R. (1972). Patterns of daily activity in mammals. Mammal Review, 1(7‐8), 171–185. https://doi.org/10.1111/j.1365-2907.1972.tb00088.x
- Ashraf, N. V. K. (1990) A preliminary survey of the two endangered viverrids of Western Ghats: Malabar Civet (Viverra civettina) and Brown Palm Civet (Paradoxurus jerdoni). Unpublished report submitted to the IUCN/ SCC Mustelid & Viverrid Specialist Group
- Ashraf, N. V. K., Kumar, A., & Johnsingh, A. J. T. (1993). Two endemic viverrids of the Western Ghats, India. Oryx, 27(2), 109–114. https://doi.org/10.1017/S0030605300020640
- Balakrishnan, M., & Sreedevi, M. B. (2007). Husbandry and management of the Small Indian Civet Viverricula indica (É. Geoffroy Saint-Hilaire, 1803) in Kerala, India. Small Carnivore Conservation, 36, 9–13. http://nebula.wsimg.com/fa5cf48f2a125523a5d6a2c8ee2fa110?AccessKeyId=35E369A09ED705622D78&disposition=0&alloworigin=1
- Bhosale, H. S., Punjabi, G. A., & Bardapurkar, R. (2013). Photographic documentation of Brown Palm Civet Paradoxurus jerdoni in Maharashtra, India, north of its known range. Small Carnivore Conservation, 49, 37–39. http://nebula.wsimg.com/f366f720221284c28474f7155b2ad652?AccessKeyId=35E369A09ED705622D78&disposition=0&alloworigin=1
- Blanford, W. T. (1885) A Monograph of the Genus Paradoxurus, F. Cuvier. Proceedings of the Zoological Society of London, 53 ( 4): 780–808. https://doi.org/10.1111/j.1096-3642.1885.tb02921.x.
- Blanford, W. T. (1888–91). Fauna of British India. Taylor and Francis.
- Brandon-Jones, D. (1996). The Asian Colobinae (Mammalia, Cercopithecidae) as indicators of Quaternary climatic changes. Biological Journal of the Linnean Society, 59(3), 327–350. https://doi.org/10.1111/j.1095-8312.1996.tb01469.x
- Bunnell, F. L., & Harestad, A. S. (1990). Activity budgets and body weight in mammals. How sloppy can mammals be? Current Mammalogy, 2, 245–305. http://pascal-francis.inist.fr/vibad/index.php?action=getRecordDetail&idt=6805766
- Caro, T. I. M. (2013). The colours of extant mammals. Semin Cell Dev Biol, 24(6–7), 542–52. doi: 10.1016/j.semcdb.2013.03.016.
- Chaitanya, R., Giri, V. B., Deepak, V., Datta-Roy, A., Murthy, B. H. C. K., & Karanth, P. (2019). Diversification in the mountains: A generic reappraisal of the Western Ghats endemic gecko genus Dravidogecko Smith, 1933 (Squamata: Gekkonidae) with descriptions of six new species. Zootaxa, 4688(1), 1–56. https://doi.org/10.11646/zootaxa.4688.1.1
- Chen, J., Fleming, T. H., Zhang, L., Wang, H., & Liu, Y. (2004). Patterns of fruit traits in a tropical rainforest in Xishuangbanna, SW China. Acta Oecologica, 26(2), 157–164. https://doi.org/10.1016/j.actao.2004.04.002
- Chen, M. T., Tewes, M. E., Pei, K. J., & Grassman, L. I. (2009). Activity patterns and habitat use of sympatric small carnivores in southern Taiwan. Mammalia, 73(1), 20–26. https://doi.org/10.1515/MAMM.2009.006
- Chunekar, H., Pardeshi, A., Gulawani, C., & Shinde, R. (2017). A note on coat colour variation in Common Palm Civet (Paradoxurus hermaphroditus). Small Carnivore Conservation, 55, 104–108. http://nebula.wsimg.com/e62331275154e1918dc4596590459aa1?AccessKeyId=35E369A09ED705622D78&disposition=0&alloworigin=1
- Colón, C. P. (2002). Ranging behaviour and activity of the Malay civet (Viverra tangalunga) in a logged and an unlogged forest in Danum Valley, East Malaysia. Journal of Zoology, 257(4), 473–485. https://doi.org/10.1017/S0952836902001073
- Corbet, G. B., & Hill, J. E. (1992). The mammals of the Indomalayan region: A systematic review (Vol. 488). oxford university press.
- Crowther, T. W., Glick, H. B., Covey, K. R., Bettigole, C., Maynard, D. S., Thomas, S. M., Smith, J. R., Hintler, G., Duguid, M. C., Amatulli, G., & Tuanmu, M. N. (2015). Mapping tree density at a global scale. Nature, 525(7568), 201–205. https://doi.org/10.1038/nature14967
- Dahanukar, N., Raut, R., & Bhat, A. (2004). Distribution, endemism and threat status of freshwater fishes in the Western Ghats of India. Journal of Biogeography, 31(1), 123–136. https://doi.org/10.1046/j.0305-0270.2003.01016.x
- Diete, R. L., Meek, P. D., Dickman, C. R., Lisle, A., & Leung, L. K. P. (2017). Diel activity patterns of northern Australian small mammals: Variation, fixity, and plasticity. Journal of Mammalogy, 98(3), 848–857. https://doi.org/10.1093/jmammal/gyx003
- Duan, R. Y., Kong, X. Q., Huang, M. Y., Fan, W. Y., & Wang, Z. G. (2014). The predictive performance and stability of six species distribution models. PloS One, 9(11), e112764. https://doi.org/10.1371/journal.pone.0112764
- Eaton, J. A., Wust, R., Wirth, R., & Duckworth, J. W. (2010). Recent records of the Javan small-toothed palm civet Arctogalidia (trivirgata) trilineata. Small Carnivore Consevation, 43, 16–22. http://nebula.wsimg.com/d671b039acfa21cc89c3480404c503f4?AccessKeyId=35E369A09ED705622D78&disposition=0&alloworigin=1
- Fielding, A. H., & Bell, J. F. (1997). A review of methods for the assessment of prediction errors in conservation presence/absence models. Environmental Conservation, 24(1), 38–49. https://doi.org/10.1017/S0376892997000088
- Ganesh, T. (1997). Occurrence of the Brown Palm Civet in the wet forest of Kalakad Mundanthurai Tiger Reserve, Tamil Nadu. Journal of Bombay Natural History Society, 94, 556. https://www.biodiversitylibrary.org/page/48601934#page/608/mode/1up
- Gathorne-Hardy, F. J., Davies, R. G., Eggleton, P., & Jones, D. T. (2002). Quaternary rainforest refugia in south-east Asia: Using termites (Isoptera) as indicators. Biological Journal of the Linnean Society, 75(4), 453–466. https://doi.org/10.1046/j.1095-8312.2002.00031.x
- Gnanaolivu, S. D., & Singh, M. (2019). First sighting of predatory attack on a Malabar Grey Slender Loris Loris lydekkerianus malabaricus by Brown Palm Civet Paradoxurus jerdoni. Asian Primates Journal, 8(1), 37–40. http://static1.1.sqspcdn.com/static/f/1200343/28250840/1580697664893/2020Jan31_Article_4.pdf?token=%2Bh3Cnq2KfxKg1MeaYX1h9U6FD3I%3D
- Groves, C. P., Rajapaksha, C., & Manemandra-Arachchi, K. (2009). The taxonomy of the endemic golden palm civet of Sri Lanka. Zoological Journal of the Linnean Society, 155(1), 238–251. https://doi.org/10.1111/j.1096-3642.2008.00451.x
- Hanley, J. A., & McNeil, B. J. (1983). A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology, 148(3), 839–843. https://doi.org/10.1148/radiology.148.3.6878708
- Haq, B. U., Hardenbol, J. A. N., & Vail, P. R. (1987). Chronology of fluctuating sea levels since the Triassic. Science, 235(4793), 1156–1167. https://doi.org/10.1126/science.235.4793.1156
- Heaney, L. R. (1986). Biogeography of mammals in SE Asia: Estimates of rates of colonization, extinction and speciation. Biological Journal of the Linnean Society, 28(1–2), 127–165. https://doi.org/10.1111/j.1095-8312.1986.tb01752.x
- Herrera, C. M. (1989). Frugivory and seed dispersal by carnivorous mammals, and associated fruit characteristics, in undisturbed Mediterranean habitats. Oikos, 55(2), 250–262. https://doi.org/10.2307/3565429
- Hewitt, G. (2000). The genetic legacy of the Quaternary ice ages. Nature, 405(6789), 907–913. https://doi.org/10.1038/35016000
- Hijmans, R. J., Cameron, S. E., Parra, J. L., Jones, P. G., & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology: A Journal of the Royal Meteorological Society, 25(15), 1965–1978. https://doi.org/10.1002/joc.1276
- Hijmans, R. J., Phillips, S., Leathwick, J., Elith, J., & Hijmans, M. R. J. (2017). Package dismo. Circles, 9(1), 1–68. https://cran.r-project.org/web/packages/dismo/
- Hutton, A. F. (1949). Notes on the snakes and mammals of the high wavy mountains, Madura District, south India Part II-Mammals. Journal of the Bombay Natural History Society, 48, 681–694.
- Jennings, A. P., & Veron, G. (2011). Predicted distributions and ecological niches of 8 civet and mongoose species in Southeast Asia. Journal of Mammalogy, 92(2), 316–327. https://doi.org/10.1644/10-MAMM-A-155.1
- Jhala, Y. V., Qureshi, Q., & Nayak, A. K. (2019), Status of Tigers, Co-Predators and Prey in India 2018: Summary Report, National Tiger Conservation Authority, Government of India, New Delhi, and Wildlife Institute of India, Dehradun TR No. 2019/05. https://ntca.gov.in/assets/uploads/Reports/AITM/Status_Tigers_India_summary_2018.pdf
- Joshi, A. R., David Smith, J. L., & Cuthbert, F. J. (1995). Influence of food distribution and predation pressure on spacing behavior in palm civets. Journal of Mammalogy, 76(4), 1205–1212. https://doi.org/10.2307/1382613
- Joshi, B. D., Matura, R., Predit, M. A., Rahul, D., Pandav, B., Sharma, V., Nigam, P., & Goyal, S. P. (2018). Palghat gap reveals presence of two diverged populations of Nilgiri tahr (Nilgiritragus hylocrius) in Western Ghats, India. Mitochondrial DNA, Part B, 3(1), 245–249. https://doi.org/10.1080/23802359.2018.1436990
- Kalle, R., Ramesh, T., Qureshi, Q., & Sankar, K. (2013). Predicting the distribution pattern of small carnivores in response to environmental factors in the Western Ghats. PLoS One, 8(11), e79295. https://doi.org/10.1371/journal.pone.0079295
- Klicka, J., & Zink, R. M. (1997). The importance of recent ice ages in speciation: A failed paradigm. Science, 277(5332), 1666–1669. https://doi.org/10.1126/science.277.5332.1666
- Kumar, A., & Yoganand, K. (1999) Distribution and abundance of small carnivores in Nilgiri Biosphere Reserve, India. ENVIS Bulletin: Wildlife and Protected Areas, mustelids, viverrids and herpestids of India, Wildlife Institute of India 74–86.
- Kumara, H. N., & Singh, M. (2004). The influence of differing hunting practices on the relative abundance of mammals in two rainforest areas of the Western Ghats, India. Oryx, 38(3), 321–327. https://doi.org/10.1017/S0030605304000560
- Kumara, H. N., & Singh, M. E. W. A. (2007). Small carnivores of Karnataka: Distribution and sight records. Journal of the Bombay Natural History Society, 104(2), 155–162. http://repository.ias.ac.in/89658/1/18p.pdf
- Linkie, M., & Ridout, M. S. (2011). Assessing tiger-prey interactions in Sumatran rainforests. Journal of Zoology, 284(3), 224–229. London. https://doi.org/10.1111/j.1469-7998.2011.00801.x
- Lucherini, M., Reppucci, J. I., Walker, R. S., Villalba, M. L., Wurstten, A., Gallardo, G., Iriarte, A., Villalobos, R., & Perovic, P. (2009). Activity pattern segregation of carnivores in the high Andes. Journal of Mammalogy, 90(6), 1404–1409. https://doi.org/10.1644/09-MAMM-A-002R.1
- Lynam, A. J., Jenks, K. E., Tantipisanuh, N., Chutipong, W., Ngoprasert, D., Gale, G. A., Steinmetz, R., Sukmasuang, R., Bhumpakphan, N., Grassman, L. I., Cutter, P., Kitamura, S., Reed, D. H., Baker, M. C., McShea, W., Songsasen, N., & Leimgruber, P. (2013). Terrestrial activity patterns of wild cats from camera-trapping. Raffles Bulletin of Zoology, 61(1), 407–415. https://lkcnhm.nus.edu.sg/wp-content/uploads/sites/10/app/uploads/2017/04/61rbz407-415.pdf
- Mathai, J., Niedballa, J., Radchuk, V., Sollmann, R., Heckmann, I., Brodie, J., Struebig, M., Hearn, A. J., Ross, J., Macdonald, D. W., & Hon, J. (2019). Identifying refuges for Borneo’s elusive Hose’s civet. Global Ecology and Conservation, 17, e00531. https://doi.org/10.1016/j.gecco.2019.e00531
- Meek, P. D., Ballard, G., Claridge, A., Kays, R., Moseby, K., O’Brien, T., O’Connell, A., Sanderson, J., Swann, D. E., Tobler, M., & Townsend, S. (2014). Recommended guiding principles for reporting on camera trapping research. Biodiversity Conservation, 23(9), 2321–2343. https://doi.org/10.1007/s10531-014-0712-8
- Meijaard, E. (2004). Biogeographic history of the Javan leopard Panthera pardus based on a craniometric analysis. Journal of Mammalogy, 85(2), 302–310. https://doi.org/10.1644/BER-010
- Menon, S., & Bawa, K. S. (1997). Applications of geographic information systems, remote-sensing, and a landscape ecology approach to biodiversity conservation in the Western Ghats. Current Science,73(2), 134–145.
- Meredith, M., & Ridout, M. (2014). Overlap: estimates of coefficient of overlapping for animal activity patterns. R package version 0.2, 4.
- Miller, K. G., Kominz, M. A., Browning, J. V., Wright, J. D., Mountain, G. S., Katz, M. E., Sugarman, P. J., Cramer, B. S., Christie-Blick, N., & Pekar, S. F. (2005). The Phanerozoic record of global sea-level change. Science, 310(5752), 1293–1298. https://doi.org/10.1126/science.1116412
- Monserud, R. A., & Leemans, R. (1992). Comparing global vegetation maps with the Kappa statistic. Ecological Modelling, 62(4), 275–293. https://doi.org/10.1016/0304-3800(92)90003-W
- Mudappa, D. (1998). Use of camera-traps to survey small carnivores in the tropical rainforest of Kalakad-Mundanthurai tiger reserve, India. Small Carnivore Conservation, 18, 9–11.
- Mudappa, D. (1999). Lesser-known carnivores of the Western Ghats. ENVIS Bulletin: Wildlife and Protected Areas, mustelids, viverrids and herpestids of India, Wildlife Institute of India, 65–70.
- Mudappa, D. (2001) Ecology of the Brown Palm Civet Paradoxurus jerdoni in the tropical rainforests of the Western Ghats, India. Ph. D. thesis, Bharathiar University.
- Mudappa, D. (2006). Day-bed choice by the brown palm civet (Paradoxurus jerdoni) in the Western Ghats, India. Mammalian Biology, 71(4), 238–243. https://doi.org/10.1016/j.mambio.2006.01.003
- Mudappa, D., Kumar, A., & Chellam, R. (2010). Diet and fruit choice of the brown palm civet Paradoxurus jerdoni, a viverrid endemic to the Western Ghats rainforest, India. Tropical Conservation Science, 3(3), 282–300. https://doi.org/10.1177/194008291000300304
- Mudappa, D., Noon, B. R., Kumar, A., & Chellam, R. (2007). Responses of small carnivores to rainforest fragmentation in the southern Western Ghats, India. Small Carnivore Conservation, 36, 18–26.
- Myers, N., Mittermeier, R. A., Mittermeier, C. G., Da Fonseca, G. A., & Kent, J. (2000). Biodiversity hotspots for conservation priorities. Nature, 403(6772), 853–858. https://doi.org/10.1038/35002501
- Nicoglou, A. (2015). Phenotypic plasticity: From microevolution to macroevolution. InHandbook of evolutionary thinking in the sciences. Springer. https://doi.org/10.1007/978-94-017-9014-7_14
- Nikhil, S., & Nameer, P. O. (2017). Small carnivores of the montane forests of Eravikulam National Park in the Western Ghats, India. Journal of Threatened Taxa, 9(11), 10880–10885. https://doi.org/10.11609/jott.2211.9.11.10880-10885
- Nowak, R. M. (2005). Dogs, wolves, coyotes, jackals, and foxes: Canidae. Walker’s Carnivores of the World, Johns Hopkins University Press 112.
- O’Donoghue, M., Boutin, S., Krebs, C. J., Murray, D. L., & Hofer, E. J. (1998). Behavioural responses of coyotes and lynx to the snowshoe hare cycle. Oikos, 82(1), 169–183. https://doi.org/10.2307/3546927
- Oehler, J. D., & Litvaitis, J. A. (1996). The role of spatial scale in understanding responses of medium-sized carnivores to forest fragmentation. Canadian Journal of Zoology, 74(11), 2070–2079. https://doi.org/10.1139/z96-235
- Papeş, M., & Gaubert, P. (2007). Modelling ecological niches from low numbers of occurrences: Assessment of the conservation status of poorly known viverrids (Mammalia, Carnivora) across two continents. Diversity and Distributions, 13(6), 890–902. 890-902.https://doi.org/10.1111/j.1472-4642.2007.00392.x
- Patou, M. L., Wilting, A., Gaubert, P., Esselstyn, J. A., Cruaud, C., Jennings, A. P., Fickel, J., & Veron, G. (2010). Evolutionary history of the Paradoxurus palm civets–a new model for Asian biogeography. Journal of Biogeography, 37(11), 2077–2097. https://doi.org/10.1111/j.1365-2699.2010.02364.x
- Phillips, S. J., Anderson, R. P., & Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190(3–4), 231–259. https://doi.org/10.1016/j.ecolmodel.2005.03.026
- Pillay, R. (2009). Observations of small carnivores in the southern Western Ghats, India. Small Carnivore Conservation, 40, 36–40.
- Pocock, R. I. (1933). The Palm Civets or ‘Toddy Cats’ of the genera Paradoxurus and Paguma inhabiting British India. Journal of the Bombay Natural History Society, 36, 856–877.
- Pocock, R. I. (1939). The fauna of British India including Ceylon and Burma (Vol. I). Taylor & Francis.
- R Development Core Team. (2019). R: a language and environment for statistical computing. R Foundation for Statistical Computing, 3.6.2ed. http://www.R-project.org.
- Rabinowitz, A. R. (1991). Behaviour and movements of sympatric civet species in Huai Kha Khaeng Wildlife Sanctuary, Thailand. Journal of Zoology, 223(2), 281–298. https://doi.org/10.1111/j.1469-7998.1991.tb04765.x
- Rajamani, N., Mudappa, D., & Van Rompaey, H. (2002). Distribution and status of the Brown Palm Civet in the Western Ghats, South India. Small Carnivore Conservation, 27, 6–11. http://nebula.wsimg.com/d42c49f79b262a71ec8426b2f9629b49?AccessKeyId=35E369A09ED705622D78&disposition=0&alloworigin=1
- Ramachandran, K. K. (1990). Recent evidence of the Brown Palm Civet, Paradoxurus jerdoni, from Silent Valley National Park, India. Mustelid and Viverrid Conservation, 3, 15.
- Raman, S., Shameer, T. T., Charles, B., & Sanil, R. (2020b). Habitat suitability model of endangered Latidens salimalii and the probable consequences of global warming. Tropical Ecology, 61(4), 570–582. https://doi.org/10.1007/s42965-020-00114-5
- Raman, S., Shameer, T. T., Sanil, R., Usha, P., & Kumar, S. (2020a). Protrusive influence of climate change on the ecological niche of endemic brown mongoose (Herpestes fuscus fuscus): A MaxEnt approach from Western Ghats, India. Modeling Earth Systems and Environment, 6(3), 1795–1806. https://doi.org/10.1007/s40808-020-00790-1
- Ray, J., & Sunquist, M. (2001). Trophic relations in a community of African rainforest carnivores. Oecologia, 127(3), 395–408. https://doi.org/10.1007/s004420000604
- Ridout, M. S., & Linkie, M. (2009). Estimating overlap of daily activity patterns from camera trap data. Journal of Agricultural, Biological, and Environmental Statistics, 14(3), 322–337. https://doi.org/10.1198/jabes.2009.08038
- Robin, V. V., Sinha, A., & Ramakrishnan, U. (2010). Ancient geographical gaps and paleo-climate shape the phylogeography of an endemic bird in the sky islands of southern India. PLoS One, 5(10), e13321. https://doi.org/10.1371/journal.pone.0013321
- Robin, V. V., Vishnudas, C. K., Gupta, P., & Ramakrishnan, U. (2015). Deep and wide valleys drive nested phylogeographic patterns across a montane bird community. Proceedings of the Royal Society B: Biological Sciences, 282( 1810), 20150861. https://doi.org/10.1098/rspb.2015.0861
- Rogowitz, G. L. (1997). Locomotor and foraging activity of the white-tailed jackrabbit (Lepus townsendii). Journal of Mammalogy, 78(4), 1172–1181. https://doi.org/10.2307/1383060
- Sanghamithra, D., & Nameer, P. O. (2018). Small carnivores of Silent Valley National Park, Kerala, India. Journal of Threatened Taxa, 10(8), 12091–12097. https://doi.org/10.11609/jott.2992.10.8.12091-12097
- Sanil, R., Shameer, T. T., & Easa, P. S. (2014). Albinism in jungle cat and jackal along the coastline of the southern Western Ghats. Cat News, 61, 23–25.
- Sayyed, A., Talmale, S. S., & Mahabal, A. (2019). Records of Brown Palm Civet Paradoxurus jerdoni in Satara district, Maharashtra: Extension of known range in Western Ghats, India. Small Mammal Mail, 34(9), 08–11. #423, In: Zoo’s Print
- Schreiber, A. (1989). Weasels, civets, mongooses, and their relatives: An action plan for the conservation of mustelids and viverrids. IUCN.
- Schuette, P., Wagner, A. P., Wagner, M. E., & Creel, S. (2013). Occupancy patterns and niche partitioning within a diverse carnivore community exposed to anthropogenic pressures. Biological Conservation, 158, 301–312. https://doi.org/10.1016/j.biocon.2012.08.008
- Shameer, T. T., Mungi, N. A., Ramesh, B., Kumar, S. V., & Easa, P. S. (2021). How can spatio-temporal overlap in mammals assist in maximizing biodiversity conservation? A case study of Periyar tiger reserve. Biologia,76, 1–11. https://doi.org/10.2478/s11756-020-00645-1
- Shankar, A., Salaria, N., Sanil, R., Chackaravarthy, S. D., & Shameer, T. T. (2020). Spatio-temporal association of fishing cats with the mammalian assemblages in the East Godavari mangrove delta, India. Mammal Study, 45(4), 1–11. https://doi.org/10.3106/ms2020-0015
- Singhvi, A. K., & Kale, V. S. (2010). Paleoclimate studies in India: Last ice age to the present. Indian National Science Academy.
- Sreehari, R., & Nameer, P. O. (2016). Small carnivores of Parambikulam tiger reserve, southern Western Ghats, India. Journal of Threatened Taxa, 8(11), 9306–9315. https://doi.org/10.11609/jott.2311.8.11.9306-9315
- Sreekumar, E. R., & Nameer, P. O. (2018). Small carnivores of Wayanad Wildlife Sanctuary, the southern Western Ghats, India. Journal of Threatened Taxa, 10(1), 11218–11225. https://doi.org/10.11609/jott.3651.10.1.11218-11225
- Swets, J. A. (1988). Measuring the accuracy of diagnostic systems. Science, 240(4857), 1285–1293. https://doi.org/10.1126/science.3287615
- Tian, C., Zhang, Y. Y., Liu, Z. X., Dayananda, B., Fu, X. B., Yuan, D., & Li, J. Q. (2020). Temporal niche patterns of large mammals in Wanglang National Nature Reserve, China. Global Ecology and Conservation, 22, e01015. https://doi.org/10.1016/j.gecco.2020.e01015
- van Donkelaar, A., Martin, R. V., Brauer, M., & Boys, B. L. (2015). Use of satellite observations for long-term exposure assessment of global concentrations of fine particulate matter. Environmental Health Perspectives, 123(2), 135–143. https://doi.org/10.1289/ehp.1408646
- van Schaik, C. P., & Griffiths, M. (1996). Activity periods of Indonesian rain forest mammals. Biotropica, 28(1), 105–112. https://doi.org/10.2307/2388775
- Vasudeva, V., Behera, G., Behera, S. P., Panda, S. K., Ramasamy, P., Karat, P. R., Gupta, S. K., & Ramesh, K. (2021). Coat colour variation in Common Palm Civet in Satkosia, eastern India calls for the need to revisit taxonomic and distribution status. Mammal Tales, 36(6), 15–17. #32, In: Zoo’s Print
- Veron, G., Laidlaw, R., Rosenthal, S. H., Streicher, U., & Roberton, S. (2004). Coat colour variation in the banded palm civet Hemigalus derbyanus and in Owston’s civet Chrotogale owstoni. Mammal Review, 34(4), 307–310. https://doi.org/10.1111/j.1365-2907.2004.00047.x
- Veron, G., Patou, M. L., Tóth, M., Goonatilake, M., & Jennings, A. P. (2015). How many species of Paradoxurus civets are there? New insights from India and Sri Lanka. Journal of Zoological Systematics and Evolutionary Research, 53(2), 161–174. https://doi.org/10.1111/jzs.12085
- Vieira, E. M., & Baumgarten, L. C. (1995). Daily activity patterns of small mammals in a cerrado area from central Brazil. Journal of Tropical Ecology, 11(2), 255–262. https://doi.org/10.1017/S0266467400008725
- Vijayakumar, S. P., Menezes, R. C., Jayarajan, A., & Shanker, K. (2016). Glaciations, gradients, and geography: Multiple drivers of diversification of bush frogs in the Western Ghats Escarpment. Proceedings of the Royal Society B: Biological Sciences, 283(1836), 20161011. https://doi.org/10.1098/rspb.2016.1011
- Williams, J. N., Seo, C., Thorne, J., Nelson, J. K., Erwin, S., O’Brien, J. M., & Schwartz, M. W. (2009). Using species distribution models to predict new occurrences for rare plants. Diversity and Distributions, 15(4), 565–576. https://doi.org/10.1111/j.1472-4642.2009.00567.x
- Winaya, A., Nicolás, C. M., & Prasetyo, D. (2020). Morphometric variations of Asian Common Palm Civet (Paradoxurus hermaphroditus, Pallas 1777) from Bali Island, Indonesia as the basis of morphometrics diversity data. Biodiversitas Journal of Biological Diversity, 21(3), 1027–1034. https://doi.org/10.13057/biodiv/d210324
- Wozencraft, W. C. (2005). Paradoxurus jerdoni. In D. E. Wilson & D. M. Reeder (Eds.), Mammal species of the world: A taxonomic and geographic reference (3rd ed., pp. 551). Johns Hopkins University Press.
- Zhou, Y., Newman, C., Palomares, F., Zhang, S., Xie, Z., & Macdonald, D. W. (2014). Spatial organization and activity patterns of the masked palm civet (Paguma larvata) in central-south China. Journal of Mammalogy, 95(3), 534–542. https://doi.org/10.1644/13-MAMM-A-185