ABSTRACT
Macrobenthos animals are an architect of a variety of roles including as a part of the food web of aquatic ecosystem and improve the structure of the sediment and can determine the quality of the water. Macrobenthos communities have shown their potential role in biomonitoring to analyze contaminant loads due to high sensitivity to organic pollutants along the coastal water area. Besides, it is also used to determine heavy metals and petroleum hydrocarbons in coastal water due to their long sustainability with chemicals are primarily related with industrial calamities and occupational activities. Based on above perspectives, this systematic review has shown interesting findings about the macrobenthic diversity including community composition in the coastal areas of Malaysia. The study has focused on the diversity and abundance of macrobenthos communities along some potential coastal areas of Malaysia which emphasis on the states of Johor, Pulau Pinang, Selangor, Pahang, Terengganu, and Sarawak. Several studies have evidently revealed that pollutants and human activities have contributed to loss of macrobenthos towards abundance (individuals/m2) and species richness. The highest abundance of macrobenthos was Coleoptera sp. (1650 ind./m2 followed by Hemiptera sp. (860 ind./m2) were observed in Sarawak and Crustacea sp. (597 ind/m2) was found in Selangor, respectively. While Crustacea (10 ind/m2) was found as the lowest in the coastal water Pahang only. A major shortcoming among the studies was sampling time along with sampling method which was observed in this systematic review of different studies of macrobenthic assemblages in the coastal waters of Malaysia. However, the existing study reveals the baseline information on macrobenthic community which are still inadequate in Malaysia. Hence, a long-term monitoring for eco-biology and species diversity of macrobenthic assemblages are necessary for their sustainable development in this fascinating tropical coastal water of Malaysia.
Keywords:
1. Introduction
1.1. Background of the study
Malaysia is one of the mega biodiversity nations with largest continental shelf areas within the trophic region that consists of Peninsular Malaysia, Sabah, and Sarawak. The total coastline of Malaysia is extended to 4,800 km, whereas 2,100 km from Peninsular Malaysia and 2,700 km from East Malaysia. Malaysia is bounded by seas on all sides except in the north where it relates to Thailand whereas Sabah and Sarawak are separated by southwestern portion of the South China Sea. The west coast of Peninsular Malaysia is bordered by the Andaman Sea to the north and Java Sea to the south (Mazlan et al., Citation2005). The neighbouring countries including Thailand, Indonesia, Singapore, and Vietnam play a significant role in development of marine-related activities. The coastal waters in Malaysia are subjectively important for bio-productivity, marine ecosystems, and natural resources. Malaysia is also situated at a base on each side of one of the world’s busiest sea pathways, the Straits of Malacca, which links Southeast and Northeast Asia, Asia and Western Europe and Asia and North America. Macrobenthic communities are inhabiting the same locality throughout their life mostly in water and sediment as they act as an indicator, nutrient cycles, translocation of materials and decomposition in the ecosystem (Hutton et al., Citation2015). Macrobenthos are known as animals without backbone or bony skeleton that live in or on the sediments (Akbar et al., Citation2013; Ahmad Azfar and Jalal, Citation2018). The term “benthos” is introduced by Ernst Haeckel who also presented the term “ecology.” It is from ancient Greek which means “Depth of the sea bottom” (Tagliapietra & Sigovini, Citation2010). Polychaete, oligochaete worms, gastropods, bivalves, and insect larvae are the typical benthic invertebrates which subjugate in aquatic bodies (Sarker Md, Citation2016). The survival of macrobenthos is determined by the biological, chemical, and physical factors of the environment (Muhammad Darif et al., Citation2016). These organisms are essential in marine ecosystem for the supply and demand of food for the bottom feeders like; demersal fish while being commercial in food industries such as prawns, crabs, and cockles (Yasin & Razak, Citation1994). They also play a specific role in the circulation and recirculation of nutrients in aquatic ecosystems by breaking down organic matter into simpler inorganic forms such as phosphate and nitrate (Barua et al., Citation2019). The word macrobenthos itself in Scopus showed 3,702 document results and consisted of 30 documents from Malaysia. It highly signifies that there is least interest in macrobenthos due to the lack of information regarding the diversity and community composition in Malaysia.
Our coastal area ecosystem is important ecologically and economically, yet it is exposed to extreme exploitation by development projects and foreign fishing vessels including illegal unreported, unregulated (IUU) fishing (Latun et al., Citation2016). Anthropogenic activities have become one of the major threats to macrobenthic as well as pollutants from ships and vessels that alter the most of biological environment and water quality. In addition, commercial fishing activities also lead to changes of habitat structure and affect the diversity, community composition, biomass, and productivity of associated biota. Moreover, the physical and chemical environment of the water body such as depth, current, organic contents of the sediments, and contaminations of bed sediments’ condition and toxicity of sediments influence the species richness and distribution of macrobenthos (Sarker Md, Citation2016). Macrobenthos communities are the most commonly used in biomonitoring to analyze contaminant loads due to high sensitivity to organic pollutants either in coastal water or fresh water (Azrina et al., Citation2006). It is also used to determine heavy metals and petroleum hydrocarbons in coastal water as these chemicals are primarily related with industrial accidents and occupational activities . Nevertheless, macrobenthic organisms have become a perfect tool for biological assessment of water quality because of the elasticity structure of invertebrates in riverine ecosystem (Bossley & Smiley, Citation2019).
This study comprehensively addresses the existing macrobenthic diversity and community composition of various species macrobenthos in each representative state in Malaysia. The priority of this review focuses on diversity and community of macrobenthos state of Malaysia comprising threats that have impacted macrobenthic organisms in Malaysian coastal water. Besides, this study is significant because of its holistic approach in determining the abundance of macrobenthic diversity that have persisted for many years in Malaysian water which includes coastal water, riverine, and estuarine area.
1.2. Importance of macrobenthos
The term assemblages of macrobenthic organisms refers to a collection or gathering of marine invertebrates that live at the bottom of a water column and visible to the naked eyes under the comparable environmental conditions and biological surroundings (Sarker Md, Citation2016). It is usually dominated by different species of polychaete worms, bivalvia. gastropods, pelecypods, anthozoans, echinoderms, sponges, and ascidians. The smallest size for macrobenthos is 500 mm, thus every other organism that are relatively smaller is considered as meiobenthos or microbenthos (Zaleha et al., Citation2009). Approximately, there are 80% of the benthic organisms are Epifauna, which live on the surface of rocky areas or seabed or in firm sediments (Hemery et al., Citation2018). Endofauna (infauna) animals live by buried themselves with soft sediments such as sand or mud. Each of the macrobenthos has particularly adapted to its niche as they have common functional structure that can be observed by its behaviour and morphological characteristics (Piló et al., Citation2016). The community of benthic invertebrates including molluscs, echinoderms, and polychaetes were commonly associated with different characteristic types of sediment on the continental shelves at all latitudes. The major macrofauna in the marine ecosystem is polychaete (Guan et al., Citation2014). These organisms have significant values to be one of the major food sources of fish and marine habitats including maintaining the ecology of fish resources.
Macrobenthos is significant to access the pollution as it acts as a biological indicator in rivers, streams, lakes, and any coastal water. The physiological functions of this macrofauna is a tool of study about the impact of environmental changes especially related to water and temperature (Tagliarolo et al., Citation2018). In addition, macroinvertebrate habitat also plays a vital core in the food web of food resources for fishes. It can be utilized to study the habitat quality, biodiversity, and ecological health of humans as one of the reliable indicators for monitoring the quality of the hydrological environment (S. W. Lee et al., Citation2019). The functional traits of macrobenthic communities determine the functioning and stability in one ecosystem due to the filtration act on both natural and anthropogenic pressures in the ecosystem (Piló et al., Citation2016).
Bristle worms or polychaetes are segmented worms commonly found at all depths of the water column especially in the benthos where they constitute approximately 35–70% of macroinvertebrate inhabitants (Díaz-Castañeda & Reish, Citation2009). Polychaetes are essential in decomposing of organic matter and that function in controlling organic carbon transferred out of marine waters and surroundings. In a recent study of polychaete worms, Marenzelleria sp. are known for the adaptation to tolerate insufficient oxygen, low salinity gradient and presence of hydrogen sulphide (Hahlbeck et al., Citation2000). Within few years, this species become dominant that manipulate most of the food web in coastal areas (Daunys et al., Citation2019). Polychaetes undergo ingestion that can lead metallic ions into the body as it can accumulate xenobiotics from the external environment into their body tissues. These metallic ions enter marine water by anthropogenic pathways and mix with sediment particles and deposit on seabed (Ramachandran, Citation1998).
Bivalvia are infauna organisms that supply food sources and act as a filter in most of the freshwater. The core importance of Bivalvia freshwater is that they can absorb heavy metals and large organic material while filter out blue-green algae, diatoms, bacteria, and fine particulate organic particles such as silt (Bogan, Citation2008). Moreover, Bivalvia is gifted with faster growth and life span which help them to adapt to any evolutionary changes but the decrease in temperature and light can affect the metabolic rate of this species. The continuous supply of food also affects the growth production as they feed on phytoplankton to survive (Moss et al., Citation2016). Besides, bivalve is often used in sediment elutriate tests by using oyster embryos such as Crassostrea iredalei and Crassostrea belcheri in Southeast Asia (Ramachandran, Citation1998).
Gastropods a phylum of Mollusca have a total diversity approximately 200,000 that make them second largest after arthropods in species richness (Strong et al., Citation2008). The submerged gastropods usually live in rivers, lakes, drainage, and irrigation which mostly can tolerate any aquatic vegetation, solid surfaces and even soft sediments. They feed on bryozoans, planorbid eggs, algae, and diatoms that make them known as macro-herbivorous grazers (Strong et al., Citation2008). Benthic gastropods are easiest way to monitor acidification in river, estuaries or even seawater by using it shells to access the geochemical discharge from acidic sediments (Marshall et al., Citation2019).
2. Factors influencing macrobenthic community and distribution in coastal water
There are several factors that affect the distribution of macrobenthos which are the physical nature of the substratum, depth, nutritive, and oxygen content and the degree of stability (Bozorgchenani et al., Citation2018). The declining of this habitat is mostly because of the construction of dams, canalization, water depth alteration because of changes of flow and fine particle deposition (Mohammad & Jalal, 2018). Additionally, the discharge of water from industry and irrigation, pollution, and the blocked area of the river due to urbanization and road building affected the abundance and species richness of macroinvertebrate (Bogan, Citation2008). Thus, these physicochemical parameters such as particle size, salinity, temperature, dissolved oxygen, and heavy metal pollution with sediment characteristics need to be studied to determine the assemblages of macrobenthos.
2.1. Particle size
Structure, roughness of the edges and shape of sediment surface is needed for the sediment sorting (S. W. Lee et al., Citation2019). It is an important tool for assessing the dominance of fossorial species. The percentage of very fine sand and the combined percentage of silt and clay were found to be used to differentiate biotic assemblages (Dernie et al., Citation2003). The feeding behaviour of macrobenthos are used for bioindicator such as Baltic clams (M. balthica) and Oregon pill bugs (G. oregonensis) as they are good in resisting physical and chemical changes to determine contaminant (Sizmur et al., Citation2019).
2.2. Salinity
Salinity is another physico-chemical parameter that is sensitively important to macrobenthic (Ritter et al., Citation2005). Stenohaline marine and freshwater species are delicate to the salinity gradient due to physiological barrier while euryhaline marine species is affected by the surrounding (Teske & Wooldridge, Citation2003). The changes in abiotic factor mainly the salinity will affect ecology, chemical, and physical characteristics of aquatic water body (Telesh & Khlebovich, Citation2010). Spatial variation of salinity and sediment composition is essential to determine the factors that influence the distribution and species richness of macrobenthos community since river is a point source. Species diversity is higher in the freshwater and lower at the mouth of the river to seawater, but the lowest salinity would be at steepest salinity gradient (Bozorgchenani et al., Citation2018). The difference in sediment characteristics such as sand and particulate organic matter.
2.3. Temperature
Temperature is an essential characteristic to determine the suitable condition for growth and production of macrofauna (Tagliarolo et al., Citation2018). A little change in temperature will modify the normal physiological functions of aquatic fauna which will reduce the population density. The highest density of macrobenthos occurs in warm temperature while highest diversity is in cold temperature (Bozorgchenani et al., Citation2018). This is because increased temperature in the summer will reduce the percentage of sediment and seawater intrusion at the estuary will increase, as a result the salinity will be increased (Norouzi, Citation2016).
2.4. Dissolved oxygen
Oxygen is a relatively vital factor that affects macrobenthos assemblages. Fluctuation in water temperature influenced the percentage of dissolved oxygen in river water and thereby altered the species richness of organisms (Azrina et al., Citation2006). The distribution of macrobenthic community is affected by oxygen deficits in intertidal sediments due to the build-up of hydrogen sulphide, inundation, poor drainage, and insufficient interstitial oxygen concentrations (Sarkar et al., Citation2005).
2.5. Heavy metal pollution
Heavy metal pollution is conceptually higher in estuaries compared to the ocean water (Hutton et al., Citation2015). Macrobenthic organisms are sensitive to organic enrichment, oil pollution and toxicity such as amphipods and polychaetes due to the high tolerance of heavy metal concentration (Ng et al., Citation2019). High concentration of Copper (Cu) in sediments is toxic to certain species of macrofauna such as (Glycera rouxii, Phylo norvegica, Sosane gracilis, Terebellides stroemi, Eriopisa elongate and Ennucula tenuis; Chen et al., Citation2010). Macrobenthic community is primarily related to natural variability and releases of anthropogenic pollution from the industrial outlets especially heavy metal becomes secondary disturbances to benthic structure.
3. Systematic review
Systematic review can be defined as a comprehensive synthesis of experiments for discovering reliable and generalizable evidence in a specific location. It is important to search and identify studies in a highly comprehensive, organized, transparent and replicable manner (J. Lee, Citation2015). Systematic review also presents methodological characteristics for easy overview and comparison among reviewed studies. For further analysis, systematic review may or may not include meta-analysis depends on the possibility of merging statistical information.
Corresponding to the PRISMA statement guideline, systematic review can be interpreted as a review of a clearly formulated question that uses systematic and explicit methods to identify, select and critically appraise relevant research, and to collect and analyse data from the studies that are included in the review. Statistical methods (meta-analysis) may or may not be used to analyse and summarize the results of the included studies. Meta-analysis is referred to the use of statistical techniques in a systematic review to integrate the results of included studies (Moher et al., Citation2015).
Based on above perspectives, the objectives of this study as outlined under following approach:
i. To retrieve published information resources on macrobenthic community assemblages in coastal areas in Malaysia.
ii. Interpret the diversity and abundance of macrobenthic assemblages in the coastal waters in Malaysia.
iii. Compare the diversity and abundance of macrobenthic community assemblages in the coastal waters in Malaysia.
4. Methodology
4.1. Information retrieval
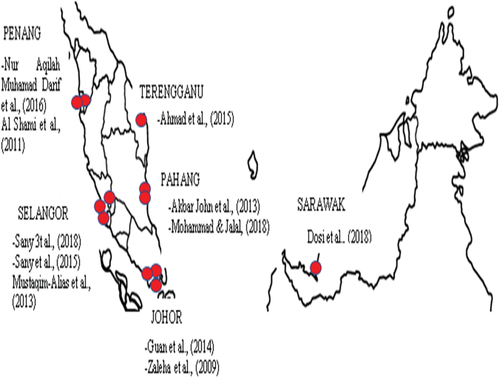
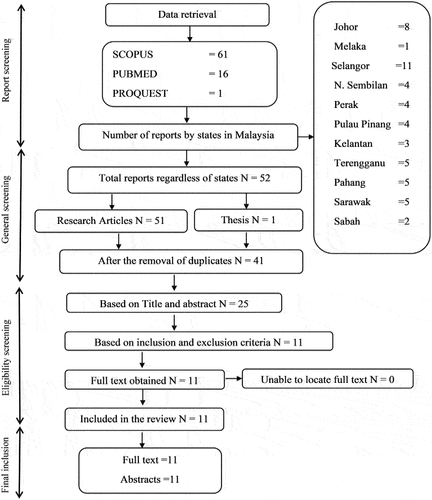
Data retrieval and meta-analysis were completed with the adoption of detailed searches in electronic databases such as SCOPUS, PubMed, and ProQuest. Following keywords in combination were used to screen published resources from different states in Malaysia: macrobenthos, macrobenthic, macrofauna, macroinvertebrate, diversity, community composition, and Malaysia. In addition, all search items were screened together with states in Malaysia where macrobenthos are recorded in literature (Perlis, Perak, Kedah, Pulau Pinang, Terengganu, Kelantan, Pahang, Negeri Sembilan, Melaka, Johor, Sarawak, and Sabah). A manual search from the retrieved data was concluded by removing duplicate references and further screened by title and abstracts. No time limits were set during the search and all published articles until the end of July 2020 were considered. shows the sampling locations in Malaysia where the macrobenthic samples were collected. The red spots in the figures identify the published resources from each representative state.
4.2. Inclusion and exclusion criteria for the selection of paper
This review required a full text article for further review on macrobenthic diversity in Malaysia. Additionally, the following inclusion criteria were formed prior to systematic review selection:
(i) diversity of macrobenthos by different diversity indices; such as Shannon-Wiener’s Index, evenness indices such as Simpson’s Diversity Index, (ii) the abundance of macrobenthos (individual/m2), and (iii) containing latitude and longitude of location. Exclusion criteria include (1) studies without comprehensive detail on sample collection method and (2) findings on sediment toxicity related to macrobenthos habitat. The most recent paper was given the priority when multiple publications from the same sampling site were available () (John et al., Citation2018).
5. Result and discussion
5.1. Potential Studies around Coastal areas of Malaysia
Major problems in the previous studies could be identified as a limited sample collection for instance, few potential studies (Ahmad et al., Citation2015; Guan et al., Citation2014; Mohamamad & Jalal, 2018; Mustaqim-Alias & Ahmad, Citation2013; Nur Aqilah Aqilah Muhamad Darif et al., Citation2016) have collected samples less than a week period.
While other studies sampled for few months (Mustaqim-Alias & Ahmad, Citation2013; Sany et al., Citation2015; Zaleha et al., Citation2009) and limited studies have addressed year-round sampling (Akbar John et al., Citation2013; Al-Shami et al., Citation2011; Dosi et al., Citation2018; Sany et al., Citation2018). The method adopted to sample the benthic varies from grab sampler (Mohammad & Jalal, 2018; Sany et al., Citation2015, Citation2018), hand corer (Akbar John et al., Citation2013; Guan et al., Citation2014; Nur Aqilah Aqilah Muhamad Darif et al., Citation2016; Zaleha et al., Citation2009), and nets (Ahmad et al., Citation2015; Al-Shami et al., Citation2011; Dosi et al., Citation2018; Mustaqim-Alias & Ahmad, Citation2013) in different studies ().
Table 1. Sampling Locations Showing the Diversity of Total Individuals at Different State.
Table 2. Total mean abundance of macrobenthos (individuals/m2) in each state in Malaysia.
5.2. Johor
Two research articles were retrieved from search engines addressing macrobenthic community composition and diversity mainly at Sungai Pulai estuary that have enormous seagrass beds (Guan et al., Citation2014; Zaleha et al., Citation2009). Seagrass beds are essential to support various species of marine organisms by providing food resources and nursery. The location of the sampling involves Sungai Duku, Tanjung Adang and Merambong in seagrass beds. Zaleha et al. (Citation2009) addressed both meiobenthic and macrobenthic diversity inhabiting the seagrass beds and addressed how environmental factors influence the community composition at different sampling stations. Three different sampling locations which addressed in this study were Sungai Duku (N07 °24.137’, E103 °22.46’), Tanjung Adang (N01 °19.762, E103 °33.894’), and Merambong (N01 °20.094’, E103 °35.982’). The sampling activity was conducted from 3rd December to 5 December 2006 during the lowest tide by using PVC hand corer and aided by quadrates frame (0.5 m x 0.5 m) along 12 m length starting from the bank to the upper site of the area. The samples collected were preserved by 10% neutralized formalin mixed with Rose Bengal stain. The identification of macrobenthos were identified to the lowest possible classification reference. In addition, sediment samples for particle size analysis and water parameters were also collected in each sampling location to identify the temperature, salinity, dissolved oxygen concentration, and pH by using YSI 556 multi-parameter.
Sungai Duku was selected for the review purposes by Zaleha et al. (Citation2009) as there are latest macrobenthos identification in Tanjung Adang and Merambong by Guan et al. (Citation2014). The mean total density of macrobenthic in Sungai Duku was low approximately 1,027 individuals/m2 in three sampling locations. The abundance of macrobenthos was elevated on the higher ground water (Site 1) compared to the edge of the water (Site 2 & Site 3). The highest total individuals of macrobenthos were Gastropoda (255 individuals/m2) followed by Polychaeta (247 individuals/m2). The diversity (H’), richness (d), and evenness index (J’) of Sungai Duku was relatively low compared to Tanjung Adang and Merambong, 0.73, 0.33, and 0.62, respectively. This was due to the low salinity concentration as it was located in the upper stream. In fact, the macrobenthic communities showed low abundance in low salinity levels (Teske & Wooldridge, Citation2003).
Guan et al. (Citation2014), which addressed macrobenthic composition, distribution and its abundance at Sungai Pulai Estuary mainly located at Tanjung Adang Shoal (N01°19ʹ48.4”, E103°34ʹ05.5) and Merambong Shoal (N01°20ʹ05.9”, E103°36ʹ05.8”). The macrobenthos sampling activity was held in October, 2013 by using a 10cm diameter corer and preserved using 4% formalin solution. The samples were identified to the lowest possible taxonon,, , . In addition, sediment particle size was determined by using pipette analysis while physical parameters such as pH, temperature, salinity and dissolved oxygen (DO) were measured in situ to study the influence of environmental factors to the macrobenthic distribution.
The sampling location of this research includes non-seagrass bed area that indicates lowest mean total density (individuals/m2) of macrobenthos. This might be influenced by the particle size of sediment as the macrobenthos population in coarser sediment compared to finer sediment (Bozorgchenani et al., Citation2018). Crustaceans dominated Tanjung Adang Shoal by 4,850 individuals/m2 whereas in Merambong Shoal showed high abundance of polychaeta (5,400 individual’s/m2). In this study, phylum Annelida also showed the high abundance of macrobenthos habitat such as Spionidae, Glyceridae, and Capitellidae. This indicated that the seagrass ecosystem was impacted by environmental pollution (Mazlan et al., Citation2005).
According to the data in , the differences between two studies were approximately 5 years apart, yet the number of individuals was relatively lowered. The common group of macrobenthic organisms in Sungai Pulai estuary were Polychaete, Bivalvia, Gastropoda, and Crustacea. Whereas less organisms found such as Oligochaeta, Amphipoda, Euphausiidae, and Decapoda. There was selectively high mean total density of macrofauna in higher ground contrasted to the water side area (Zaleha et al., Citation2009). Whereas, according to recent study, the dominant taxa at Sungai Pulai estuary consist of Spionidae, Glyceridae, and Capitellidae (Guan et al., Citation2014).
The abundance and composition of macrobenthic habitat in Sungai Pulai estuary were affected by physical factors such as temperature, turbidity, salinity, dissolved oxygen, and water movement. Moreover, this seagrass ecosystem always suffers from severe environmental changes as it was located near the coastline of Tanjung Pelepas that caused deterioration of species richness and community composition of macrobenthos. The recent industrial development from that area led to an unstable environment of the benthic community that indicated the moderate value of diversity. Highest diversity of Sungai Pulai estuary was at Merambong shoal. The variation of salinity also induced the community composition of macrofauna as Sungai Pulai estuary was located mainly at intense high and low tides. Thus, the diversity of macrobenthos in Sungai Pulai estuary indicated as moderately diverse.
5.3. Pulau Pinang
Two research studies were retrieved from search engines addressing soft sediment communities and macroinvertebrates in two different locations, Tanjung Bungah, and Juru River Basin (Al-Shami et al., Citation2011; Nur Aqilah Aqilah Muhamad Darif et al., Citation2016). Both sampling locations were contaminated due to human activities such as fishing activities, water, and air pollution. For instance, Tanjung Bungah is known as a great fishing village whereas Juru River Basin is situated in an industrial area. Thus, domestic waste that were directly discharged into these rivers became one of the essential factors which influenced the distribution of the macrobenthic community. The study by Al-Shami et al. (Citation2011), addressed the influence of agricultural, industrial, and anthropogenic stresses on the community composition and diversity of macrobenthos in Juru River Basin located approximately at 05 ° 22ʹN latitude and 100° 28ʹE longitude. Five sampling locations were selected at Juru River Basin including Ceruk Tok Kun River (CTKR) as a reference site. Those are: Pasir River (PR), Permatang Rawa River (PRR), Kilang Ubi River (KUR), and Juru River (JR). Juru River was selected for the review purposes as it shows selectively high diversity index compared to other sampling stations excluding Ceruk Tok Kun River (CTKR). The sampling of macrobenthic communities were collected from November 2007 until November 2008, approximately 13 months by using D-shaped aquatic nets and preserved by using 80% of ethanol. The samples were identified according to morphological characteristics and lowest possible taxa (Morse et al., Citation2007). The research study also includes sediment characteristic identification and water quality index to identify the influence of environmental parameters on macrobenthos abundance.
The total amount of macroinvertebrates collected at the five (5) sampling locations of River Basin was 19,928 individuals. CTKR denotes the highest abundance and diversity of macrobenthos as a reference site followed by Juru River. The biological monitoring water party (BMWP) which significantly uses families of macroinvertebrates as biological indicators to measure water quality stated that PRR, KUR, and JR were moderately polluted while PR had poor water quality. Only CTKR falls into good water quality condition. Chironomidae, Culicidae, Syrphidae, Ephemeroptera, Plecoptera, and Oligochaeta showed high abundance in five sampling locations. Oligochaeta and Chironomidae significantly could survive in high levels of biological oxygen demand (BOD) and chemical oxygen demand (COD). Moreover, these organisms are also moderately impacted by agricultural waste as it could tolerate low oxygen levels with high concentration of heavy metals in sediment such as Zn, Cu, and Ni (Yule et al., Citation2015). The Shannon-Weiner diversity index of these areas are quite low yet the Juru River has a high abundance of macrobenthos habitat. Juru River has been declared by Malaysian Department of Environment (DOE) as “very polluted according to the water quality index (WQI). It is highly contaminated with non-residual heavy metals such as Cd, Cu, Pb, and Zn due to chemicals thrown from industrial factories by Perai Industrial Estate. Hence, it can be concluded that there were three levels of stress that consists of slightly polluted river in Ceruk Tok Kun River (CTKR) (level 1), low habitat quality and quantity in Juru River (JR) (level 2), and severe environmental stresses because of agricultural, industrial, municipal, and sewage discharges in Pasir River (PR), Permatang Rawa River (PRR), Kilang Ubi River (KUR) (level 3).
Nur Aqilah Aqilah Muhamad Darif et al. (Citation2016) addressed the abundance and spatial distribution of soft sediment communities including meiobenthos and macrobenthos in Tanjung Bungah. The sampling locations were conducted at 05°28.048ʹN latitude and 100°16.730’ longitude on 6 November 2013 at six site locations in Tanjung Bungah area. The samples were collected by using a PVC hand corer (10 cm diameter x 10 cm depth) and preserved with 70% of ethanol. The sampling was identified to lowest possible taxon using standard references for both benthic habitats. In addition, in-situ physical parameters were also carried out to identify the influence of environmental factors to the macrobenthos and meiobenthos organisms. Total mean abundance of macrobenthos in Tanjung Bungah was 110 individuals that comprised families of Annelida, Bivalvia, Crustacea, Gastropoda, Nematoda, Nemertea, and Polychaeta. Crustaceans showed the most abundance in the six (6) sampling locations due to the high salinity and high dissolved oxygen levels. The soft sediment communities including annelids, crustaceans, and nemerteans were affected by dissolved oxygen concentration. Whereas, nematodes showed negative correlation with dissolved oxygen in Tanjung Bungah. Nematodes can survive in considerably polluted areas and severe eutrophication as it could tolerate sediment containing sulphide (Díaz-Castañeda & Reish, Citation2009).
5.4. Selangor
Macrobenthic assemblages at West Port, Klang Strait, and Sungai Congkak were addressed in (Mustaqim-Alias & Ahmad, Citation2013; Sany et al., Citation2015, Citation2018). West Port and Klang Strait are located near the north end of Malacca Strait in Southeast Asia while Sungai Congkak is located at Hulu Langat, Selangor. Mustaqim-Alias and Ahmad (Citation2013) study revealed macroinvertebrates diversity including water quality index in the recreational area of Sungai Congkak. The sampling was located at N 03° 13’ latitude and E101° 51’ longitude comprises eight sampling stations. The macroinvertebrates were collected by using Surber’s net facilitates by quadrates and then were preserved by using 70 % ethanol prior to the identification.. Besides, water quality parameters were identified to study the relationship between water quality index (WQI) and ecological indices of macroinvertebrates diversity. Sungai Congkak River had 3,754 individuals comprising Ephemeroptera, Coleoptera, Plecoptera, and Tricoptera. There was the low presence of macrobenthos that was typically found in polluted water in this river, indicating that the sampling location was cleaned and suitable for recreational purposes. The high quantity of Ephemeroptera (mayflies) often be used to indicate good water quality (Nor Zaiha et al., Citation2015).
Macrobenthic assemblages were also used to identify ecological quality assessment in West Port (03°00ʹN, 101°24ʹE; Sany et al., Citation2015). The sampling activity of macrobenthos was conducted during the dry and rainy season (26-27 November 2011, 28-29 February 2012, 28-29 May 2012, and 15-17 August 2012) by using Peterson grab sampler (0.07m2). There were nine sampling stations and this review was based on Station 6 that located along the mangrove edge as it showed high abundance of macrobenthic diversity, abundance, and richness, (4.00,1,736, and 28.00, respectively). The samples were preserved with 99.9% ethanol with Rose Bengal stain and macrobenthos were identified to the lowest practical taxonomic level. In addition, the physicochemical parameters were measured to assess pollution levels based on the benthic response to this disturbance.
The total abundance of all sampling stations was 2,144 individuals that indicated 81% from the total abundance on Station 6. The diversity index at this sampling location can be classified as it was slightly disturbed and polluted. The abundance, richness, and diversity of West Port from November, 2011 until August, 2012 signified an upward trend. The environmental parameters such as total organic carbon (TOC), particle size, and depth influenced the distribution of macrobenthos abundance, diversity, and richness. In addition, the high concentration of Fe and Mn prompted the increase of macrobenthos diversity (Sany et al., Citation2015). Anthropogenic effects and environmental factors suppressed the development of the macrobenthic community.
Macrobenthic community’s assemblage variation in relation to the changes in environmental variability in Klang Strait coastal water (03° 00° N, 101° 24′ E), which was primarily observed three main sites with 21 sampling stations at North Port, West Port, and South Port (Sany et al., Citation2018). The North Port and West Port sampling stations located at Mangrove Islands were prominent with cargo ships, fishing boats and industrial waste (Sany et al., Citation2015). The South Port which is known as local harbour was highly affected by port activities including waste from sewage pipes. The sampling activity for macrobenthos, sediment analysis and water quality parameters were conducted from November, 2012 until November, 2015. Macrobenthos and sediment samples were collected by using Peterson grab sampler (0.07 m2) while water parameters measured by using Secchi disc and fish sounder to measure water depth and current. The macrobenthos samples were preserved with 99.9% ethanol and stained with Rose Bengal. The sorting and identification of macrobenthic communities was done to the lowest possible classification.
The sampling collected 33 species including bivalves, gastropods, and crustaceans. Diversity and species richness were lowered in the inter-monsoon season compared to other seasons along two years of sampling. The average abundance of individual species recorded 1,436 individuals/m2. Xenophthalmus pinnotheroides and Lumbricillus sp were recorded as the dominant species during all seasons. Thus, induced that variation of monsoon does not affect the macrobenthic diversity. Macroinvertebrate at North Port and West Port showed higher abundance, richness, and diversity as it has high organic content and shallow water. Besides, the high percentage of total organic carbon and fine sediments also decreased the abundance of macroinvertebrates organisms. The community composition of macrobenthic increased with decreasing gradients of total organic carbon (TOC; Brauko et al., Citation2015). Therefore, macrobenthos diversity in Klang Strait were affected by environmental factors and spatial factors as there are fewer species at berth line.
The total individuals for Klang Strait are higher compared to West Port yet the diversity is relatively high at West Port. And this observation is questionable as the H’ value recorded to be 4.0. Sungai Congkak is a recreational area in Selangor state and flows toward the main river, Sungai Langat. The diversity index for Sungai Congkak is low compared to West Port and Klang Strait but has an abundance of macrobenthos. The physico-chemical parameters of Sungai Congkak indicate that the study area is not affected by biological pollution and physical contamination based on dissolved oxygen (DO), biological oxygen demand (BOD), chemical oxygen demand (COD), ammoniacal nitrogen (AN) and total suspended solid (TSS; Mustaqim-Alias & Ahmad, Citation2013). Generally, West Port is classified as moderately polluted and strong correlation between macrobenthos diversity including abundance, richness. Environmental parameters indicated these parameters controlled the spatial distribution of macrobenthos in the West Port. Whereas Klang Strait portrayed that the abundance and biomass of macrobenthos organisms constituted 35.69% of bivalves followed by crustaceans, polychaetes, and gastropods. Besides, the BIO-ENV procedure at Klang Strait showed the highest correlation between total organic carbon (TOC), depth, and fine sand fraction size. The environmental factors and macrobenthic diversity at Klang Strait were influenced by spatial factors and magnitude of environmental pressures.
5.5. Pahang
Two research papers were retrieved from search engines regarding macrobenthic diversity and community composition in Balok beach and Pahang estuary (Akbar John et al., Citation2013; Mohammad & Jalal, 2018). The first research at Balok beach was regarding macrobenthos diversity in horseshoe crab nesting ground. The sampling was located at N3°56.194’ latitude and E 103°22.608’ longitude. The sediments of the Balok beach were observed to be soft sediments that contributed to easiness of horseshoe crab development. According to Malaysian Meteorological Department (MMD), monsoon season occurred from November to January while non-monsoon seasons with low rainfall happened during April to June. The sampling of macrobenthos was conducted between March, 2010 to February, 2011 comprises 10 sampling locations at the Balok beach by using hand scoop during low tide time. The macrobenthos sampled were preserved with 70% ethanol and identified to lowest possible taxon. The macrobenthos diversity were identified by using the Shannon Diversity Index, Simpson Diversity Index, Margalef Diversity Index, Mcintosh Diversity Index and Berger Parker Index.
Bivalves denote the most dominant macrobenthos followed by gastropods, polychaeta and crustaceans at 10 sampling locations. The highest diversity index of macrobenthic was recorded during June, 2010 (0.673) followed by September, 2010 (0.663) and the lowest diversity index was observed during March, 2010 (0.545). It was observed that the macrobenthos diversity elevated during monsoon sessions and fluctuated throughout dry sessions. Besides, monsoonal wind and monsoon shifting also impacted the sediment characteristics that may disrupt the abundance of macrobenthic community composition. The low diversity index also signified that the macrobenthic diversity along Balok station were under environmental stress due to developmental activities (Abdullah et al., Citation2015).
Next, the second paper from Mohammad and Jalal (2018) was about macrobenthic diversity and community composition at Pahang estuary located in Pekan, Pahang. The sampling activity is located at N03°33ʹ01.78’ latitude and E103°25ʹ56.48’ longitude in Pahang estuary which consists of three zones with the distances approximately 3 km from each zone. The macrobenthic sampling was conducted by using grab sampler to collect the sediment and samples were identified to the lowest possible classification. Zone 1 (mouth of the sea) was identified as low in diversity of the macrobenthic community with the diversity index (H’) of 0.3 where only polychaete worms and bivalves were identified from this area. Polychaete represented by Neries sp. bivalve class was represented by Yoldia sp. in this zone. Zone 2 (Mangrove area), showed the number and types of benthic communities were low because of human disturbance, but their existence was due to the presence of detritus that acts as food and habitat provided by the Mangrove. This location was identified as a lowest diversity index of macrobenthos (polychaete, bivalves, and gastropods) with the value of 0.31. Zone 3 (riverine area) has shown highest diversity among all three zones, with the diversity index of 0.38 along with three classes of macrobenthos, which were polychaete, bivalves, and gastropods. Polychaete was represented by Neries sp., bivalves by Yoldia sp., and gastropods by Nassarius sp., respectively (Mohammad & Jalal, 2018). Besides, the influence of type of sediment and trace metal pollution were affecting the distribution of macrobenthic communities due to industrial purposes, housing, and recreation (Abdullah et al., Citation2015).
5.6. Terengganu
The research done at Terengganu state relating to macrobenthos as biological indicators was mainly from Sungai Ikan, Hulu Terengganu, Terengganu (N 05° 00ʹ39.97’, E 102° 52ʹ24.55’). The paper includes water quality index (WQI) in determining the influence of environmental factors to the macroinvertebrate diversity (Ahmad et al., Citation2015). Sungai Ikan is a small river and point source of Kenyir Lake. Thus, the sampling location includes five stations in a 250m long river. The coordinates in are according to the Station 2. The sampling activity comprises benthic macroinvertebrate and water quality was done once on 4 November 2012 with three replicates. The water quality involves dissolved oxygen (DO), pH, temperature, biological oxygen demand (BOD), chemical oxygen demand (COD), total suspended solid (TSS), nitrate, and ammoniacal nitrogen. The sampling gear for macrobenthos was a Surber net that was placed in the river for three minutes. The sample then was preserved with 70% ethanol and the identification according to the family and order, .
The total recorded macrobenthos is approximately 1,353 individuals and the highest abundance is at Station 2 (394 individuals/m2). Ephemeroptera denotes 57.1% of total abundance followed by Diptera (22.9%), Trichoptera (8.0%) and Coleoptera (7.6%). The abundance and community composition of macrobenthic in Sungai Ikan are not well-distributed although the result shows good water quality index. This can be induced from a study depicted that fast-moving water has less macrobenthos diversity. Ephemeroptera became the most abundant and was found in all stations due to the tolerance of this species towards fast moving water. Moreover, good water quality also influences the richness of Ephemeroptera (Appalasamy et al., Citation2018). Next, Chironomidae also can be found at all stations, although some study suggests that it can be bio-indicator as it always appeared in polluted river ecosystems (Azrina et al., Citation2006).
According to the water quality of Sungai Ikan, biological oxygen demand and chemical oxygen demand of this sampling area is 0.33 mg/L and 2.20 mg/L, respectively. The average temperature of Sungai Ikan is 22.95±0.25°C, which signifies that it is normal in condition as upstream rivers naturally have temperatures not more than 25°C. In addition, canonical correlation was conducted to identify the relationship between influencing of water quality with the environmental factors that induced the diversity of macrobenthos. The results signify that macrobenthos in Sungai Ikan are not influenced by water quality (Ahmad et al., Citation2015).
5.7. Sarawak
The study was retrieved from a search engine related to the macrobenthic diversity in Maludam River which is a major river system in Maludam National Park (Dosi et al., Citation2018). It is the largest peat dome in Northern Borneo that covers an area of 43,147 ha. The sampling was conducted at three sites, Site A (01° 34.894′ N, 111° 05.086′ E), Site B (01° 35.799′ N, 111° 04.415′ E) and site C (01° 37.043′ N, 111° 03.746′ E). Site B was selected as it has the highest diversity compared to Site A and Site C. The sampling activity was conducted during wet and dry sessions from April, 2011 to November, 2014 involving 14 sampling visits. Macrobenthos were collected by using a kick net (frame 40 x 32cm, mesh size 0.4mm). The samples were preserved with 85% of ethanol before sorting and identification according to morphological characteristics and lowest possible taxonomic level.
The half of the macrobenthos captured were aquatic beetles and another 26% of captures represented aquatic bugs whereas the dominant of the macroinvertebrate species were Odonata which signifies 16 species. Total number of individuals in three sampling sites is 3,257 m2. The diversity index varied during wet and dry seasons, which indicates 2.189 and 2.440, respectively. According to the research study, Pandanus species is a major plant community around the water. The black in colour of river water is due to production of tannin whereas the seabed comprises semi-decomposed peat, logs, leaves, branches, and submerged vegetation. The high concentration of tannin became a huge problem to the colonization of macrobenthos. There is a huge gap in determining the macrobenthos diversity in Maludam river as headwaters are not sampled due to the difficulties and inaccessible area during low water levels. In addition, the use of kick-net sampling also was not effective compared to the use of a grab sampler yet the diversity of macrofauna in Maludum river. It has shown consistently high despite the stress of the environment in this area and survives in providing peat swamp forest.
6. Conclusion and recommendation
Macrobenthic community composition has been extensively used to determine the quality of the aquatic environment for a long time. Based on this review paper studies, macrobenthos respond relatively rapidly to environmental conditions, so that they can be considered as very useful organisms for monitoring the marine ecosystem. However, the review papers have shown the high and low diversity of the macrobenthic community in the different coastal waters of Malaysia which reveals the alarming conditions of the Malaysian coastal area. Because some estuarine and riverine areas have been undergoing poor conditions in water quality and sand dredging, dumping of wastes from fishing boats, and other massive human activities along the estuary and riverine area. These activities prove the greatest threats to bottom habitat loss, degradation of water quality, and declining indigenous fish population and macrobenthic communities. Major drawback in the previous studies can be identified as a limited sample collection for instance, few studies. It could be a great concern about the abundance of microbenthic assemblages to compare between sapling locations.
However, the present study reveals the baseline information of the macro-benthic community which is not adequate in Malaysia. Furthermore, more extensive studies need to initiate towards massive Borneo coastal areas to get more updated information regarding macro-benthos distribution and pollution status along the coastal areas of Sabah, Sarawak and Brunei Darussalam. A balanced approach towards standardization of technique coupled with year-round sampling through continuous monitoring would be helpful in identifying the fate of macro-benthic diversity, distribution, and assemblages along the coastal waters of ASEAN region. A long-term continuous monitoring for “Eco-Biology” and species diversity of macro-benthic assemblages are urgently needed to maintain the “Sustainable Aquatic Ecosystem Health” to fulfil the Sustainable Development Goal (SDG-14) along the coastal waters of Malaysia and south-east Asia as well.
Acknowledgements
The authors are also grateful to the Ministry of Higher Education, Malaysia (MOHE) and Research Management Centre, International Islamic University Malaysia for funding through FRGS (Fundamental Research Grant Scheme) grant [FRGS/1/2018/STG03/UIAM/01/1]. The finding of the paper was an extension of relevant fisheries and environment-based research activities of the approved project area in the Balok river compared with other rivers of the coastal areas in Malaysia.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Abdullah, M. Z., Louis, V. C., & Abas, M. T. (2015). Metal pollution and ecological risk assessment of Balok river sediment, Pahang Malaysia. American Journal of Environmental Engineering, 5(3A), 1–7. https://doi.org/10.5923/c.ajee.201501.01
- Ahmad, A. K., Siti Hafizah, A., & Shuhaimi-Othman, M. (2015). Use of benthic macroinvertebrate as biological indicators in Sungai Ikan, Hulu Terengganu, Terengganu | Potensi makroinvertebrat bentik sebagai penunjuk biologi di Sungai Ikan, Hulu Terengganu, Terengganu. Sains Malaysiana, 44(5), 663–670. https://www.ukm.my/jsm/pdf_files/SM-PDF-44-5-2015/04%20A.K.%20Ahmad.pdf
- Ahmad Azfar, M., & Jalal, K. C. A. (2018). Macrobenthic diversity and community composition in the Pahang Estuary, Malaysia. Journal of Coastal Research, 82(4), 206–211. https://doi.org/10.2112/SI82-030.1
- Akbar, J. B., Jalal, K. C. A., & Kamaruzzaman, B. Y. (2013). Macrobenthic diversity in Horseshoe Crab nesting ground - Balok station, Pahang, Malaysia. Oriental Journal of Chemistry, 29(4), 1311–1318.
- Akbar John, B., Jalal, K. C. A., & Kamaruzzaman, B. Y. (2013). Macrobenthic diversity in horseshoe crab nesting ground-balok station, Pahang, Malaysia. Oriental Journal of Chemistry, 29(4), 1311–1318. http://dx.doi.org/10.13005/ojc/290406
- Al-Shami, S. A., Md Rawi, C. S., Ahmad, A. H., Abdul Hamid, S., & Mohd Nor, S. A. (2011). Influence of agricultural, industrial, and anthropogenic stresses on the distribution and diversity of macroinvertebrates in Juru River Basin, Penang, Malaysia. Ecotoxicology and Environmental Safety, 74(5), 1195–1202. https://pubmed.ncbi.nlm.nih.gov/21419486/
- Appalasamy, S., Arumugam, N., Sukri, S., & Rak, A. E. (2018). Physico-chemical water quality and macroinvertebrate distribution along Sungai Asah in Pulau Tioman, Johor, Malaysia. Songklanakarin Journal of Science and Technology, 40(6), 1265–1270. https://doi.org/10.14456/sjst-psu.2018.155
- Aqilah Muhamad Darif, N., Shakila Abdul Samad, N., Salleh, S., Mohmmad, M., Alia Ahmad Nordin, N., Mariam Mohamed Javeed, A., Hilal Mohd Zainudin, M., & Jonik, M. G. G. (2016). The abundance and spatial distribution of soft sediment communities in Tanjung Bungah, Malaysia: A preliminary study. Tropical Life Sciences Research, 27(3), 71–77. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5131656/
- Azrina, M. Z., Yap, C. K. Ã., Ismail, A. R., Ismail, A., & Tan, S. G. (2006). Anthropogenic impacts on the distribution and biodiversity of benthic macroinvertebrates and water quality of the Langat River. Peninsular Malaysia, 64(2) , 337–347. https://doi.org/10.1016/j.ecoenv.2005.04.003
- Barua, P., Mohasin Meah, M., & Rahman, S. H. (2019). Abundance of macrobenthos with special reference to some physico-chemical parameters of south-eastern coastal area, Bangladesh. Asian Journal of Water, Environment and Pollution, 16(4), 51–60. https://doi.org/10.3233/AJW190048
- Bogan, A. E. (2008). Global diversity of freshwater mussels (Mollusca, Bivalvia) in freshwater. Hydrobiologia, 595(1), 139–147. https://link.springer.com/article/10.1007/s10750-007-9011-7
- Bossley, J. P., & Smiley, P. C. (2019). Impact of student-induced disturbance on stream macroinvertebrates differs among habitat types. Scientific Reports, 9(1), 1–15. https://www.nature.com/articles/s41598-018-38210-1
- Bozorgchenani, A., Seyfabadi, J., & Shokri, M. R. (2018). Effects of thermal discharge from Neka power plant (southern Caspian Sea) on macrobenthic diversity and abundance. Journal of Thermal Biology, 75(4) , 13–30. https://www.sciencedirect.com/science/article/abs/pii/S0306456517304989
- Brauko, K. M., Souza, F. M., De, Muniz, P., Camargo de, M. G., & Lana da, P. C. (2015). Spatial variability of three benthic indices for marine quality assessment in a subtropical estuary of Southern Brazil. Marine Pollution Bulletin, 91(2), 454–460. https://www.sciencedirect.com/science/article/abs/pii/S0025326X14007097
- Chen, K., Tian, S., & Jiao, J. J. (2010). Macrobenthic community in Tolo Harbour, Hong Kong and its relations with heavy metals. Estuaries and Coasts, 33(3), 600–608. https://link.springer.com/article/10.1007/s12237-010-9271-8
- Daunys, F., Schiedek, O., & Zettler. (2019). Effect of species invasion on transport of solutes at different levels of soft sediment macrofauna diversity: Results from an experimental approach. Water, 11(8), 1544. https://www.mdpi.com/2073-4441/11/8/1544
- Dernie, K. M., Kaiser, M. J., Richardson, E. A., & Warwick, R. M. (2003). Recovery of soft sediment communities and habitats following physical disturbances. Journal of Experimental Marine Biology and Ecology, 286(7) , 415–434. https://www.sciencedirect.com/science/article/abs/pii/S0022098102005415
- Díaz-Castañeda, V., & Reish, D. J. (2009). Polychaetes in environmental studies. Annelids in Modern Biology, 7, 203–227. https://doi.org/10.1002/9780470455203.ch11
- Dosi, E. M., Grinang, J., Nyanti, L., Khoon, K. L., Harun, M. H., & Kamarudin, N. (2018). A preliminary study of the macroinvertebrate fauna of freshwater habitats in Maludam National Park, Sarawak. Mires and Peat, 6(4) , 22. https://doi.org/10.19189/MaP.2017.OMB.316
- Guan, W. S., Lee, D. M., Ghaffar, M. A., Md Ali, M., & Che Cob, Z. (2014 February 2015). Macrobenthos composition, distribution and abundance within sungai pulai estuary, Johor, Malaysia. AIP Conference Proceedings, 1614, 591–596. https://doi.org/10.1063/1.4895269
- Hahlbeck, E., Arndt, C., & Schiedek, D. (2000). Sulphide detoxification in Hediste diverse color and Marenzelleria viridis two dominant polychaete worms within the shallow coastal waters of the southern Baltic Sea. Comparative Biochemistry and Physiology Part B, 125(4), 457–471. https://pubmed.ncbi.nlm.nih.gov/10904859/
- Hemery, L. G., Henkel, S. K., & Cochrane, G. R. (2018). Benthic assemblages of mega epifauna on the Oregon continental margin. Continental Shelf Research, 159(March 2017), 24–32. https://www.sciencedirect.com/science/article/abs/pii/S0278434317301188
- Hutton, M., Venturini, N., García-Rodríguez, F., Brugnoli, E., & Muniz, P. (2015). Assessing the ecological quality status of a temperate urban estuary by means of benthic biotic indices. Marine Pollution Bulletin, 91(2), 441–453. https://doi.org/10.1016/j.marpolbul.2014.10.042
- John, B. A., Nelson, B. R., Sheikh, H. I., Cheung, S. G., Wardiatno, Y., Dash, B. P., Tsuchiya, K., Iwasaki, Y., & Pati, S. (2018). A review on fisheries and conservation status of Asian horseshoe crabs. Biodiversity and Conservation, 27(14), 3573–3598. https://link.springer.com/article/10.1007/s10531-018-1633-8
- Latun, A. R., Ali, M., Tamimi, M., Ahmad, A., & Katoh, M. (2016). Boosting National Mechanisms to Combat IUU Fishing: Dynamism of the Southeast Asian Fisheries Sector. FIsh for the People, 14(1), 36–43. http://repository.seafdec.org/handle/20.500.12066/979
- Lee, J. (2015). The effects of music on pain: A review of systematic reviews and meta-analysis a dissertation submitted to the Temple University graduate board in partial fulfilment of the requirements for the degree. DOCTOR OF PHILOSOPHY by Examining Committee Members.
- Lee, S. W., Park, G., & Choi, K. H. (2019). Biomass of plankton and macrobenthos and benthic species diversity in relation to environmental gradients in a nationwide coastal survey. Regional Studies in Marine Science, 26(8) , 100502. https://www.sciencedirect.com/science/article/abs/pii/S0048969719331547
- Marshall, D. J., Abdelhady, A. A., Wah, D. T. T., Mustapha, N., Gӧdeke, S. H., De Silva, L. C., & Hall-Spencer, J. M. (2019). Biomonitoring acidification using marine gastropods. Science of the Total Environment, 692(9) , 833–843.
- Mazlan, A. G., Zaidi, C. C., Wan-Lotfi, W. M., & Othman, B. H. R. (2005). On the current status of coastal marine biodiversity in Malaysia. Indian Journal of Marine Sciences, 34(1), 76–87.
- Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., PRISMA, L. A., & Shekelle, P. (2015, January). Evaluation of ASTM Standard Test method E 2177, 6 retroreflectivity of pavement markings in a condition of 7 wetness. Systematic Reviews, 4(1), 1–9. https://journals.sagepub.com/doi/abs/10.3141/2272-10
- Morse, J. C., Bae, Y. J., Munkhjargal, G., Sangpradub, N., Tanida, K., Vshivkova, T. S., Wang, B., Yang, L., & Yule, C. M. (2007). Freshwater biomonitoring with macroinvertebrates in East Asia. Frontiers in Ecology and the Environment, 5 (1), 33–42. [33: FBWMIE]2.0.CO;2. https://esajournals.onlinelibrary.wiley.com/doi/abs/10.1890/1540-9295%282007%295%5B33%3AFBWMIE%5D2.0.CO%3B2
- Moss, D. K., Ivany, L. C., Judd, E. J., Cummings, P. W., Bearden, C. E., Kim, W. J., … Driscoll, J. R. (2016). Lifespan, growth rate, and body size across latitude in marine bivalvia, with implications for phanerozoic evolution. Proceedings of the Royal Society B: Biological Sciences, 283(1836). https://doi.org/10.1098/rspb.2016.1364
- Muhamad Darif, N. A., Abdul Samad, N. S., Salleh, S., Mohammad, M., Ahmad Nordin, N. A., Mohamed Javeed, A. M., Jonik, M. G. G., & Mohd Zainudin, M. H. (2016). The abundance and spatial distribution of soft sediment communities in Tanjung Bungah, Malaysia: A preliminary study. Tropical Life Sciences Research, 27(3), 71–77.
- Mustaqim-Alias, M., & Ahmad, A. K. (2013). Benthic macroinvertebrates diversity and water quality assessment at Sungai Congkak recreational area, Hulu Langat, Selangor. AIP Conference Proceedings, 1571, 608–613. https://doi.org/10.1063/1.4858721
- Ng, C. S. L., Toh, K. B., Toh, T. C., Ng, J. Y., Cheo, P. R., Tun, K., & Chou, L. M. (2019). Distribution of soft bottom macrobenthic communities in tropical marinas of Singapore. Urban Ecosystems, 22(3), 443–453. https://link.springer.com/article/10.1007/s11252-019-0828-4
- Nor Zaiha, A., Mohd Ismid, M. S., & Salmiati. (2015). Temporal distribution of benthic macroinvertebrate communities from tropical forest stream in Gunung Pulai recreational forest, Johor, Peninsular Malaysia. Sains Malaysiana, 44(9), 1223–1228. https://www.researchgate.net/publication/283317355_Temporal_Distribution_of_Benthic_Macroinvertebrate_Communities_from_Tropical_Forest_Stream_in_Gunung_Pulai_Recreational_Forest_Johor_Peninsular_Malaysia
- Norouzi, M. (2016, November). The effects of salinity, pH and temperature changes and the macrobenthos of the Shirud River Deltaic region. International Congress on Applied Ichthyology & Aquatic Environment.
- Piló, D., Ben-Hamadou, R., Pereira, F., Carriço, A., Pereira, P., Corzo, A., Carvalho, S., & Carvalho, S. (2016). How functional traits of estuarine macrobenthic assemblages respond to metal contamination? Ecological Indicators, 71, 645–659. https://www.sciencedirect.com/science/article/abs/pii/S1470160X16304113
- Ramachandran, S. D. (1998). Toxicity Associated with Sediments from Malaysian Estuarine Environment, 5. https://research.library.mun.ca/11311/
- Ritter, C., Montagna, P. A., & Applebaum, S. (2005). Short-term succession dynamics of macrobenthos in a salinity-stressed estuary. Journal of Experimental Marine Biology and Ecology, 323(1), 57–69.
- Sany, S. B. T., Hashim, R., Salleh, A., Rezayi, M., & Safari, O. (2015). Ecological quality assessment based on macrobenthic assemblages’ indices along West Port, Malaysia coast. Environmental Earth Sciences, 74(2), 1331–1341.
- Sany, S. B. T., Tajfard, M., Hashim, R., Rezayi, M., Rahman, M. A., & Karlen, D. J. (2018). The response of macrobenthic communities to environmental variability in tropical coastal waters. Estuaries and Coasts, 41(4), 1178–1192. https://link.springer.com/article/10.1007/s12237-017-0346-7
- Sarkar, S. K., Bhattacharya, A., Giri, S., Bhattacharya, B., Sarkar, D., Nayak, D. C., & Chattopadhaya, A. K. (2005). Spatiotemporal variation in benthic polychaetes (Annelida) and relationships with environmental variables in a tropical estuary. Wetlands Ecology and Management, 13(1), 55–67. https://doi.org/10.1007/s11273-003-5067-y
- Sarker Md, J. (2016). Macrobenthic community structure - an approach to assess coastal water pollution in Bangladesh. Fisheries and Aquaculture Journal, 07(1), 157. https://doi.org/10.4172/2150-3508.1000157
- Sizmur, T., Campbell, L., Dracott, K., Jones, M., O’Driscoll, N. J., Gerwing, T., & Hewitt, J. (2019). Relationships between potentially toxic elements in intertidal sediments and their bioaccumulation by benthic invertebrates. PLoS One, 14(9), e0216767. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0216767
- Strong, E. E., Gargominy, O., Ponder, W. F., & Bouchet, P. (2008). Global diversity of gastropods (Gastropoda; Mollusca) in freshwater. Hydrobiologia, 595(1), 149–166.
- Tagliapietra, D., & Sigovini, M. (2010). Benthic fauna: Collection and identification of macrobenthic invertebrates. NEAR Curriculum in Natural Environmental Sciences. Terre Et Environ, 88(8) , 253–261.
- Tagliarolo, M., Porri, F., & Scharler, U. M. (2018). Temperature-induced variability in metabolic activity of ecologically important estuarine macrobenthos. Marine Biology, 165(1). https://doi.org/10.1007/s00227-017-3276-9
- Telesh, I. V., & Khlebovich, V. V. (2010). Principal processes within the estuarine salinity gradient: A review. Marine Pollution Bulletin, 61(4–6), 149–155.
- Teske, P. R., & Wooldridge, T. H. (2003). What limits the distribution of subtidal macrobenthos in permanently open and temporarily open/closed South African estuaries? Salinity vs. sediment particle size. Estuarine, Coastal and Shelf Science, 57(1–2), 225–238. https://www.sciencedirect.com/science/article/abs/pii/S0272771402003475
- Yasin, A. H., & Razak, S. A. (1994). Distribution of Macrobenthos in the South China Sea, Area I: Gulf of Thailand and East Coast of Peninsular Malaysia. Area, 1990, 285–293. http://repository.seafdec.org/handle/20.500.12066/4308?locale-attribute=my
- Yule, C. M., Gan, J. Y., Jinggut, T., & Lee, K. V. (2015). Urbanization affects food webs and leaf-litter decomposition in a tropical stream in Malaysia. Freshwater Science, 34(2), 702–715. https://doi.org/10.1086/681252
- Zaleha, K., Farah Diyana, M. F., Amira Suhaili, R., & Amirudin, A. (2009). Benthic community of the sungai pulai seagrass bed, Malaysia. Malaysian Journal of Science, 28(2), 143–159. https://doi.org/10.22452/mjs.vol28no2.4