?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
The present study was carried out to assess the floristic diversity and community characteristics in the forest and alpine zone of Gulmarg Wildlife Sanctuary (GWLS), Kashmir Himalaya. A total of 123 sites were selected along an elevational gradient (2300–4200 m a.m.s.l) in each and every accessible aspect and habitats from July 2018 to June 2022. The present study recorded 364 species of vascular plants belonging to 227 genera and 74 families. Of the total species, 22 were trees, 34 shrubs, 290 herbs and 14 ferns. A total of 18 communities (10 within forest and 8 within alpine zone) were identified. Within the identified forest communities, species richness ranged from 44 to 198; tree density and total basal area ranged from 185–810 Ind ha−1 and 20.28–159.8 m2 ha−1, respectively. Density of shrubs and herbs in forest zone ranges from 886–2040 Ind ha−1 and 27.79–87.75 Ind m−2, respectively. Within the alpine communities, species richness ranged from 26–93; total density of shrubs and herbs ranged from 1410.0–5540 Ind ha−1 and 31.73–102.2 Ind m−2, respectively. Within the forest zone, diversity of trees, shrubs and herbs ranged from 0.88–1.67, 0.27–1.82 and 3.29–4.81, respectively. Within the alpine communities, species diversity of shrubs and herbs ranged from 0.73–1.33 and 2.21–3.69, respectively. Present study presents a first comprehensive floristic and community assessment of GWLS, and provides a template, which can be replicated in other protected as well as unprotected areas of the Kashmir Himalaya.
Introduction
Botanical assessments involving structural studies of vegetation zones and floristic composition are essential for determining significant elements of plant biodiversity, protecting threatened and economically important species and monitoring protected areas (Anwar et al., Citation2019; Melese & Ayele, Citation2017; Pukkala, Citation2018; Ramachandran & Swarupanandan, Citation2013). A complete floristic inventory of a region, serves as a base for investigations in other fields like photochemistry, taxonomy, ecology, ethnobotany, conservation and management (Anup et al., Citation2018; Ijaz et al., Citation2018; Taj et al., Citation2018). Because floral diversity is one of the most significant biotic component of ecosystems (Navarro-Cerrillo et al., Citation2019; P. P. Joshi et al., Citation2018), accurate floristic inventories can provide better understanding of climatic, topographic, and edaphic status of an area (Goncalves et al., Citation2018; Liu & Wang, Citation2018; Naghdi et al., Citation2018). Besides, botanical assessments and phytosociological studies are very essential to describe the population dynamics of species and for understanding how these species interact with other species within a same community (Salisu et al., Citation2021). The floristic composition and phytosociological interrelationships are quite dynamic in nature and tend to change with respect to biotic and abiotic components of any ecological system. The plant community of a region is a function of time and climate (Harrison et al., Citation2020); however, a complex of factors, namely, vegetation type, slope, aspect, precipitation, edaphic factors, and altitude determines the community composition, structure, and distribution pattern of diversity in mountainous vegetation (A. M. Khan et al., Citation2019; Nepali et al., Citation2021; Z. A. Malik & Nautiyal, Citation2016).
India ranks sixth among the 12 mega-biodiversity centres of the world concentrating four terrestrial biodiversity hotspots viz., The Himalayas, Indo-Burma, Western Ghats and Sundaland (J. S. Singh & Chaturvedi, Citation2017). The Himalayas cover approximately 18% of its total geographical area (V. S. Negi et al., Citation2021), and accounts for more than one third of its forest cover and harbours 40% of its endemics (Anthwal et al., Citation2010; Haq, Calixto, et al., Citation2020; V. S. Negi et al., Citation2021). The Indian Himalayan Region (IHR) ranging from Arunachal Pradesh to Jammu and Kashmir and elevation range of>8000 m amsl is one of the major repositories of biodiversity (Barman et al., Citation2021; Devi et al., Citation2019; Kaur et al., Citation2022). It comprises of three biogeographical zones (i.e., Trans Himalaya, Himalaya, and Central Himalaya) and eight biogeographic provinces i.e., Trans-Himalaya (Ladakh Mountains), Trans-Himalaya (Tibetian Plateau), Trans-Himalaya (Cold arid regions of Eastern Himachal Pradesh and Uttarakhand), Trans-Himalaya (Sikkim Plateau), North-West Himalaya, West Himalaya, Central Himalaya, and Eastern Himalaya (A. Kumar et al., Citation2017; Rodgers & Panwar, Citation1988). Owing to its variation in topography as well as having a distinct geographical and ecological state, IHR region is regarded as one of the richest ecosystems in terms of its biodiversity elements. Its floral components includes about 8000 species of angiosperm, 44 species of gymnosperm, 600 species of pteridophytes, 1737 species of bryophytes, 1159 species of lichens and 6,900 species of fungi with 40%, 15.91%, 25%, 32.53%, 11.22% and 27.38% endemics, respectively (D. K. Singh & Hajra, Citation1996; Mehta et al., Citation2020; Samant et al., Citation2007). According to Bargali et al. (Citation2022), IHR harbours about half of the angiosperms of India, with nearly 30% species as endemics. The region is considered significant for ecological security of India as it provides ecosystem services and benefits to millions of people residing within or outside the region, and is thus facing an enormous pressure (Dhyani & Dhyani, Citation2016).
Kashmir Himalaya is located in the extreme northwest of the IHR with varied geography and considerable area under forests, meadows and glaciers (G. H. Dar & Khuroo, Citation2020; Khuroo, Citation2015). Owing to its wide habitat heteriogenity, it is regarded as one of the ecologically complex and biologically diverse ranges within the Himalayan Biodiversity Hotspot (A. A. Dar & Parthasarathy, Citation2022; Haq et al., Citation2020). Owing to its topographical variations and habitat heterogeneity with a broad altitudinal gradient, the valley supports a rich floristic diversity (Mir et al., Citation2020). Despite of representing only 0.4% of the total geographic area of India, the region encompasses 12% of total angiosperms of India (G. H. Dar & Khuroo, Citation2013) signifying its rich floristic diversity. Like other parts of Himalayan region, biodiversity of Kashmir Himalaya is also undergoing serious alterations owing to various drivers of biodiversity loss. Over the decades, many plant species have become threatened due to habitat loss, habitat fragmentation, deforestation, introduction of invasive species, overexploitation, overgrazing, land-use change, huge tourist influx, and building of roads, coupled with political disturbances (A. R. Dar, Citation2008; Khuroo et al., Citation2018; Tali et al., Citation2019; Hamid et al., Citation2020; S. A. Dar, Bhat, Aneaus, et al., Citation2020 b, Mir et al., Citation2020). A growing number of regional, national, and international awareness campaigns and policy initiatives are being implemented in response to the loss of biodiversity worldwide (Kullberg & Moilanen, Citation2014). Keeping in view the importance and values of biological diversity, the CBD agreed in 2010 to have 17% of land covered by well-connected Protected Area (PA) Networks by 2020 (Saura et al., Citation2019). Most of the biodiversity-rich areas have been notified as protected areas to conserve the species, habitats, and ecosystems. The percentage of protected connected areas has increased globally from 6.5% in 2010 to 7.7% in 2018 (Saura et al., Citation2019). India has established a network of PAs including 104 National Parks, 566 Wildlife Sanctuaries, 97 Conservation Reserves and 214 Community reserves (https://wii.gov.in; http://wiienvis.nic.in/ assessed on 14th July, 2022). About 171, 921 km2 area of India is under the protected area network, which accounts for 5.03% of the country’s total geographic area. Gulmarg Wildlife Sanctuary (GWLS) proposed to be declared as Biosphere Reserve in 1984 was declared as sanctuary, vide notification no. S.R.O.147, Dated: 14 March 1987.Owing to its heart touching scenic beauty, GWLS is regarded as one of the renowned tourist destinations of Kashmir having huge influx of local, domestic as well as foreign tourists annually. However, detailed floristic studies of the GWLS have not been carried out so far except some reports and general papers on few particular aspects (D. A. Dar, Citation2018; Hamid, Citation2020; N. A. Bhat, Citation1984; Nanda et al., Citation2019; Naqshi et al., Citation1984; Saima et al., Citation2014). Thus, a comprehensive study of the floristic assessment and vegetation structure of the sanctuary is still lacking. To fulfill these lacunae, the present study was undertaken to assess the floristic diversity and community characteristics in the forest as well as the alpine zone of GWLS.
Materials and methods
Study area
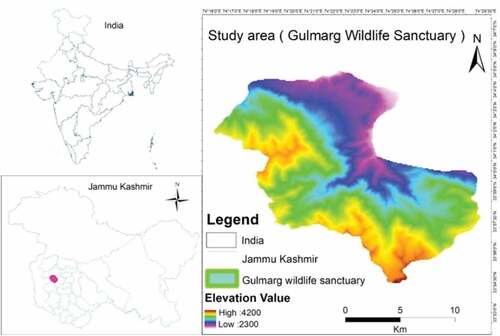
Gulmarg Wildlife Sanctuary (GWLS) falls 52 Kms to the South-West of District Baramulla, western side of Kashmir Himalaya on the Pir Panjal range, at an altitudinal range of 2300–4200 m a.m.s.l (). It extends up to an area of 180 km2 having the top catchment of Ferozpur Nallah and forests surrounding the Gulmarg Bowl. The sanctuary has two administrative units Ferozpora/Tangmarg and Block Gulmarg and is divided into 20 compartments numbering 31–41 (Lower elevation compartments) and 50–58 (Upper elevation compartments). Further, about 120 km2 area of GWLS falls in alpine while 60 km2 falls in lower area (www.jkwildlife.com, assessed on 15thApril, 2019). The climate of the study area is continental temperate type, with average precipitation about 1049 mm yr−1 (Hamid, Citation2020).
Selection of sites and habitats
Sites were selected along an elevational gradient in each and every accessible aspect and habitats from July 2018 to June 2022. Habitat of sites was identified on the basis of physical features and the dominant vegetation. The sites that had closed canopies with high humus and moisture content were considered as “shady moist habitats”, whereas the low content of humus and moisture as “dry habitats.” The sites that had>50% area as boulders were considered as “bouldary habitats,” whereas the sites that had>50 rocks were considered as “rocky.” In addition, open sites with sufficient moisture content were considered as “moist habitats.” The sites facing high anthropogenic pressures like deforestation, overgrazing, and trampling were considered as “degraded or exposed habitats.” Georeference and altitude of each site were recorded with the help of Global Positioning System (Garmin). Aspect of sites was recorded using a compass.
Surveys, sampling, and plant identification
Habit wise sampling of the plant species was carried out for the assessment of floristic diversity. The plant species composition of each selected site was studied by random sampling using quadrat method, as this method is less biased and most popularly followed (Bhatta et al., Citation2012). The size and number of quadrats were determined following (Kersaw, Citation1973; Misra, Citation1968). In the forest zone, a 50 m × 50 m plot was laid at each site. Within each plot, 10 random quadrats (10 m × 10 m) were laid for trees, 10 quadrats (5 m × 5 m) for shrubs and saplings and 20 quadrats (1 m × 1 m) for herbs and seedlings nested in the same plot following standard sampling methods (Curtis & Mcintosh, Citation1950; Misra, Citation1968; Rawal et al., Citation2018; Samant et al., Citation2002). Circumference at breast height (cbh at 1.37 m from ground) for each tree individual was measured. In the alpine zone, a 20 m × 20 m plot was laid at each site. Within each plot, 10 random quadrats (5 m × 5 m) were laid for shrubs and 20 quadrats (1 m × 1 m) for herbs (M. S. Rana & Samant, Citation2009). Vascular plant species encountered during the field surveys were collected, and tagged and brought to the laboratory and were mounted on herbarium sheets following standard herbarium techniques (B. Smith & Chinnappa, Citation2015; C. E. Smith, Citation1971; Jain & Rao, Citation1976). All the collected specimens were identified up to species level using local floras (Dhar & Kachroo, Citation1983; J. B. Singh & Kachroo, Citation1994; N. A. Bhat, Citation1984; N. P. Singh et al., Citation2002; Naqshi et al., Citation1984) and further authentication was done at Centre for Biodiversity and Taxonomy, University of Kashmir. Correct nomenclature and family to each identified plant species was assigned using the Plants of the World Online database (POWO 2022; https://powo.science.kew.org).
Quantitative analysis for delineation of plant communities
The data were analyzed for frequency, relative frequency, density, relative density, abundance, relative abundance, basal area, relative basal area and Importance Value Index, following standard ecological methods (Curtis & Mcintosh, Citation1950; Grieg-Smith, Citation1957; Kersaw, Citation1973; Misra, Citation1968; Muller-Dombois & Ellenberge, Citation1974; Samant et al., Citation2002).
Frequency and Relative Frequency: Frequency determines the degree of dispersion of individual species in an area and is expressed in terms of percentage (%) occurrence. It was calculated by the equation:
Relative frequency (RF) is the degree of species distribution in an area in relation to the frequency of all of the species. RF was calculated using the formula:
Density and Relative Density: Density determines the numerical strength of a species. It was calculated using the equation:
Relative Density (RD) is the measure of numerical strength of a species in relation to the total number of individual of a species in a unit area and was calculated using the formula:
Abundance and Relative Abundance: Abundance determines how commonly a species is distributed. It was calculated using the equation:
Relative Abundance (RA) is the ratio of the species to number of quadrat studied for given vegetation type and was calculated using the formula:
Basal Area (BA): BA determines the ground area actually covered by the stem of a tree. CBH of all individual trees was measured with a measuring tape and basal area was calculated using the formula:
where BA is Basal Area, CBH is the circumference at Breast Height and value of π is 3.1416
Total Basal Area (TBA) was calculated using the formula:
Relative Basal Area (RBA): It is the basal area covered by a species with respect to the sum of the area covered by rest of the species. It was calculated using the formula:
Importance Value Index: Importance Value Index (IVI) has been calculated as the sum of relative frequency, relative density and relative basal area. The abundance data of different sites were pooled to get community averages in terms of density, total basal area and IVI following Pant (Citation2005).
Communities were delineated on the basis of IVI in forest zone. The sites at which a single species contribute≥50% of the total IVI were categorized as pure communities of that species whereas the sites at which two or more species contribute≥50% of the total IVI were categorized as mixed communities of those species. However, in the alpine zone, communities were identified on the basis of relative density instead of IVI following M. S. Rana and Samant (Citation2009).
Species richness, species diversity (Hʹ) and concentration of dominance (Cd): Species richness was calculated as the total number of species within a community (Pant, Citation2005). Species diversity and concentration of dominance were determined following (Citation1963) and Simpson (Citation1949), respectively.
Shannon-Weaver index of species diversity was calculated using the equation
where H′ = Shannon’s information index of species diversity; ni = Total number of individuals of a particular species; and N = Total number of individuals of all the species.
The equation used to calculate concentration of dominance was
where Cd = concentration of dominance; ni = total number of individuals of particular species; and N = total number of individuals of all species.
Results
Site characteristics
A total of 123 sites have been sampled in the Gulmarg Wildlife Sanctuary (GWLS) between 2300 and 4200 m a.m.s.l (Table S1). Out of these, 63 sites were sampled in forest zone and 60 sites were sampled in alpine zone of the sanctuary. Within the forest zone, shady moist habitats represented the maximum sites (19), followed by dry habitats (13), degraded (11), bouldary (10), rocky (6) and riverine habitats (4). Within the alpine zone, bouldary habitats represented the maximum sites (24), followed by rocky (17), dry (8), riverine and moist (4 each) and degraded habitats (3) (). South aspect was represented by maximum sites (18) in the forest zone, followed by North and Northwest (13 each), Southeast (7) and East, Northeast, West and Southwest (3 each). In the alpine zone, Southeast aspect represented the maximum sites (18), followed by North (13), East (11), South (8), Northwest (6), Southwest (2) and Northeast and West aspects (1 each).
(A) Shady Moist; (B) Riverine; (C) Bouldary; (D) Rocky; (E) Degraded/Exposed; (F) Moist
Floristic diversity
The present study recorded 364 species of vascular plants belonging to 227 genera and 74 families, which includes angiosperms (62 families, 211 genera, 344 species), Gymnosperms (3 families, 6 genera, 6 species), and Pteridophytes (9 families, 10 genera, 14 species) (Table S2). Of the total species, 22 were trees, 34 shrubs, 290 herbs, and 14 ferns ().
Table 1. Taxonomic description of floristic diversity of GWLS, Kashmir Himalaya.
Among the angiospermic families, Asteraceae (58 spp.); Lamiaceae (21 spp.); Rosaceae (21 spp.); Ranunculaceae (18 spp.); Poaceae (17 spp.); Caryophyllacea (15 spp.), Brassicaceae (14 spp.), Polygonaceae (13 spp.), Fabaceae (11 spp.), Apiaceae (10 spp.), Plantaginaceae (9 spp.), Gentianaceae and Caprifoliaceae (8 spp., each), Papaveraceae and Boraginaceae (7 spp., each), Salicaceae and Berberidaceae (6 spp., each), Saxifragaceae, Primulaceae and Iridaceae (5 spp., each), and Violaceae, Ericaceae and Balsaminaceae (4 spp., each) were dominant. Among genera, Ranunculus (7 spp.), Anaphalis (6 spp.), Silene, Nepeta, Lonicera, Corydalis and Iris (5 spp., each), and Artemisia, Berberis, Cirsium, Geranium, Geum, Impatiens, Myosotis, Poa, Potentilla, Primula, Salix and Viola (4 sp., each) were dominant.
Among gymnosperms, the family Pinaceae (4 spp.) was dominant and Taxaceae and Cupressaceae was represented by single species, each. All the genera were represented by single species. Among pteridophytes, Pteridaceae (4 spp.), and Aspleniaceae (3 spp.) were dominant families. Athyriaceae, Cystopteridaceae, Polypodiaceae, Equisetaceae, Lycopodiaceae, Osmundaceae, and Dryopteridaceae were represented by single species, each. Among genera, Adiantum (4 spp.) and Asplenium (3 spp.) were dominant.
Community diversity, species composition, and structure
A total of 18 communities (10 within forest and 8 within alpine zone) have been identified from the GWLS between 2300 and 4200 m amsl altitudinal ranges ( & ). The forest communities were represented by evergreen coniferous communities (Abies pindrow, Pinus wallichiana, Abies pindrow-Pinus wallichiana mixed, Abies pindrow-Picea smithiana mixed, and Pinus wallichiana-Cedrus deodara mixed communities); evergreen coniferous-broad leaved mixed communities (Aesculus indica-Taxus wallichiana mixed, Aesculus indica-Pinus wallichiana mixed, Taxus wallichiana-Prunus cornuta-Aesculus indica mixed, and Abies pindrow-Acer caesium mixed communities) and broad leaved deciduous communities (Betula utilis community). The alpine zone communities were represented by “krumholz” (Viburnum grandiflorum, Salix denticulata, and Rhododendron campanulatum communities), scrubs (Juniper squamata, Rhododendron anthopogon, and Rhododendron anthopogon-Juniperus squamata mixed communities) and herbaceous communities (Bistorta affinis-Saussurea atkinsonii-Bergenia stracheyi mixed, and Bistorta affinis-Swertia petiolata mixed communities). The community types, representation, habitat(s), aspect(s), altitudinal ranges and major associates have been presented in .
Table 2. Community types, distribution, and major associates in GWLS, Kashmir Himalaya.
(A) Abies pindrow community; (B) Pinus wallichiana-Cedrus deodara mixed community; (C) Pinus wallichiana community; (D) Abies pindrow-Pinus wallichiana mixed community; (E) Abies pindrow-Picea smithiana mixed community (F) Betula utilis community
(A) Viburnum grandiflorum community; (B) Salix denticulata community; (C) Juniperus squamata community; (D) Rhododendron anthopogon community; (E) Bistorta affinis-Swertia petiolata mixed community
Forest communities: composition, structure, and regeneration pattern
Abies pindrow community
Abies pindrow community was represented at 19 sites with an altitudinal range of 2303–3050 m a.m.s.l. The representative habitats of the community includes Shady Moist, Dry, Bouldary, Rocky, Riverine, and Degraded in South, Southwest, Southeast, North, Northwest, Northeast, and East aspects. A total of 198 plant species (8 trees; 13 shrubs; 177 herbs) were recorded in this community. The total density and total basal area of trees were 185 Ind ha−1 and 158. 8 m2 ha−1, respectively. Abies pindrow was the dominant tree and Picea smithiana and Pinus wallichiana were the major tree associates. Total shrub density was 1830.0 Ind ha−1 and total herb density was 87.75 Ind m−2.
In this community, total sapling and seedling densities were 258 Ind ha−1 and 331 Ind ha−1, respectively. Among the saplings, Pinus wallichiana had the highest density (118.0 Ind ha−1) followed by Abies pindrow (53.0 Ind ha−1) and Taxus wallichiana (22.0 Ind ha−1). Among the seedlings, Abies pindrow had the highest density (105.0 Ind ha−1) followed by Pinus wallichiana (103.0 Ind ha−1) and Taxus wallichiana (65.0 Ind ha−1). Morus nigra and Prunus cornuta shows no regeneration while as Ulmus wallichiana and Salix alba shows new recruitments.
Pinus wallichiana community
Pinus wallichiana community was represented at six sites with an altitudinal range of 2315–2561 m a.m.s.l. The representative habitats of the community include Shady Moist, Bouldary, Riverine, and Degraded in South, Southeast, Northwest, and East aspects. A total of 125 species (10 trees; 12 shrubs; 103 herbs) were recorded in this community. The total density and total basal of trees were 245 Ind ha−1 and 124. 4 m2 ha−1, respectively. Pinus wallichiana was the dominant tree, and Abies pindrow and Picea smithiana were the major tree associates. Total shrub density was 1614.0 Ind ha−1 and total herb density was 46.75 Ind m−2.
In this community, total sapling and seedling densities were 400 Ind ha−1 and 575 Ind ha−1, respectively. Among the saplings, Pinus wallichiana had the highest density (226.6 Ind ha−1) followed by Abies pindrow (60.0 Ind ha−1) and Robinia pseudoacacia (33.3 Ind ha−1). Among the seedlings, Pinus wallichiana had the highest density (229.1 Ind ha−1) followed by Abies pindrow (37.5 Ind ha−1) and Aesculus indica (62.5 Ind ha−1). Picea smithiana shows poor regeneration while as Corylus colurna, Juglans regia, and Populus ciliata show no regeneration. Aesculus indica, Cedrus deodara, Ailanthus altissima, Prunus cornuta, and Quercus leucotrichophora show new recruitments.
Abies pindrow-Pinus wallichiana mixed community
Abies pindrow-Pinus wallichiana mixed community was represented at nine sites with an altitudinal range of 2335–2714 m a.m.s.l. The representative habitats of the community include Shady Moist, Bouldary, Dry and Degraded in South and Northwest aspects. A total of 114 species (07 trees; 08 shrubs; 99 herbs) were recorded in this community. The total density and total basal of trees were 384 Ind ha−1 and 112. 6 m2 ha−1. Abies pindrow was the dominant tree, and Pinus wallichiana and Taxus wallichiana were the major tree associates. Total shrub density was 2882.0 Ind ha−1 and total herb density was 47.05 Ind m−2.
In this community, total sapling and seedling densities were 900 Ind ha−1 and 670 Ind ha−1, respectively. Among the saplings, Pinus wallichiana had the highest density (310.0 Ind ha−1) followed by Cedrus deodara (175.0 Ind ha−1) and Abies pindrow (140.0 Ind ha−1). Among the seedlings, Pinus wallichiana had the highest density (218.7 Ind ha−1) followed by Abies pindrow (101.2 Ind ha−1) and Taxus wallichiana (100.0 Ind ha−1). Quercus robur shows no regeneration while as Aesculus indica shows new recruitment.
Pinus wallichiana-Cedrus deodara mixed community
Pinus wallichiana-Cedrus deodara mixed community was represented at four sites with an altitudinal range of 2370–2424 m a.m.s.l. The representative habitats of the community include Shady Moist, Dry and Degraded in South and North aspects. A total of 100 species (09 trees; 09 shrubs; 82 herbs) were recorded in this community. The total density and total basal of trees were 505.0 Ind ha−1 and 49.3 m2 ha−1. Pinus wallichiana was the dominant tree and Cedrus deodara and Abies pindrow were the major tree associates. Total shrub density was 1112.0 Ind ha−1 and total herb density was 38.18 Ind m−2.
In this community, total sapling and seedling densities was 1450 Ind ha−1 and 866 Ind ha−1, respectively. Among the saplings, Cedrus deodara had the highest density (450.0 Ind ha−1) followed by Pinus wallichiana (300.0 Ind ha−1) and Taxus wallichiana (166.6 Ind ha−1) and Aesculus indica (160.0 Ind ha−1). Among the seedlings, Cedrus deodara had the highest density (271.6 Ind ha−1) followed by Pinus wallichiana (158.3 Ind ha−1) and Abies pindrow (150.0 Ind ha−1). Juglans regia shows poor regeneration while as Ulmus wallichiana shows new recruitment.
Abies pindrow-Picea smithiana mixed community
Abies pindrow-Picea smithiana mixed community was represented at four sites with an altitudinal range of 2540–2686 m a.m.s.l. The representative habitats of the community include Shady Moist, Dry and Degraded in South, Northwest and Northeast aspects. A total of 92 species (06 trees; 07 shrubs; 79 herbs) were recorded in this community. The total density and total basal area of trees were 328.0 Ind ha−1 and 40.78 m2 ha−1. Abies pindrow was the dominant tree and Picea smithiana and Pinus wallichiana were the major tree associates. Total shrub density was 2660.0 Ind ha−1 and total herb density was 41.35 Ind m−2.
In this community, total sapling and seedling densities were 468 Ind ha−1 and 447 Ind ha−1, respectively. Among the saplings, Taxus wallichiana had the highest density (131.4 Ind ha−1) followed by Abies pindrow (120.0 Ind ha−1) and Pinus wallichiana (102.8 Ind ha−1). Among the seedlings, Abies pindrow had the highest density (230 Ind ha−1) followed by Pinus wallichiana (128.5 Ind ha−1) and Taxus wallichiana (67.86 Ind ha−1). Cedrus deodara and Picea Smithiana show poor regeneration, whereas Salix alba shows new recruitments.
Aesculus indica-Pinus wallichiana mixed community
Aesculus indica-Pinus wallichiana mixed community was represented at three sites with an altitudinal range of 2416–2452 m a.m.s.l. The representative habitats of the community include Shady Moist in North and West aspects. A total of 44 species (05 trees; 03 shrubs; 36 herbs) were recorded in this community. The total density and total basal of trees were 700.0 Ind ha−1 and 106.6 m2 ha−1. Aesculus indica was the dominant tree, and Pinus wallichiana and Cedrus deodara were the major tree associates. Total shrub density was 2040.0 Ind ha−1 and total herb density was 23.77 Ind m−2.
In this community, total sapling and seedling densities were 1046.0 Ind ha−1 and 852.0 Ind ha−1, respectively. Among the saplings, Pinus wallichiana had the highest density (400.0 Ind ha−1) followed by Abies pindrow (226.6 Ind ha−1) and Aesculus indica (177.3 Ind ha−1). Among the seedlings, Aesculus indica had the highest density (333.0 Ind ha−1) followed by Taxus wallichiana (186.6 Ind ha−1) and Abies pindrow (180.0 Ind ha−1).
Aesculus indica-Taxus wallichiana mixed community
Aesculus indica-Taxus wallichiana mixed community was represented at two sites with an altitudinal range of 2466–2484 m a.m.s.l. The representative habitats of the community include Shady Moist and Degraded in Northwest and East aspects. A total of 56 species (07 trees; 05 shrubs; 44 herbs) were recorded in this community. The total density and total basal area of trees were 750.0 Ind ha−1 and 19.62 m2 ha−1. Aesculus indica was the dominant tree, and Taxus wallichiana and Cedrus deodara were the major tree associates. Total shrub density was 4020.0 Ind ha−1 and total herb density was 32.15 Ind m−2.
In this community, total sapling and seedling densities were 580.0 Ind ha−1 and 638.0 Ind ha−1, respectively. Among the saplings, Taxus wallichiana had the highest density (320.0 Ind ha−1) followed by Aesculus indica (140.0 Ind ha−1) and Cedrus deodara (120.0 Ind ha−1). Among the seedlings, Cedrus deodara had the highest density (250.0 Ind ha−1) followed by Taxus wallichiana (200.0 Ind ha−1) and Aesculus indica (175.0 Ind ha−1). Aesculus indica shows poor regeneration; Abies pindrow, Juglans regia, Platanus orientalis, and Prunus cornuta shows no regeneration and Acer caesium shows new recruitment.
Taxus wallichiana-Prunus cornuta-Aesculus indica mixed community
Taxus wallichiana-Prunus cornuta-Aesculus indica mixed community was represented at two sites with an altitudinal range of 2457–2519 m a.m.s.l. The representative habitats of the community include Shady Moist in North and West aspects. A total of 44 species (05 trees; 04 shrubs; 35 herbs) were recorded in this community. The total density and total basal of trees were 521.0 Ind ha−1 and 38.06 m2 ha−1. Taxus wallichiana was the dominant tree and Prunus and Aesculus indica were the major tree associates. Total shrub density was 1376.0 Ind ha−1 and total herb density was 34.15 Ind m−2.
In this community, total sapling and seedling densities were 416.0 Ind ha−1 and 450.0 Ind ha−1, respectively. Among the saplings, Aesculus indica had the highest density (168.0 Ind ha−1) followed by Taxus wallichiana (136.0 Ind ha−1) and Prunus cornuta (72.0 Ind ha−1). Among the seedlings, Pinus wallichiana had the highest density (150.0 Ind ha−1) followed by Aesculus indica (120.0 Ind ha−1) and Cedrus deodara (100.0 Ind ha−1). Taxus wallichiana and Prunus cornuta shows poor regeneration.
Abies pindrow-Acer caesium mixed community
Abies pindrow-Acer caesium mixed community was represented at five sites with an altitudinal range of 2719–2880 m a.m.s.l. The representative habitats of the community include Shady Moist, Dry and Riverine in North, West and Southwest aspects. A total of 101 species (06 trees; 06 shrubs; 89 herbs) were recorded in this community. The total density and total basal area of trees were 316.0 Ind ha−1 and 77.1 m2 ha−1. Abies pindrow was the dominant tree and Acer caesium and Picea smithiana were the major tree associates. Total shrub density was 1435.0 Ind ha−1 and total herb density was 43.14 Ind m−2.
In this community, total sapling and seedling densities were 205.0 Ind ha−1 and 110.0 Ind ha−1, respectively. Among the saplings, Acer caesium had the highest density (70.0 Ind ha−1) followed by Abies pindrow (45.0 Ind ha−1) and Pinus wallichiana (35.0 Ind ha−1). Among the seedlings, Acer caesium had the highest density (55.0 Ind ha−1) followed by Taxus wallichiana (30.0 Ind ha−1) and Abies pindrow (20.0 Ind ha−1). Abies pindrow and Acer caesium show poor regeneration, Picea smithiana shows no regeneration and Cedrus deodara shows new recruitment.
Betula utilis community
Betula utilis community was represented at nine sites with an altitudinal range of 3190–3576 m a.m.s.l. The representative habitats of the community include Bouldary and Rocky in Northwest, and Southeast aspects. A total of 57 species (04 trees; 07 shrubs; 46 herbs) were recorded in this community. The total density and total basal area of trees were 810.0 Ind ha−1 and 23.6 m2 ha−1. Betula utilis was the dominant tree, and Abies pindrow and Pinus wallichiana were the major tree associates. Total shrub density was 886.0 Ind ha−1 and total herb density was 27.79 Ind m−2.
In this community, total sapling and seedling densities were 540.0 Ind ha−1 and 320.0 Ind ha−1, respectively. Among the saplings, Betula utilis had the highest density (413.6 Ind ha−1) followed by Abies pindrow (88.7 Ind ha−1) and Pinus wallichiana (78.0 Ind ha−1). Among the seedlings, Betula utilis had the highest density (198.0 Ind ha−1) followed by Pinus wallichiana (88.6 Ind ha−1) and Abies pindrow (23.4 Ind ha−1). Betula utilis and Abies pindrow show poor regeneration; Pinus wallichiana shows good regeneration and Acer caesium shows new recruitments.
Alpine communities: structure and composition
Viburnum grandiflorum community
Viburnum grandiflorum community was represented at seven sites with an altitudinal range of 3025–3190 m a.m.s.l. The representative habitats of the community include Bouldary, Rocky, and Exposed in South, Southeast, and East aspects. A total of 93 species (8 shrubs; 85 herbs) were recorded in this community. Total shrub density was 1410.0 Ind ha−1 and total herb density was 79.13 Ind m−2.
Salix denticulata community
Salix denticulata community was represented at two sites with an altitudinal range of 3170–3195 m a.m.s.l. The representative habitats of the community include Bouldary and Rocky in North aspects. A total of 50 species (8 shrubs; 42 herbs) were recorded in this community. Total shrub density was 5540.0 Ind ha−1 and total herb density was 50.84 Ind m−2.
Rhododendron campanulatum community
Rhododendron campanulatum community was represented at four sites with an altitudinal range of 3187–3246 m a.m.s.l. The representative habitats of the community include Bouldary and Moist in North and Northwest aspects. A total of 60 species (5 shrubs; 55 herbs) were recorded in this community. Total shrub density was 2440.0 Ind ha−1 and total herb density was 49.2 Ind m−2.
Juniperus squamata community
Juniperus squamata community was represented at six sites with an altitudinal range of 3280–3728 m a.m.s.l. The representative habitats of the community include Bouldary and Rocky in North and Southeast aspects. A total of 46 species (5 shrubs; 41 herbs) were recorded in this community. Total shrub density was 4020.0 Ind ha−1 and total herb density was 64.9 Ind m−2.
Rhododendron anthopogon community
Rhododendron anthopogon community was represented at nine sites with an altitudinal range of 3754–4196 m a.m.s.l. The representative habitats of the community include Bouldary, Rocky and Dry in North, East, Northwest, and Southeast aspects. A total of 31 species (3 shrubs; 28 herbs) were recorded in this community. Total shrub density was 2560.0 Ind ha−1 and total herb density was 70.74 Ind m−2.
Bistorta affinis-Saussurea atkinsonii-Bergenia stracheyi mixed community
Bistorta affinis-Saussurea atkinsonii-Bergenia stracheyi mixed community was represented at six sites with an altitudinal range of 3640–3810 m a.m.s.l. The representative habitats of the community include Riverine and Moist in East, Southeast, and Northeast aspects. A total of 35 herbaceous species were recorded in this community. The total herb density was 49.34 Ind m−2.
Rhododendron anthopogon-Juniperus squamata mixed community
Rhododendron anthopogon-Juniperus squamata mixed community was represented at 21 sites with an altitudinal range of 3435–4184 m a.m.s.l. The representative habitats of the community include Riverine, Rocky, Bouldary, and Dry in East, Southeast, South, Southwest, North and Northwest aspects. A total of 92 species (7 shrubs; 85 herbs) were recorded in this community. Total shrub density was 4431.0 Ind ha−1 and total herb density was 102.2 Ind m−2.
Bistorta affinis-Swertia petiolata mixed community
Bistorta affinis-Swertia petiolata mixed community was represented at five sites with an altitudinal range of 3825–4140 m a.m.s.l. The representative habitats of the community include Dry and Bouldary in South and Southeast aspects. A total of 26 herbaceous species were recorded in this community. The total herb density was 31.73 Ind m−2.
Species diversity (Hʹ)
Amongst the forest communities, species diversity for trees ranged from 0.88–1.67, saplings, 0.74–1.85, seedlings, 0.83–1.84, shrubs, 0.27–1.82 and herbs, 3.29–4.81. Highest diversity of trees was recorded in Aesculus indica-Taxus wallichiana mixed community (1.67), and lowest in Betula utilis community (0.88). Species diversity of saplings was recorded highest in Pinus wallichiana-Cedrus deodara mixed community (1.85), and lowest in Betula utilis community (0.74). Species diversity of seedlings was recorded highest in Pinus wallichiana-Cedrus deodara mixed community (1.84), and lowest in Betula utilis community (0.83). Species diversity of shrubs was recorded highest in Betula utilis community (1.82), and lowest in Aesculus indica-Pinus wallichiana mixed community (0.27). Species diversity of herbs was recorded highest in Abies pindrow community (4.81), and lowest in Aesculus indica-Pinus wallichiana mixed community (3.29). Community wise species diversity of trees, saplings, seedlings, shrubs and herbs in forest communities is presented in .
Table 3. Community wise species diversity (Hʹ) of trees, saplings, seedlings, shrubs and herbs in GWLS, Kashmir Himalaya.
Amongst the alpine communities, species diversity of shrubs ranged from 0.73 to 1.33 and species diversity of herbs ranged from 2.21 to 3.69. Species diversity of shrubs was recorded highest in Juniperus squamata community (1.33), and lowest in Rhododendron anthopogon community (0.73). Species diversity of herbs was recorded highest in Viburnum grandiflorum community (3.69), and lowest in Bistorta affinis-Swertia petiolata mixed community (2.21). Community wise species diversity of trees, saplings, seedlings, shrubs and herbs in alpine communities is presented in .
Concentration of dominance (Cd)
Amongst the forest communities, Cd for trees ranged from 0.22–0.56, saplings, 0.18–0.61, seedlings, 0.18–0.49, shrubs, 0.19–0.85, and herbs, 0.009–0.045. Cd for trees was recorded highest in Pinus wallichiana community (0.56), and lowest in Aesculus indica-Taxus wallichiana mixed community (0.22). Cd for saplings was recorded highest in Betula utilis community (0.61), and lowest in Pinus wallichiana-Cedrus deodara mixed community (0.18). Cd for seedling was recorded highest in Betula utilis community (0.49), and lowest in Pinus wallichiana-Cedrus deodara mixed and Abies pindrow-Pinus wallichiana mixed community (0.18 each). Cd for shrubs was recorded highest in Aesculus indica-Pinus wallichiana mixed community (0.85), and lowest in Betula utilis community (0.19). Cd for herbs was recorded highest in Betula utilis community (0.045), and lowest in Abies pindrow community (0.009). Community wise Concentration of Dominance (Cd) of shrubs and herbs in forest communities has been presented in .
Table 4. Community wise concentration of dominance (Cd) of trees, saplings, seedlings, shrubs and herbs in GWLS, Kashmir Himalaya.
Amongst the alpine communities, Cd of shrubs and herbs ranged from 0.32–0.65 and 0.04–0.22, respectively. Cd of shrubs was recorded highest in Viburnum grandiflorum (0.65), and lowest in Juniperus squamata (0.32). Cd of herbs was recorded highest in Bistorta affinis-Swertia petiolata mixed (0.22) and lowest in Viburnum grandiflorum (0.04). Community wise Cd of shrubs and herbs in alpine communities has been presented in .
Discussion
The present study presents first-hand information on the floristic and community diversity of the sanctuary. The sanctuary embodies broad elevation range with complex topography, providing a large variation in climate and other variables to harbour higher species richness in reasonable lesser area. During the study, 364 plant species, including 344 angiosperms, 06 gymnosperms and 14 pteridophytes along with 18 plant communities (10 forest and 08 alpine communities) were reported from the sanctuary. Number of species recorded during the present study was higher than earlier reports from Kashmir Himalaya. A. H. Malik et al. (Citation2015) reported 285 plant species from Warwan Valley, Jammu and Kashmir; J. A. Dar and Sundarapandian (Citation2016) reported 177 plant species from two forest divisions (Anantnag and Lidder) of Kashmir Himalaya; Haq et al. (Citation2019, Citation2021) reported 181 and 183 plant species from Keran valley and Dachigam National Park of Kashmir Himalaya, respectively. Reported number of plant species was also higher from other parts of Himalaya region like Bhabha Valley (Chawla et al., Citation2008), Naran Valley, Pakistan (S. M. Khan et al., Citation2011), Dudhatoli forests of Garhwal Himalaya (Ghildiyal & Gairola, Citation2013), Sangla Valley (Sharma et al. 2014), Dhauladhar Mountains (Ahmad et al., Citation2020), Kedarnath Wildlife Sanctuary (J. A. Bhat et al., Citation2020) and Nanda Devi Biosphere Reserve (Maletha et al., Citation2022). However, the number of species reported was lower than reported by M. S. Rana and Samant (Citation2010) from Manali Wildlife Sanctuary, Lal and Samant (Citation2015 and Lal & Samant, Citation2019) from Kais Wildlife Sanctuary, N. Sharma et al. (Citation2019) from Eastern Himalaya, and Das et al. (Citation2020) from Great Himalayan National Park of IHR. The dominant families include Asteraceae (58 spp.), Lamiaceae (21 spp.), Rosaceae (21 spp.), Ranunculaceae (18 spp.), Poaceae (17 spp.), Caryophyllacea (15 spp.), Brassicaceae (14 spp.), Polygonaceae (13 spp.), Fabaceae (11 spp.), and Apiaceae (10 spp.). Dominance of these families in Kashmir Himalaya has been reported by other workers also (Altaf et al., Citation2022; G. H. Dar & Khuroo, Citation2013; G. H. Dar et al., Citation2007). These plant families have been reported to be dominant in other parts of Himalayan region also (D. K. Singh & Pusalkar, Citation2020; D. S. Rawat et al., Citation2013; Gairola et al., Citation2009; Samant et al., Citation1998; Shaheen et al., Citation2012). Due to the larger ecological amplitudes and wide distributions of these plant families, possibilities of encountering the members of these families increase (Mumshad et al., Citation2021). Dominance of herbaceous growth forms in the present study is in accordance with the several other studies conducted in other parts of IHR (B. Rawat et al., Citation2021; Haq et al., Citation2021; N. Sharma et al., Citation2019; Samant et al., Citation2007; Z. A. Malik & Bhatt, Citation2015). This can be explained by the fact that herbs are considered to be common growth forms in most of the mountainous region because of their capacity to adapt to a wide range of environmental conditions (Ahmad et al., Citation2021). Further, the continental temperate climate of Himalayan region favours predominance of herbaceous elements rather than arboreal ones, as it is true for sub-tropical and tropical climates (Mehraj et al., Citation2018).
Community structure
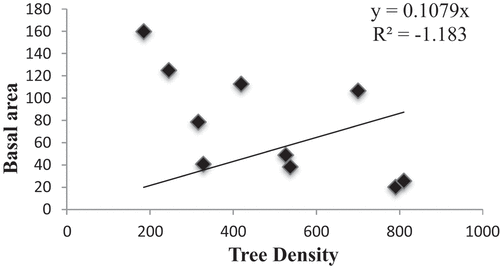
Amongst the identified forest communities, tree density ranged from 185 to 810 Ind ha−1 which is comparable with other studies from different parts of IHR; Adhikari et al. (Citation1991) from Kumaun Himalaya; H. C. Joshi (Citation2002) from Nanda Devi Biosphere Reserve; Samant et al. (Citation2002) from Nanda Devi Biosphere Reserve; M. S. Rana (Citation2007) from Manali Wildlife Sanctuary; A. Sharma (Citation2008) from Hirb and Shoja Catchments; and J. A. Dar and Sundarapandian (Citation2016) from temperate forests of Kashmir Himalaya. Total basal area ranged from 20.28 to 159.8 m2 ha−1 which is comparable with Kunwar and Sharma (Citation2004) from Dolpha district, mid-west Nepal; C. S. Rana and Gairola (Citation2009) from ParshuramKund area, Arunachal Pradesh; Hanief et al. (Citation2016) from Darhal Watershed, Jammu and Kashmir, but was higher than Gairola et al. (Citation2011) from Garhwal Himalaya; Raturi (Citation2012) from Rudrapryag of Garhwal Himalaya; J. A. Dar and Sundarapandian (Citation2016) from temperate forests of Kashmir Himalaya; D. A. Dar and Sahu (Citation2018) from temperate forests of northern Kashmir Himalaya; and D. A. Dar (Citation2018) from Gulmarg Forest range. Total basal area was highest (159.8 m2 ha−1) in Abies pindrow community which is comparable with 124.3 m2 ha−1, reported by Das et al. (Citation2021), from Abies pindrow dominated forests in western Himalaya. There is a negative correlation between basal area and density (−0.65, p < 0.01, R2 = 1.18) ().
Density of shrubs in forest zone ranges from 886–2040 Ind ha−1 which is comparable with A. Sharma (Citation2008) (630–2470 Ind ha−1) from Hirb and Shoja Catchments, Sakshi (Citation2009) (460–2180 Ind ha−1) from central parts of Himachal Pradesh, and A. Singh (Citation2007) (480–2611 Ind ha−1) from western Himalaya. Total herb density ranged from 27.79 to 87.75 Ind m−2 which is again comparable with A. Kumar (Citation2020) from Kalatop-Khajjiar Wildlife Sanctuary, Himachal Pradesh, but lower than H. C. Joshi (Citation2002) from Nanda Devi Biosphere Reserve of West Himalaya, H. C. Joshi and Samant (Citation2004) Nanda Devi Biosphere Reserve, West Himalaya and Pant (Citation2005) from Mornaula Reserve Forest, Kumaun. Amongst the alpine communities, the total density of shrubs ranged from 1410.0 to 5540 Ind ha−1 which is comparable to M. S. Rana and Samant (Citation2009) (880–6800 Ind ha-1) from Manali Wildlife Sanctuary and is higher than M. S. Rana et al. (Citation2011) (341–1200 Ind ha−1) from northwestern Himalaya. Total herb density ranged from 31.73 to 102.2 Ind m−2 which is lower than G. S. Rawat and Adhikari (Citation2005) (28–744 Ind m−2) from Changthang plateau, eastern Ladakh, M. S. Rana and Samant (Citation2009) (149–327.1 Ind m−2) from Manali Wildlife Sanctuary and M. S. Rana et al. (Citation2011) (114–218 Ind m−2) from alpine area of the northwestern Himalaya.
Regeneration patterns
Regeneration status of tree species of any forest type may be determined with the help of recruitment of saplings and seedlings. When there are many seedlings, saplings, and mature trees in a forest, the regeneration is good or satisfactory; when there are few or no seedlings or saplings, the regeneration is poor; and when there are no seedlings or saplings at all, there is no regeneration (Akash et al., Citation2020; Wani et al., Citation2022). In GWLS, the number of plant species in seedling and sapling layers varied from identified community to community. Based on the recruitment of saplings and seedlings, the communities can be categorized into following types:
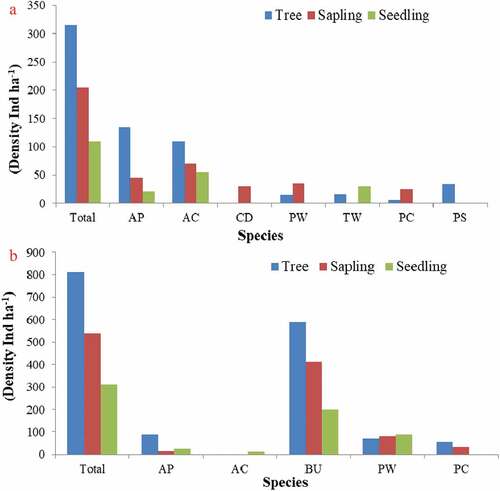
The communities having highest regeneration of the dominant plant species particularly in sapling layer followed by other species (). The communities of this category were Pinus wallichiana community, and Abies pindrow-Picea smithiana mixed community. Good regeneration status of dominant tree species in the communities was due to the well-established seedling, saplings, and mature trees, which reveals that these communities may be sustained in future unless there is any major environmental interference due to anthropogenic activities.
Figure 6. Forest communities having highest regeneration of the dominant plant species (A) Pinus wallichiana community; (B) Abies pindrow-Picea smithiana mixed community.
Abbreviations used: AA = Ailanthus altissima; AC = Acer caesium; AI = Aesculus indica; AP = Abies pindrow; CA = Celtis australis; CC = Corylus colurna; CD = Cedrus deodara; JR = Juglans regia; PS = Picea smithiana; PW = Pinus wallichiana; Pci = Populus ciliata; PC = Prunus cornuta; QL = Quercus leucotrichophora; RP = Robinia pseudoacacia; SA = Salix alba; TW = Taxus wallichiana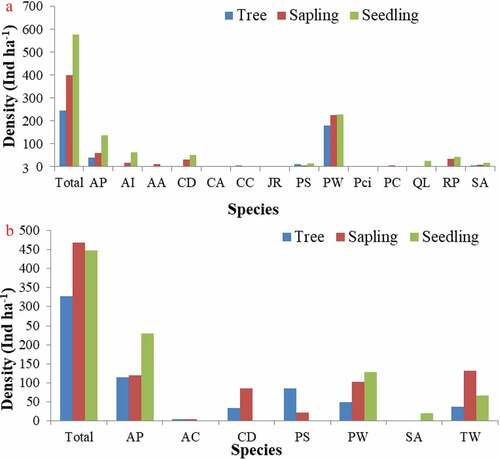
The communities having sufficient regeneration of dominant species but highest regeneration was found in co-dominant plant species particularly in the sapling layer (). The communities in this category were Abies pindrow community, Abies pindrow-Pinus wallichiana mixed, Pinus wallichiana-Cedrus deodara mixed, Aesculus indica-Pinus wallichiana community, Aesculus indica-Taxus wallichiana mixed community and Taxus wallichiana-Prunus cornuta-Aesculus indica mixed. The co-dominant species within these communities may replace the dominant species in coming future.
Figure 7. Forest communities having sufficient regeneration of dominant species but highest regeneration in co-dominant plant species(a) Abies pindrow community; (B) Abies pindrow-Pinus wallichiana mixed community (C) Pinus wallichiana-Cedrus deodara mixed community (D) Aesculus indica-Pinus wallichiana mixed community (E) Aesculus indica-Taxus wallichiana mixed community (F) Taxus wallichiana-Prunus cornuta-Aesculus indica mixed community.
Abbreviations used: AA = Ailanthus altissima; AC = Acer caesium; AI = Aesculus indica; AP = Abies pindrow; CD = Cedrus deodara; JR = Juglans regia; MN = Morus nigra; PC =Prunus cornuta; PO = Platanus orientalis; PS = Picea smithiana; PW = Pinus wallichiana; QL = Quercus leucotrichophora; QR = Quercus robur; SA = Salix alba; SB = Salix babylonica; TW= Taxus wallichiana; UW= Ulmus wallichiana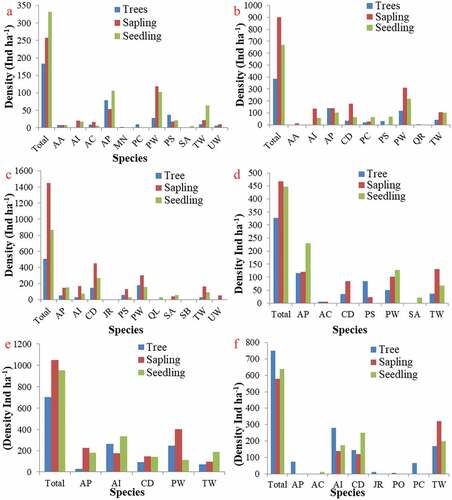
The communities having poor or no regeneration of dominant species were Abies pindrow-Acer caesium mixed and Betula utilis community (). Presently dominant species within these communities may likely be replaced by other plant species. Further, survival, growth and reproduction ability of the plant species showing poor or no regeneration may be at risk in future.
Species richness, species diversity (Hʹ) and concentration of dominance (Cd)
Within the identified forest communities, species richness ranged from 44 to 198. Species richness of trees ranged from 4 to 10 which is comparable with other studies conducted by A. A. Dar and Parthasarathy (Citation2022) (5–10), J. A. Dar and Sundarapandian (Citation2016) (2–7), D. A. Dar (Citation2018) (2–7), but is lower than C. S. Rana and Gairola (Citation2009) (13–26). In GWLS, species richness of shrubs ranged from 3–13 which is again comparable with C. S. Rana and Gairola (Citation2009) (10–12), and J. A. Dar and Sundarapandian (Citation2016) (3–9). Species richness of herbs ranged from 35 to 177 which is comparable with B. Rawat et al. (Citation2015) (18–107) from Nanda Devi Biosphere Reserve and higher than J. A. Dar and Sundarapandian (Citation2016) (20–84) from temperate forests of Kashmir Himalaya. Within the alpine communities, species richness ranged from 26 to 93 which is higher than Rahman et al. (Citation2021) (14–27) from alpine pastures and cold desert of northwestern Himalaya, Pakistan. Species richness of shrubs ranged from 3 to 8. Species richness of herbs ranged from 26 to 85 which is comparable with Sherman et al. (Citation2008) (19–105) from Hengduan mountains, China but is higher than G. S. Rawat and Adhikari (Citation2005) (4–15) from Tso Kar basin, Changthang plateau, eastern Ladakh. Higher species richness in alpine communities of GWLS may be due to the dominance of cushion forming shrubs like Rhododendron anthopogon and Juniperus squamata which act as micro-refuges for plant species in alpine areas and thus frequently increases species richness (Molenda et al., Citation2012).
Within the forest zone, diversity of trees (Hʹ) ranged from 0.88–1.67 which is comparable with Ajaz et al. (Citation2022) (1.37–160) from Overa Wildlife Sanctuary, but higher than D. A. Dar (Citation2018) (0.13–0.81) from Gulmarg Forest Range. The higher diversity in the present study may be due to the habitat wise comprehensive sampling and higher number of sampling sites. Diversity of trees is however comparable with Kumar (2018) (0.31–1.43), J. A. Dar and Sundarapandian (Citation2016) (0.17–1.06), C. M. Sharma et al. (Citation2010) (0.28–1.75), (Citation1994) (0.4–2.8) and is lower than Malik and Bhatt (Citation2015) (2.30–3.53), and J. A. Bhat et al. (Citation2020) (2.09–3.37) from other parts of Indian Himalayan Region. Diversity of shrubs ranged from 0.27 to 1.82 which is again comparable with Adhikari et al. (Citation1991) (0.05–1.33) from Kumaun Himalaya, Pokhriyal et al. (Citation2009) (0.67–0.76) from Phakot and Pathri Rao watersheds of Garhwal Himalaya and J. A. Dar and Sundarapandian (Citation2016) (0.53–1.22) from seven temperate forest types of Western Himalaya. However, diversity of shrubs is lower than Malik and Bhatt, (Citation2015) (2.74–3.78) and J. A. Bhat et al. (Citation2020) (2.62–4.20) from Kedarnath Wildlife Sanctuary. Diversity of herbs ranged from 3.29 to 4.81 which is higher than J. A. Dar and Sundarapandian (Citation2016) (0.53–1.22) from seven temperate forest types of Western Himalaya. Within the alpine communities, species diversity of shrubs ranged from 0.73 to 1.33, which is lower than Tambe and Rawat (Citation2010) (1.62–2.39) from Khangchendzonga landscape, Sikkim Himalaya. Species diversity of herbs among alpine communities ranged from 2.21 to 3.69 which is comparable with Rahman et al. (Citation2021) (2.075–2.785) from alpine pastures of Northwest Himalaya, Pakistan and Tambe and Rawat (Citation2010) (1.44–2.48) from Khangchendzonga landscape, Sikkim Himalaya; however the values are higher than Vashistha et al. (Citation2012) (0.070–0.532) from alpine ecosystem of north-west Himalaya and G. S. Rawat and Adhikari (Citation2005) (0.143–1.679) from Tso Kar basin, Changthang plateau, eastern Ladakh. Within forest communities, concentration of dominance (Cd) of trees ranged from 0.22 to 0.56 which is comparable with J. A. Dar and Sundarapandian (Citation2016) (0.36–0.94), Gairola et al. (Citation2009) (0.129–0.467) and V. Negi et al. (Citation2022) (0.27–0.75). However, the values are higher than Malik and Bhatt (Citation2015) (0.06–0.1) and (Citation2009) (0.10–0.20) and lower than earlier reported values (0.63–0.94) from Gulmarg Forest Range (D. A. Dar, Citation2018). In the present study, sub-alpine tree community (Betula utilis community) has lower Hʹ values and higher Cd which may be due to dominance of a single tree species, as a high Cd value indicates dominance of a single species and thus a low diversification of communities (Connell & Orias, Citation1964). Cd of shrubs ranged from 0.19–0.85 which is again comparable with J. A. Dar and Sundarapandian (Citation2016) (0.43–0.75) from seven temperate forest types of Western Himalaya but higher than Malik and Bhatt (Citation2015) (0.08–0.19) from Kedarnath Wildlife Sanctuary. Cd of herbs (0.009–0.45) is again comparable with J. A. Dar and Sundarapandian (Citation2016) (0.08–0.35). Dominance of evergreen forests in temperate zones of Himalayas is due to the cold and frost prone climate that favours emergence and development of evergreen vegetation (Saxena & Singh, Citation1982). However, temperate forests generally have higher Cd in comparison to other forest types and may be due to lower evolution, diversification rate and environmental severity (Connell, Citation1978). Within the alpine zone, Cd of shrubs ranged from 0.32 to 0.65 which is lower than Tambe and Rawat (Citation2010) (0.69–0.89) from Khangchendzonga landscape, Sikkim Himalaya. Cd of herbs ranged from 0.04 to 0.22 which is comparable with Vashistha et al. (Citation2012) (0.0001–0.277) alpine ecosystem of north-west Himalaya, and lower than Tambe and Rawat (Citation2010) (0.72–0.91) from Khangchendzonga landscape, Sikkim Himalaya and Rahman et al. (Citation2021) (0.822–0.895) from the alpine pastures of northwestern Himalaya, Pakistan.
The Indian Himalayan Region (IHR) is known for the representative, natural, unique, ecologically and economically important plant diversity (Pant, Citation2005). This may be due to unique topography of the region supporting diverse habitats, species, communities, and ecosystems. In general, different forest and alpine communities have been reported by various workers from sub-tropical, temperate, sub-alpine, and alpine zones in different parts of Himalaya (M. Kumar et al., Citation2004; Mehmood et al., Citation2021; Ram et al., Citation2004; Rawal et al., Citation1994, Citation2003; S. M. Khan et al., Citation2013). However, most of the studies have been carried out in transacts along an altitudinal gradient without considering habitat(s) for vegetation sampling except a few studies (Arya, Citation2002; H. C. Joshi & Samant, Citation2004; H. C. Joshi, Citation2002; Kala & Mathur, Citation2002; M. S. Rana et al., Citation2011; Pant & Samant, Citation2012; Pant, Citation2005) which deals with the habitat wise sampling of the vegetation. Further, it has been found that studies along transects without considering the habitat(s) for vegetation sampling do not cover the total number of species occurring in the area. Therefore, habitat-wise quantification of the vegetation has been considered as an appropriate method to understand the actual status of the vegetation (H. C. Joshi & Samant, Citation2004; Samant et al., Citation2002).
Conclusion
The present study conducted in the Gulmarg Wildlife Sanctuary (GWLS) provides an array of first-hand information on the floristic diversity, species composition and structural diversity within the sanctuary. Presence of 364 vascular plant species along with 18 plant communities (10 forest and 08 alpine communities) within the sanctuary indicates a high species richness community diversity of the region. There is a negative correlation between basal area and density. Based on the recruitment of saplings and seedlings, the forest communities were categorized in to communities having highest regeneration of the dominant plant species particularly in sapling layer followed by other species, communities having sufficient regeneration of dominant species but highest regeneration was found in co-dominant plant species and communities having poor or no regeneration of dominant species. Within the forest zone, diversity (Hʹ) of trees, shrubs and herbs ranged from 0.88–1.67, 0.27–1.82, and 2.21–3.69, respectively. Assessment and analysis of changes in structure and composition of different plant communities provide base line information for developing priorities for conservation and management in protected areas. Further, as no in-depth explorations for community characterization of forest and as well as alpine ecosystems of Kashmir Himalaya have been conducted; present study provides a template, which can be replicated in other protected as well as unprotected areas of the Kashmir Himalaya. However, as the different biotic and abiotic factors determine the community structure, species richness, and distribution, the use of multivariate analysis and statistical techniques for investigation will enable ecologists to efficiently understand vegetation-environment relationships.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Adhikari, B. S. (1991). High altitude forest: Composition, diversity and profile structure in a part of Kumaun Himalaya. Tropica Ecologica, 32(1), 86–97.
- Ahmad, M., Sharma, P., Rathee, S., Singh, H. P., Batish, D. R., Lone, G. R., Kaur, S., Jaryan, V., & Kohli, R. K. (2021). Niche width analyses facilitate identification of high-risk endemic species at high altitudes in western Himalayas. Ecological Indicators, 126, 107653. https://doi.org/10.1016/j.ecolind.2021.107653
- Ahmad, M., Uniyal, S. K., Batish, D. R., Singh, H. P., Jaryan, V., Rathee, S., Sharma, P., & Kohli, R. K. (2020). Patterns of plant communities along vertical gradient in Dhauladhar mountains in lesser Himalayas in North-Western India. The Science of the Total Environment, 716, 136919. https://doi.org/10.1016/j.scitotenv.2020.136919
- Ajaz, S., Ahmad, K., Qaisar, K. N., Mugloo, J. A., Rafeeq, J., Gangoo, S. A., & Khan, I. (2022). Floristic Diversity under different habitats in overa wildlife sanctuary of J&K India. Indian Forester, 148(11), 969–985. https://doi.org/10.36808/if/2022/v148i11/164125
- Akash, Akash., Navneet, & Bhandari, B. S. (2020). Natural regeneration dynamics of tree species along the altitudinal gradient in a subtropical moist deciduous forest of northern India. Current Science, 119(12), 2019–2023. https://doi.org/10.18520/cs/v119/i12/2019-2023
- Altaf, A., Haq, S. M., Shabnum, N., & Jan, H. A. (2022). Comparative assessment of phyto diversity in Tangmarg forest division in Kashmir Himalaya, India. Acta Ecologica Sinica, 42(6), 609–615. https://doi.org/10.1016/j.chnaes.2021.04.009
- Anthwal, A., Gupta, N., Sharma, A., Anthwal, S., & Kim, K. -H. (2010). Conserving biodiversity through traditional beliefs in sacred groves in Uttarakhand Himalaya, India. Resources Conservation and Recycling, 54(11), 962–971. https://doi.org/10.1016/j.resconrec.2010.02.003
- Anup, K. C., Manandhar, R., Paudel, R., & Ghimire, S. (2018). Increase of forest carbon biomass due to community forestry management in Nepal. Journal of Forestry Research, 29(2), 429–438. https://doi.org/10.1007/s11676-017-0438-z
- Anwar, S., Khan, S. M., Ahmad, Z., Ullah, Z., & Iqbal, M. (2019). Floristic composition and ecological gradient analyses of the liakot forests in the kalam region of district Swat, Pakistan. Journal of Forestry Research, 30(4), 1407–1416. https://doi.org/10.1007/s11676-019-00919-8
- Arya, S. C. 2002. Assessment of habitat diversity, distribution of vegetation and human dependence in alpine meadows of Nanda Devi Biosphere Reserve, West Himalaya. Ph. D. Thesis submitted to Kumaun University, Nainital.
- Bargali, H., Kumar, A., & Singh, P. (2022). Plant studies in Uttarakhand, Western Himalaya–A comprehensive review. Trees, Forests and People, 8, 100203. https://doi.org/10.1016/j.tfp.2022.100203
- Barman, T.,Samant, S.S & Singh, A. (2021). Structural diversity and regeneration pattern of forest communities in Parbati Valley, North Western Himalaya, India: Implications for conservation. Journal of the Geological, 48(2), 332–348.
- Bhat, N. A. 1984. Floristic Composition of Gulmarg (Baramulla). PhD. Thesis, University of Kashmir,
- Bhat, J. A., Kumar, M., Negi, A. K., Todaria, N. P., Malik, Z. A., Pala, N. A., Kumar, A., & Shukla, G. (2020). Species diversity of woody vegetation along altitudinal gradient of the Western Himalayas. Global Ecology and Conservation, 24, e01302. https://doi.org/10.1016/j.gecco.2020.e01302
- Bhatta, K. P., Chaudhary, R. P., & Vetaas, O. R. (2012). A comparison of systematic versus stratified-random sampling design for gradient analyses: A case study in subalpine Himalaya, Nepal. Phytocoenologia, 42(3–4), 191–202. https://doi.org/10.1127/0340-269X/2012/0042-0519
- Chawla, A., Rajkumar, S., Singh, K. N., Lal, B., Singh, R. D., & Thukral, A. K. (2008). Plant species diversity along an altitudinal gradient of Bhabha Valley in western Himalaya. Journal of Mountain Science, 5(2), 157–177. https://doi.org/10.1007/s11629-008-0079-y
- Connell, J. H. (1978). Diversity in coral reefs and tropical rainforests. Science, 199(4335), 1302–1310. https://doi.org/10.1126/science.199.4335.1302
- Connell, J. H., & Orias, E. (1964). The ecological regulation of species diversity. The American Naturalist, 98(903), 399–414. https://doi.org/10.1086/282335
- Curtis, J. T., & Mcintosh, R. P. (1950). The interrelations of certain analytic and synthetic phytosociological characters. Ecol, 31(3), 434–455. https://doi.org/10.2307/1931497
- Dar, G. H. (2007). Medicinal flora of the Kashmir Himalaya: A taxonomic overview. The Journal of Himalayan Ecology and Sustainable Development, 2, 13–20.
- Dar, A. R. (2008). Narrow endemic angiosperms of the Kashmir Himalaya: Threat assessment and conservation. In M. Z. Chisti & F. Ahmad (Eds.), Science for Better Tomorrow (pp. 31–39). Universal Printers, Khanyar.
- Dar, D. A. 2018. Carbon Stock Assessment of Gulmarg Forest Range, Kashmir Himalaya, India. Ph. D Thesis, Central University of Gujarat.
- Dar, S. A., Bhat, S. U., Aneaus, S., & Rashid, I. (2020). A geospatial approach for limnological characterization of Nigeen Lake, Kashmir Himalaya. Environmental Monitoring and Assessment, 192(2), 1–18. https://doi.org/10.1007/s10661-020-8091-y
- Dar, G. H., & Khuroo, A. (2013). Floristic diversity in the Kashmir Himalaya: Progress, problems and prospects. Sains Malay, 42(10), 1377–1386.
- Dar, G. H., & Khuroo, A. A. (2020). An introduction to biodiversity of the Himalaya: Jammu and Kashmir state. In G. H Dar, & A. A Khuroo (Eds.), Biodiversity of the Himalaya: Jammu and Kashmir State (pp. 3–26). Springer.
- Dar, A. A., & Parthasarathy, N. (2022). Tree species composition, stand structure and distribution patterns across three Kashmir Himalayan forests, India. Écoscience, 29(4), 1–14. https://doi.org/10.1080/11956860.2022.2048534
- Dar, D. A., & Sahu, P. (2018). Assessment of biomass and carbon stock in temperate forests of Northern Kashmir Himalaya, India. Journal of the International Academy of Ecology and Environmental Sciences, 8(2), 139.
- Dar, J. A., & Sundarapandian, S. (2016). Patterns of plant diversity in seven temperate forest types of Western Himalaya, India. Journal of Asia-Pacific Biodiversity, 9(3), 280–292. https://doi.org/10.1016/j.japb.2016.03.018
- Das, D. S. (2020). Species richness patterns of different life-forms along altitudinal gradients in the Great Himalayan National Park, Western Himalaya, India. Taiwania, 62(2), 154–162.
- Das, D. S. (2021). Population structure and regeneration status of tree species in old growth Abies pindrow dominant forest: A case study from western Himalaya, India. Trees, Forests and People, 5, 100101. https://doi.org/10.1016/j.tfp.2021.100101
- Devi, K. (2019). Diversity, structure and regeneration pattern of tree communities in Kanawar Wildlife Sanctuary of Himachal Pradesh, Northwest Himalaya, India. Journal of the Geological, 46(1), 94–103.
- Dhar, U., & Kachroo, P. (1983). Alpine Flora of Kashmir. Scientific Publishers.
- Dhyani, S., & Dhyani, D. (2016). Significance of provisioning ecosystem services from moist temperate forest ecosystems: Lessons from upper Kedarnath valley, Garhwal, India. Energy, Ecology and Environment, 1(2), 109–121. https://doi.org/10.1007/s40974-016-0008-9
- Gairola, S. (2009). Species richness and diversity along an altitudinal gradient in moist temperate forest of Garhwal Himalaya. The American Journal of Science, 5, 119–128.
- Gairola, S., Sharma, C. M., Ghildiyal, S. K., & Suyal, S. (2011). Tree species composition and diversity along an altitudinal gradient in moist tropical montane valley slopes of the Garhwal Himalaya, India. Forest Science and Technology, 7(3), 91–102. https://doi.org/10.1080/21580103.2011.597109
- Ghildiyal, S. K., & Gairola, S. (2013). Phytodiversity along an altitudinal gradient in Dudhatoli forest of Garhwal Himalaya, Uttarakhand, India. Inter Jour of Med & Arom Plants, 3(4), 439–451.
- Goncalves, F. M. (2018). Species diversity, population structure and regeneration of woody species in fallows and mature stands of tropical woodlands of southeast Angola. Journal of Forestry Research, 29(6), 1569–1579. https://doi.org/10.1007/s11676-018-0593-x
- Grieg-Smith, P. (1957). Quantitative plant ecology. Academic press.
- Hamid, M. 2020. Studies of alpine vegetation along an elevation gradient in Kashmir Himalaya. Ph. D Thesis, Department of Botany, University of Kashmir.
- Hamid, M., Khuroo, A. A., Malik, A. H., Ahmad, R., & Singh, C. P. (2020). Assessment of alpine summit flora in Kashmir Himalaya and its implications for long-term monitoring of climate change impacts. Journal of Mountain Science, 17(8), 1974–1988. https://doi.org/10.1007/s11629-019-5924-7
- Hanief, M. (2016). Natural regeneration dynamics of dominant tree species along an altitudinal gradient in three different forest covers of Darhal watershed in north western Himalaya (Kashmir), India. Tropical Plant Research, 3(2), 253–262.
- Haq, S. M. (2020). Forest ecosystems of Jammu and Kashmir State. In G. H. Dar, & A. A. Khuroo (Eds.), Biodiversity of the Himalaya: Jammu and Kashmir State (pp. 191–208). Springer.
- Haq, S. M., Calixto, E. S., & Kumar, M. (2020). Assessing biodiversity and productive over a small scale gradient in the protected forests of Indian western Himalayas. Journal of Sustainable Forestry, 40(7), 1–20. https://doi.org/10.1080/10549811.2020.1803918
- Haq, S. M., Malik, A. H., Khuroo, A. A., & Rashid, I. (2019). Floristic composition and biological spectrum of Keran-a remote valley of northwestern Himalaya. Acta Ecologica Sinica, 39(5), 372–379. https://doi.org/10.1016/j.chnaes.2018.12.001
- Haq, S. M., Shah, A. A., Yaqoob, U., & Hassan, M. (2021). Floristic quality assessment index of the Dagwan Stream in Dachigam National Park of Kashmir Himalaya. Proceedings of the National Academy of Sciences, India Section B: Biological Sciences, 91(3), 657–664. https://doi.org/10.1007/s40011-021-01247-w
- Harrison, S. et al. (2020). Climate and plant community diversity in space and time. Proceedings of the National Academy of Sciences, 117(9), 4464–4470.
- Ijaz, F., Rahman, I., Iqbal, Z., Alam, J., Ali, N., & Khan, S. M. (2018). Ethnoecology of the healing forests of Sarban Hills, Abbottabad, Pakistan: An economic and medicinal appraisal. In M Ozturk, & K Hakim (Eds.), Plant and Human Health (Vol. 1 pp. 675–706)Springer.
- Jain, S. K., & Rao, R. R. (1976). Handbook of field and herbarium methods. Today and Tomorrows Printers and Publishers.
- Joshi, H. C. 2002. Assessment of habitat diversity, forest vegetation and Human dependence in the Buffer zone of Nanda Devi Biosphere Reserve of West Himalaya. Ph. D thesis, Kumaon University, Nainital.
- Joshi, P. P., Murthy, I. K., Hegde, G. T., Sathyanarayan, V., Bhat, S., Patil, V., Esteves, T., & Ravindranath, N. H. (2018). Biophysical quantification of biodiversity and ecosystems services of forest ecosystems in the Western Ghats: A case study of Uttara Kannada District, India. Journal of Forestry Research, 29(3), 735–748. https://doi.org/10.1007/s11676-017-0488-2
- Joshi, H. C., & Samant, S. S. (2004). Assessment of forest vegetation and conservation priorities of communities in part of Nanda Devi Biosphere Reserve, West Himalaya. Part I. Journal of Sustainable Development and World Ecology, 11(3), 326–336. https://doi.org/10.1080/13504500409469835
- Kala, C. P., & Mathur, V. B. (2002). Patterns of plant species distribution in the trans-Himalayan region of Ladakh, India. Journal of Vegetation Science, 13(6), 751–754. https://doi.org/10.1111/j.1654-1103.2002.tb02104.x
- Kaur, D. (2022). Climate Change: Concerns and Influences on Biodiversity of the Indian Himalayas. In S. Rani, & R. Kumar (Eds.), Climate Change (pp. 265–281). Springer.
- Kersaw, K. A. (1973). Quantitative and dynamic plant ecology (Second edition ed.). Edward Arnold Limited.
- Khan, S. M. (2011). Species and community diversity of vascular flora along environmental gradient in Naran Valley: A multivariate approach through indicator species analysis. Pakistan Journal of Botany, 43(5), 2337–2346.
- Khan, S. M., Page, S., Ahmad, H., & Harper, D. (2013). Identifying plant species and communities across environmental gradients in the Western Himalayas: Method development and conservation use. Ecological Informatics, 14, 99–103. https://doi.org/10.1016/j.ecoinf.2012.11.010
- Khan, A. M., Qureshi, R., & Saqib, Z. (2019). Multivariate analyses of the vegetation of the western Himalayan forests of Muzaffarabad district, Azad Jammu and Kashmir, Pakistan. Ecological Indicators, 104, 723–736. https://doi.org/10.1016/j.ecolind.2019.05.048
- Khuroo, A. A. (2015). Himadri site in Kashmir Himalaya. ENVIS Newsletter on Him Ecol, 12(2), 4.
- Khuroo, A. A., Shapoo, G. A., Rasheed, S., Kaloo, Z. A., & Rafiq, S. (2018). Goodyerafusca (Orchidaceae): A new record for Kashmir Himalaya, India. Lankesteriana, 18(2), 151–154. https://doi.org/10.15517/lank.v18i2.34219
- Kullberg, P., & Moilanen, A. (2014). How do recent spatial biodiversity analyses support the convention on biological diversity in the expansion of the global conservation area network? Natureza & Conservação, 12(1), 3–10. https://doi.org/10.4322/natcon.2014.002
- Kumar, M. (2004). A study on community structure and diversity of a sub-tropical forest of Garhwal Himalayas. Indica For, 130(2), 207–214.
- Kumar, A. (2017). Biogeographic delineation of the Indian Trans-Himalaya: Need for revision. Current Science, 113(6), 1032–1033.
- Kumar, A. 2020. Ecological assessment valuation and conservation prioritization of floristic diversity in Kalatop-Khajjiar Wildlife sanctuary of Himachal Pradesh, Northwestern Himalaya. Ph. D Thesis, Kumaun University.
- Kunwar, R. M., & Sharma, S. P. (2004). Quantitative analysis of tree species in two community forests of Dolpa district, mid-west Nepal. Himalayan Journal of Sciences, 2(3), 23–28. https://doi.org/10.3126/hjs.v2i3.226
- Lal, M., & Samant, S. S. (2015). Diversity, uses and prioritization of medicinal plants in Kais Wildlife Sanctuary, North Western Himalaya, India. In P. Sharma, P. K. Bharti, & N. Singh (Eds.), Medicinal Plants: Distribution, Utilization and Significance, North Western Himalaya, India (pp. 98–112). Discovery Publishing House Pvt. Ltd.
- Lal, M., & Samant, S. S. (2019). Compositional and structural diversity of forest vegetation in Kai Wildlife Sanctuary, North Western Himalaya: Conservation implications. Journal of Biodiversity, 10(1–2), 1–14. https://doi.org/10.31901/24566543.2019/10.1-2.083
- Liu, S., & Wang, H. (2018). N, P, and K characteristics of different age groups of temperate coniferous tree species in northwestern China. Journal of Forestry Research, 29(2), 471–478. https://doi.org/10.1007/s11676-017-0442-3
- Maletha, A., Maikhuri, R. K., Bargali, S. S., Sharma, A., Negi, V. S., & Rawat, L. S. (2022). Vegetation dynamics and soil nutrient availability in a temperate forest along altitudinal gradient of Nanda Devi Biosphere Reserve, Western Himalaya, India. Plos One, 17(10), e0275051. https://doi.org/10.1371/journal.pone.0275051
- Malik, Z. A., & Bhatt, A. B. (2015). Phytosociological analysis of woody species in Kedarnath Wildlife Sanctuary and its adjoining areas in Western Himalaya, India. Journal of Forest and Environmental Science, 31(3), 149–163. https://doi.org/10.7747/JFES.2015.31.3.149
- Malik, Z. A., & Nautiyal, M. C. (2016). Species richness and diversity along the altitudinal gradient in Tungnath, the Himalayan benchmark site of HIMADRI. Tropical Plant Research, 3(2), 396–407.
- Malik, A. H., Rashid, I., Ganie, A. H., Khuroo, A. A., & Dar, G. H. (2015). Benefitting from geoinformatics: Estimating floristic diversity of Warwan valley in Northwestern Himalaya, India. Journal of Mountain Science, 12(4), 854–863. https://doi.org/10.1007/s11629-015-3457-2
- Mehmood, A., Shah, A. H., Shah, A. H., Khan, S. U., Khan, K. R., Farooq, M., Ahmad, H., & Sakhi, S. (2021). Classification and ordination analysis of herbaceous flora in district Tor Ghar, western Himalaya. Acta Ecologica Sinica, 41(5), 451–462. https://doi.org/10.1016/j.chnaes.2021.07.003
- Mehraj, G., Khuroo, A. A., Qureshi, S., Muzafar, I., Friedman, C. R., & Rashid, I. (2018). Patterns of alien plant diversity in the urban landscapes of global biodiversity hotspots: A case study from the Himalayas. Biodiversity and Conservation, 27(5), 1055–1072. https://doi.org/10.1007/s10531-017-1478-6
- Mehta, P., Sekar, K. C., Bhatt, D., Tewari, A., Bisht, K., Upadhyay, S., Negi, V. S., & Soragi, B. (2020). Conservation and prioritization of threatened plants in Indian Himalayan Region. Biodiversity and Conservation, 29(6), 1723–1745. https://doi.org/10.1007/s10531-020-01959-x
- Melese, S. M., & Ayele, B. (2017). Woody plant diversity, structure and regeneration in the Ambo state forest, south Gondar zone, Northwest Ethiopia. Journal of Forestry Research, 28(1), 133–144. https://doi.org/10.1007/s11676-016-0280-8
- Mir, A. H., Tyub, S., & Kamili, A. N. (2020). Ecology, distribution mapping and conservation implications of four critically endangered endemic plants of Kashmir Himalaya. Saudi Journal of Biological Sciences, 27(9), 2380–2389. https://doi.org/10.1016/j.sjbs.2020.05.006
- Misra, R. (1968). Ecological Work Book. Oxford and IBH Publishing Company.
- Molenda, O., Reid, A., & Lortie, C. J. (2012). The alpine cushion plant Silene acaulis as foundation species: A bug’s-eye view to facilitation and microclimate. Plos One, 7(5), e37223. https://doi.org/10.1371/journal.pone.0037223
- Muller-Dombois, D., & Ellenberge. (1974). Aims and Methods of Vegetation ecology. 1449-1453. John Willey and sons.
- Mumshad, M. (2021). Phyto-ecological studies and distribution pattern of plant species and communities of Dhirkot, Azad Jammu and Kashmir, Pakistan. Plos One, 16(10), e0257493. https://doi.org/10.1371/journal.pone.0257493
- Naghdi, R., Solgi, A., Zenner, E. K., & Behjou, F. K. (2018). Soil physical properties degrade further on skid trails in the year following operations. Journal of Forestry Research, 29(1), 93–101. https://doi.org/10.1007/s11676-017-0413-8
- Nanda, A. B., Nelofar, N., & Lone, R. A. (2019). “Study of Phenology of Woody Flora of Gulmarg and Its Neighbourhood” for Landscape Use. Journal of Scientific Research and Reports, 22(4), 1–7. https://doi.org/10.9734/jsrr/2019/v22i430095
- Naqshi, A. R., et al. (1984). Plants of Gulmarg. Journal of Economic and Taxonomic Botany, 5, 709–741.
- Navarro-Cerrillo, R. M., Esteves Vieira, D. J., Ochoa-Gaona, S., de Jong, B. H. J., & Del Mar Delgado Serrano, M. ª. (2019). Land cover changes and fragmentation in mountain neotropical ecosystems of Oaxaca, Mexico under community forest management. Journal of Forestry Research, 30(1), 143–155. https://doi.org/10.1007/s11676-017-0568-3
- Negi, V. S. (2021). Review and synthesis of climate change studies in the Himalayan region. Environment, Development and Sustainability, 24, 1–32.
- Negi, V., Bhardwaj, D. R., Sharma, P., & Pala, N. A. (2022). Tree species composition and diversity in natural temperate forests of the North-Western Himalayas. Acta Ecologica Sinica, 42(6), 653–660. https://doi.org/10.1016/j.chnaes.2021.09.014
- Nepali, B. R., Skartveit, J., & Baniya, C. B. (2021). Impacts of slope aspects on altitudinal species richness and species composition of Narapani-Masina landscape, Arghakhanchi, West Nepal. Journal of Asia-Pacific Biodiversity, 14(3), 415–424. https://doi.org/10.1016/j.japb.2021.04.005
- Pant, S. 2005. Assessment of plant diversity and ethnobotany of Mornaula Reserve Forest in Kumaon, West Himalaya. Ph.D. Thesis, Kumaon University, Nainital.
- Pant, S., & Samant, S. S. (2012). Diversity and regeneration status of tree species in Khokhan Wildlife Sanctuary, north-western Himalaya. Tropica Ecologica, 53(3), 317–331.
- Pokhriyal, P. (2009). Comparative studies on species richness, diversity and composition of Anogeissus latifolius mixed forests in Phakot and Pathri Rao watersheds of Garhwal Himalaya. Current Science, 1349–1355.
- Pukkala, T. (2018). Effect of species composition on ecosystem services in European boreal forest. Journal of Forestry Research, 29(2), 261–272. https://doi.org/10.1007/s11676-017-0576-3
- Rahman, I., Afzal, A., Abd_allah, E. F., Iqbal, Z., Alqarawi, A. A., Hashem, A., Calixto, E. S., Ali, N., & Asmarayani, R. (2021). Composition of plant communities driven by environmental gradients in alpine pastures and cold desert of Northwestern Himalaya, Pakistan. Pakistan Journal of Botany, 53(2), 655–664. https://doi.org/10.30848/PJB2021-2(35)
- Ram, J., et al. (2004). Plant diversity in six forest types of Uttaranchal, Central Himalaya, India. Current Science, 86(7), 975–978.
- Ramachandran, V. S., & Swarupanandan, K. (2013). Structure and floristic composition of old-growth wet evergreen forests of Nelliampathy Hills, Southern Western Ghats. Journal of Forestry Research, 24(1), 37–46. https://doi.org/10.1007/s11676-013-0323-3
- Rana, M. S. 2007. Assessment of floristic diversity and conservation prioritization of communities for conservation in Manali Wildlife Sanctuary of Himachal Pradesh in Northwestern Himalaya. Thesis submitted to Kumaun University, Nainital.
- Rana, C. S., & Gairola, S. (2009). Forest community structure and composition along an elevational gradient of Parshuram Kund area in Lohit district of Arunachal Pradesh, India. Journal of Natural Sciences, 1(8), 44–52.
- Rana, M. S., & Samant, S. S. (2009). Prioritization of habitats and communities for conservation in the Indian Himalayan Region: A state-of-the-art approach from Manali Wildlife Sanctuary. Current Science, 97(3), 326–335.
- Rana, M. S., & Samant, S. S. (2010). Threat categorization and conservation prioritization of floristic diversity in the Indian Himalayan region: A state of art approach from Manali Wildlife Sanctuary. Journal for Nature Conservation, 18(3), 159–168. https://doi.org/10.1016/j.jnc.2009.08.004
- Rana, M. S., Samant, S. S., & Rawat, Y. S. (2011). Plant communities and factors responsible for vegetation pattern in an alpine area of the northwestern Himalaya. Journal of Mountain Science, 8(6), 817–826. https://doi.org/10.1007/s11629-011-2078-7
- Raturi, G. P. (2012). Forest community structure along an altitudinal gradient of district Rudrapryag of Garhwal Himalaya, India. Ecol, 2(3), 76–84. https://doi.org/10.3923/ecologia.2012
- Rawal, R. S. (1994). Broad community identification of high altitude forest vegetation in Pindari region of Kumaun (Central Himalaya). Proc-Ind Nat Sci Acad Part B, 60, 553–556.
- Rawal, R. S. (2003). Himalayan forest database–thinking beyond dominants. Current Science, 84(8), 990–994.
- Rawal, R. S. (2018). Plant species diversity and rarity patterns along elevation range covering treeline ecotone in Uttarakhand: Conservation implications. Tropica Ecologica, 59(2), 1–15.
- Rawat, D. S. (2013). Plant Diversity in the Lohba Range of Kedarnath Forest Division in Garhwal Himalaya, Uttarakhand, India. Annals of Plant Sciences, 2(8), 302–320.
- Rawat, B. (2021). Spatial prediction of plant species richness and density in high-altitude forests of Indian west Himalaya. Trees, Forests and People, 6, 100132. https://doi.org/10.1016/j.tfp.2021.100132
- Rawat, G. S., & Adhikari, B. S. (2005). Floristics and distribution of plant communities across moisture and topographic gradients in Tso Kar basin, Changthang plateau, eastern Ladakh. Arctic, Antarctic, and Alpine Research, 37(4), 539–544. https://doi.org/10.1657/1523-0430(2005)037[0539:FADOPC]2.0.CO;2
- Rawat, B., Gairola, S., & Rawal, R. S. (2015). Assessing conservation values of forest communities in Nanda Devi Biosphere Reserve: Plant diversity, species distribution and endemicity. Journal of Mountain Science, 12(4), 878–890. https://doi.org/10.1007/s11629-014-3000-x
- Rodgers, W. A., & Panwar, H. S. 1988. Planning a wildlife protected area network in India.
- Saima. et al. (2014). Impact of Livestock and Human Activities on the Floral Distribution of Gulmarg Wildlife Sanctuary, Kashmir. Inter Jour of Innov Res & Dev, 3, 499–502.
- Sakshi. 2009. Eco-ethnobotanical assessment and conservation priorities of floristic diversity along an altitudinal gradient in central part of Himachal Pradesh. Thesis submitted to Kumaun University, Nainital.
- Salisu, N. (2021). Phytosocial diversity and distribution of herbaceous species in dryland ecosystem of Kebbi, North-western Nigeria. IOSR Jour of Envir Sci, Toxi & Food Tech, 15(7), 53–60.
- Samant, S. S. (2002). Studies on the structure, composition and changes of vegetation in Nanda Devi Biosphere Reserve, west Himalaya. In J. K. Sharma, P. S. Easa, C. Mohanan, N. Saudharan, & R. K. Rai, et al. (Eds.), Biosphere Reserves in India and 190 their Management (pp. 133–139). Kerala Forest Research Institute, Peechi and Ministry of Environment and Forests.
- Samant, S. S., Dhar, U., & Rawal, R. S. (1998). Biodiversity status of a protected area in West Himalaya: Askot Wildlife Sanctuary. International Journal of Sustainable Development & World Ecology, 5(3), 194–203. https://doi.org/10.1080/13504509809469983
- Samant, S. S., Pant, S., Singh, M., Lal, M., Singh, A., Sharma, A., & Bhandari, S. (2007). Medicinal Plants in Himachal Pradesh, North Western Himalaya, India. International Journal of Biodiversity Science & Management, 3(4), 234–251. https://doi.org/10.1080/17451590709618177
- Saura, S., Bertzky, B., Bastin, L., Battistella, L., Mandrici, A., & Dubois, G. (2019). Global trends in protected area connectivity from 2010 to 2018. Biological Conservation, 238, 108183. https://doi.org/10.1016/j.biocon.2019.07.028
- Saxena, A. K., & Singh, J. S. (1982). A phytosociological analysis of woody species in forest communities of a part of Kumaun Himalaya. Vegetation, 50, 3–22. https://doi.org/10.1007/BF00120674
- Shaheen, H., Ullah, Z., Khan, S. M., & Harper, D. M. (2012). Species composition and community structure of western Himalayan moist temperate forests in Kashmir. Forest Ecology and Management, 278, 138–145. https://doi.org/10.1016/j.foreco.2012.05.009
- Shannon, E. C., & Wiener, W. (1963). The mathematical theory of communication. University of Lillignoia Pres, Urban.
- Sharma, A. 2008. Studies on Floristic diversity and prioritization of communities for conservation in Hirb and Shoja Catchments, District Kullu of Himachal Pradesh, North Western Himalaya. Ph. D. Thesis, Kumaun University, Nainital.
- Sharma, C. M., Baduni, N. P., Gairola, S., Ghildiyal, S. K., & Suyal, S. (2010). Tree diversity and carbon stocks of some major forest types of Garhwal Himalaya, India. Forest Ecology and Management, 260(12), 2170–2179. https://doi.org/10.1016/j.foreco.2010.09.014
- Sharma, N., Behera, M. D., Das, A. P., & Panda, R. M. (2019). Plant richness pattern in an elevation gradient in the Eastern Himalaya. Biodiversity and Conservation, 28(8–9), 2085–2104. https://doi.org/10.1007/s10531-019-01699-7
- Sherman, R., Mullen, R., Haomin, L., Zhendong, F., & Yi, W. (2008). Spatial patterns of plant diversity and communities in alpine ecosystems of the Hengduan Mountains, northwest Yunnan, China. Journal of Plant Ecology, 1(2), 117–136. https://doi.org/10.1093/jpe/rtn012
- Simpson, E. H. (1949). Measurement of diversity. Nature, 163(4148), 163–688. https://doi.org/10.1038/163688a0
- Singh, N. P. (2002). Flora of Jammu and Kashmir. Botanical Survey of India.
- Singh, A. (2007). Assessment of plant diversity and conservation status of forest vegetation in a proposed cold desert biosphere reserve of the Western Himalaya. Ph. D Thesis, Kumaun University, Nainital.
- Singh, J. S., & Chaturvedi, R. K. (2017). Diversity of ecosystem types in India: A review. Proceedings of the Indian National Science Academy, 83(1), 569–594. https://doi.org/10.16943/ptinsa/2017/41287
- Singh, D. K., & Hajra, P. K. (1996). Floristic diversity. In G. Gujral & V. Sharma (Eds.), Biodiversity Status in the Himalaya (pp. 23–38). British Council.
- Singh, J. B., & Kachroo, P. (1994). Forest Flora of Pir Panjal Range (Northwestern Himalaya. Bishen Singh Mahendra Pal Singh.
- Singh, D. K., & Pusalkar, P. K. (2020). Floristic Diversity of the Indian Himalaya. In G. Dar & A. Khuroo (Eds.), Biodiversity of the Himalaya: Jammu and Kashmir State. Topics in Biodiversity and Conservation (Vol. 18). Springer. https://doi.org/10.1007/978-981-32-9174-4.
- Smith, C. E. 1971. Preparing herbarium specimens of vascular plants (No. 348). Agricultural Research Service, US Department of Agriculture.
- Smith, B., & Chinnappa, C. C. (2015). Plant collection, identification, and herbarium procedures. In E. Yeung, C. Stasolla, M. Summer, & B. Huang (Eds.), Plant microtechniques and protocols (pp. 541–572). Springer.
- Taj, R. et al (2018). Plant resources and human ecology of Tarnawai area, District Abbottabad, Pakistan. In M. Ozturk, & K. Hakeem (Eds.), Plant and human health (Vol. 1 pp. 731–756) Springer.
- Tali, B. A., Khuroo, A. A., Nawchoo, I. A., & Ganie, A. H. (2019). Prioritizing conservation of medicinal flora in the Himalayan biodiversity hotspot: An integrated ecological and socioeconomic approach. Environmental Conservation, 46(2), 147–154. https://doi.org/10.1017/S0376892918000425
- Tambe, S., & Rawat, G. S. (2010). The alpine vegetation of the Khangchendzonga landscape, Sikkim Himalaya. Mountain Research and Development, 30(3), 266–274. https://doi.org/10.1659/MRD-JOURNAL-D-09-00058.1
- Vashistha, R. K., Rawat, N., Chaturvedi, A. K., Nautiyal, B. P., Prasad, P., & Nautiyal, M. C. (2012). Phytostructure of diverse growth forms in an alpine ecosystem of north-west Himalaya, India. Plant Biosystems - an International Journal Dealing with All Aspects of Plant Biology, 146(1), 124–133. https://doi.org/10.1080/11263504.2011.584915
- Wani, Z. A. (2022). Tree diversity and regeneration dynamics in Gulmarg Wildlife Sanctuary, Kashmir Himalaya. Acta Ecol Sin.