Abstract
To investigate the roles of hypothalamic neuropeptide expression in body mass regulation in Eothenomys miletus, body mass, food intake, serum leptin levels and hypothalamic neuropeptide expression were measured in seasonal-acclimatized and lab-acclimated animals. The results showed that body mass, body fat mass and food intake showed seasonal variations. Serum leptin levels also appeared similar seasonal variation, showing a similar trend to body fat mass. Expression of hypothalamic neuropeptide Y (NPY) and agouti-related protein (AgRP) levels showed significant seasonal differences, while pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) expression showed no significant differences among seasons. Leptin had negative correlation with NPY and AgRP expression, positive correlation with POMC and CART expression among seasons. Cold and food restriction reduced body mass, body fat mass and serum leptin levels, and increased NPY and AgRP expression, but decreased POMC and CART expression. Photoperiods had no significant effect on these characters. Leptin was positively correlated with body fat mass, CART and POMC, and negatively correlated with NPY and AgRP under different temperatures, photoperiods and food quantity acclimation. All of these results showed that E. miletus reduced body mass and body fat mass, and increased food intake to survive in winter under seasonal changes. Temperature and food resources were the key factors influencing the body mass and energy metabolism in E. miletus. Leptin may play an important role in regulating body mass and energy metabolism by acting on hypothalamic neuropeptide expression in E. miletus.
Introduction
Phenotypic plasticity is the ability of an organism to change its phenotype in response to environmental changes, mainly in the aspects of morphology, physiology, behavior, phenology and so on (Rafael et al. Citation2013). Energy metabolism of small mammals may be the most suitable field for studying phenotypic plasticity changes (Cortés et al. Citation2011). Small mammals need to take in and assimilate energy to survive in the wild, and the assimilation energy is distributed to their maintenance (Speakman & Król Citation2005), growth and reproduction (Speakman Citation2008). Stable energy homeostasis of animals can respect the status of body mass, food intake and thermogenic properties, which shows the seasonal changes of the phenotype (Rene et al. Citation2014). Temperature and photoperiod were the important ecological factors to affect energy metabolism of small mammals; previous studies showed that short photoperiod and cold temperature reduced body mass and increased the thermogenic capacity of animals significantly (Zhu et al. Citation2012b). In addition, food resource is also one of the key factors affecting the energy metabolism of small mammals. Particularly for small mammals experiencing obvious seasonal changes in the environment, the abundance of food is essential for their survival (Zhao et al. Citation2013).
Leptin is a hormone secreted by the white fat cells. It can reflect the body fat content, and leptin levels in the animals can regulate the balance of body mass, which is mainly regulated by the energy intake and energy expenditure (Ruth Citation2014). A high concentration of leptin can be combined with the hypothalamus to regulate energy metabolism through blood circulation, and ultimately to regulate the feeding and thermogenic capacity of animals (Rezai-Zadeh et al. Citation2014). The hypothalamus plays an important role in energy homeostasis in small mammals, which is the central regulation of energy metabolism balance (Villa et al. Citation2012). The hypothalamic arcuate nucleus (ARC) can regulate food intake under environmental changes (Duan et al. Citation2014). Within the ARC, there are two types of neuropeptides, orexigenic neuropeptides: neuropeptide Y (NPY) and agouti-related protein (AgRP) (Marcelin et al. Citation2013); and anorectic neuropeptides: pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) (Yoo et al. Citation2007). Leptin is secreted into the blood, and then enters the brain, where it can affect the expression of NPY, AgRP, CART, POMC, so as to control the food intake and energy expenditure of small mammals (Trayhurn & Bing Citation2006).
During seasonal changes, animals often change the expression of the hypothalamus neuropeptide to adapt to changes in the field. Cricetus cricetus, for example, changes its NPY expression in different seasons in order to affect its activity rhythm and body mass balance (Ribelayga et al. Citation1998). Animals change the leptin concentration to adjust the expression of AgRP during seasonal changes; for example, high concentrations of leptin can reduce AgRP expression significantly, thereby reducing the appetite of animals, thus inhibiting food intake (Kitamura et al. Citation2006). Expression of POMC was significantly increased at higher temperatures, which inhibited food intake under seasonal acclimation (Endo & Park Citation2004). The study also showed that the adjustment of CART expression in Siberian hamsters played an important role in the seasonal changes in body mass and energy metabolism (Khorooshi et al. Citation2008). Moreover, different temperature, photoperiod and food resource can also affect the expression of neuropeptides: NPY expression in the rat increased significantly under cold acclimation (Bing et al. Citation1997). Injection of NPY at different photoperiod acclimation levels increased food intake significantly in Phodopus sungorus (Boss-Williams & Bartness Citation1996). NPY expression increased significantly in the food restriction group compared with that of the free feeding group (Abizaid et al. Citation1997). Siberian hamsters increased AgRP expression in order to maintain energy homeostasis under food restriction (Mercer et al. Citation2000).
Eothenomys miletus (Milne-Edwards, 1868) is a native species in the Hengduan mountain region (Zhu et al. Citation2008). It was previously reported that serum leptin levels in E. miletus showed changes under seasonal (Zhu et al. Citation2014b) or cold acclimation (Zhu et al. Citation2010, Citation2012a), and leptin levels were positively correlated with body fat mass, and negatively correlated with energy intake. But serum leptin levels showed no significant difference under acclimation to different photoperiods (Zhu et al. Citation2011). Food restriction reduced serum leptin levels significantly in E. miletus (Zhu et al. Citation2014a). All of the previous studies showed that serum leptin levels experienced seasonal changes in E. miletus with phenotypic plasticity (Zhu et al. Citation2014c). However, we know nothing about changes of hypothalamic neuropeptide expression under seasonal-acclimatized and lab-acclimated animals, and its roles in body mass regulation in E. miletus. So, in the present study we investigated: (1) whether hypothalamic neuropeptide gene expression showed seasonal variations in E. miletus; (2) if hypothalamic neuropeptide gene expression in northern small mammals shows significant seasonal changes, while that of tropical small mammals shows no changes among different seasons, then does hypothalamic regulation show species-specific patterns in E. miletus located in the Hengduan mountains region? and (3) which ecological factors affected the hypothalamic neuropeptide gene expression regulation in E. miletus for their survival. In the present study, body mass, food intake, serum leptin levels and hypothalamic neuropeptide expression were measured. We hypothesized that E. miletus can adjust its hypothalamic neuropeptide gene expression to regulate body mass during seasonal changes, and environmental factors may have effects on the hypothalamic neuropeptide gene expression. We predicted that leptin, NPY, AgRP, POMC and CART mRNA levels may play a role in the regulation of energy metabolism in E. miletus.
Materials and methods
Animals and experimental designs
Eothenomys miletus were captured in farmland (26°15´–26°45´N, 99°40´–99°55´E; altitude 2590 m) in Jianchuan County, Yunnan province. All animals were healthy adults. The animals were kept individually in plastic cages (350 mm × 300 mm × 250 mm) in a room with natural temperature and photoperiod: spring (5.4°C), summer (23.9°C), fall (16.6°C) and winter (−3.8°C). The habitat environment of the Hengduan mountainous region has a small annual range of temperature, large diurnal temperature range and relatively abundant food resources, and photoperiodic changes were not obvious. Food and water were provided ad libitum. All pregnant, lactating or young individuals were excluded. All animal procedures were licensed under the Animal Care and Use Committee of School of Life Sciences, Yunnan Normal University (Permit No. 13-0901-011).
Experiment 1
Experiment 1 was performed in 2014. E. miletus were wild-captured in March, June, September and December in 2014 (referred to hereafter as the spring, summer, autumn and winter groups, respectively). After the measurement of body mass and food intake, subjects were sacrificed by puncture of the posterior vena cava within 4 days after capture for all four seasons, and blood and tissue samples were taken for measurement of physiological parameters. A total of 37 adult E. miletus were used in the present study (spring, n = 11; summer, n = 11; autumn, n = 7; winter, n = 8).
Experiment 2
Animals used in Experiment 2 were offspring of adult E. miletus that were captured in June 2014 and then transported to School of Life Sciences of Yunnan Normal University, and housed individually in plastic boxes (350 mm × 300 mm × 250 mm). In order to test the singular or associated effects of ambient temperature, photoperiod and food restriction on hypothalamic neuropeptide gene expression, 55 adult weight-matched E. miletus were housed individually (maintained at 12L: 12D (lights on at 08:00am), and 25 ± 1°C), and kept for at least 2 weeks to familiarize themselves with the environment. After the acclimatization period, the animals were randomly assigned to the following eight groups: (1) long photoperiod (LD, 16L: 8D), warm (25 ± 1°C) and food ad libitum; (2) short photoperiod (SD, 8L: 16D), warm and food ad libitum; (3) LD, warm and 80% of the daily food intake; (4) SD, warm and 80% of the daily food intake; (5) LD, cold (5 ± 1°C) and food ad libitum; (6) SD, cold and food ad libitum; (7) LD, cold and 80% of the daily food intake; (8) SD, cold and 80% of the daily food intake. Animals were acclimated for 4 weeks.
Food intake and body fat mass
Food intake was measured by food equity (Rousseau et al. Citation2003). Each animal was put in a metabolic cage (20 cm × 15 cm × 15 cm) with no nest materials, and fed laboratory mice chow pellets. Animals were fed a fixed quantity at a set time (9.5–10.5g, 11:00 am), and the next day body mass was assessed, and residual food collected. Residual food was dried in a vacuum dryer until the mass was invariable. Total body fat was extracted from the dried carcass by ether extraction in a Soxhlet apparatus (Zhang & Wang Citation2007).
Measurement of serum leptin levels
Serum leptin levels were determined by radioimmunoassay (RIA) with the 125I Multi-species Kit (Cat. No. XL-85K, Linco Research Inc.). The lowest level of leptin that can be detected by this assay is 1.0 ng/mL when using a 100-μL sample size. And the inter- and intra-assay variabilities for leptin RIA were < 3.6% and 8.7%, respectively.
Measurements of hypothalamic neuropeptide gene expression
Total RNA was isolated from the hypothalamus using a TRIzol Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. To remove any contaminating DNA, RNA samples were treated with DNase I (Promega, USA) at 37°C for 30 min followed by another cycle of TRIzol extraction to eliminate residual DNase I. An equal amount (3 μg) of total RNA was transcribed into first-strand cDNA for each sample using the M-MLV First Strand Kit (Invitrogen) according to the manufacturer’s instructions.
Primers set for β-actin and four hypothalamic genes were used for real-time Quantitative Polymerase Chain Reaction (q-PCR), as shown in (Huang et al. Citation2013). Standard curves were constructed for each gene via serial dilutions of cDNA (1–26-fold dilutions). Analysis of standard curves between target genes and β-actin showed that they had similar amplification efficiency, which ensures the validity of the comparative quantity method. Real-time q-PCR was completed using the SYBR Green I qPCR kit (Invitrogen) in the ABI Prism® 7000 Sequence Detection system (Applied Biosystems, Carlsbad CA, USA). Real-time qPCR was carried out in 20 μL reaction agent comprised of 9.5 μL RNase-free ddH2O, 9.0 μL Platinum® Quantitative PCR SuperMix-UDG (including Rox), 0.5 μL cDNA templates, 0.5 μL 10 μmoL/L forward primer, and 0.5 μL 10 μmoL/L reserse primer. Each sample was analyzed in triplicate. Thermal cycling conditions were: 50°C for 120 s, 95°C for 120 s, 45 cycles of 95°C for 15 s, and 60°C for 45 s.
Table I. Gene-specific primers used for real-time Quantitative Polymerase Chain Reaction (qPCR).
Statistical analysis
Data were analyzed using the software package SPSS 15.0. Prior to all statistical analyses, data were examined for assumptions of normality and homogeneity of variance using Kolmogorov–Smirnov and Levene tests, respectively. Since no gender effects were found on almost all measured parameters, data from females and males were combined. In Experiment 1, body mass was analyzed using one-way analysis of variance (ANOVA). Body fat mass, food intake, serum leptin levels and hypothalamic neuropeptide gene expression were analyzed using one-way analysis of covariance (ANCOVA) with body mass as a covariate, followed by Tukey’s Honestly Significant Difference (HSD) post-hoc tests. In Experiment 2, body mass was analyzed using three-way ANOVA. Body fat mass, serum leptin levels and hypothalamic neuropeptide gene expression were analyzed using three-way ANOVA with body mass as a covariate. To detect possible associations of serum leptin levels with body fat mass, and hypothalamic neuropeptide gene expression, we used Pearson correlation analysis. Results are presented as means ± Mean Standard Error of Mean (SEM), and P < 0.05 was considered to be statistically significant.
Results
Experiment 1
Body mass, body fat mass and food intake
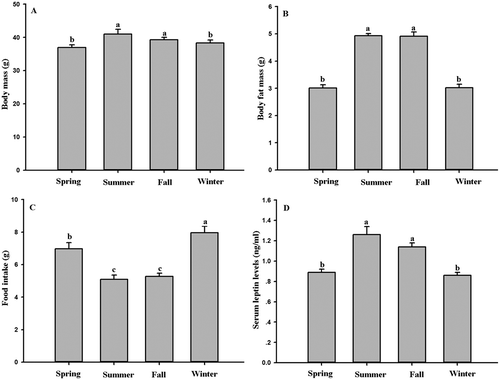
There were significant differences of body mass between seasons (F3,32 = 4.45, P < 0.01, )); it was lowest in winter, and highest in summer. Body fat mass also showed significant seasonal changes (F3,32 = 63.48, P < 0.01, )), being lowest in winter, and highest in summer. Body fat mass decreased 38.94% in winter compared with that in summer. Significant changes of food intake were found in different seasons (F3,32 = 15.22, P < 0.01, )); it was was lowest in summer, and highest in winter.
Serum leptin levels and hypothalamic neuropeptide gene expression
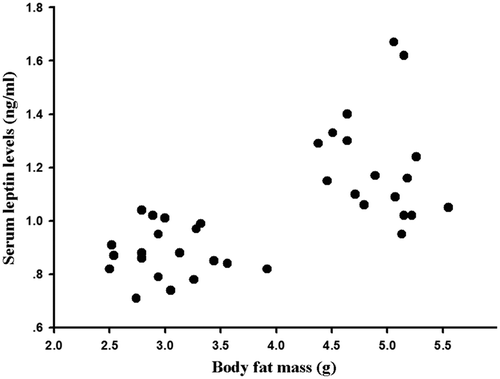
There were significant differences of serum leptin levels during seasonal variations (F3,32 = 12.97, P < 0.01, )). Like body fat mass, serum leptin levels were lowest in winter, and highest in summer. Serum leptin levels were positively correlated with body fat mass (r = 0.662, P < 0.01, ).
Figure 2. Correlation of serum leptin levels with body fat mass in Eothenomys miletus in different seasons.
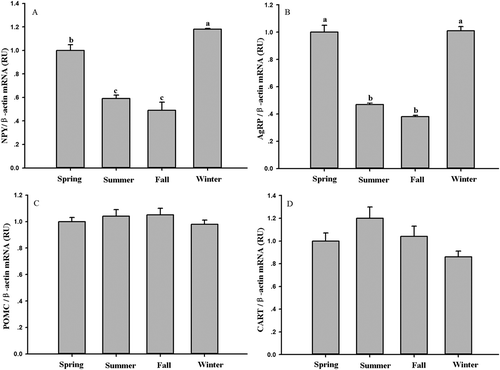
Hypothalamic neuropeptide NPY and AgRP gene expression showed significant seasonal changes (NPY: F3,32 = 44.71, P < 0.01, ); AgRP: F3,32 = 62.29, P < 0.01, )), but no significant differences were found in POMC and CART expression between seasons (POMC: F3,32 = 0.57, P > 0.05, ); CART: F3,32= 2.56, P > 0.05, )). Serum leptin levels were negatively correlated with NPY expression (r = −0.58, P < 0.01, )), and negatively correlated with AgRP expression (r = −0.65, P < 0.01, )). Leptin levels were positively correlated with POMC expression (r = 0.389, P < 0.05, )), and positively correlated with CART expression (r = 0.499, P < 0.05, )).
Experiment 2
Body mass, body fat mass and food intake
Before the experiment, body mass in the eight groups showed no significant differences (Temperature: F1,47 = 0.05, P > 0.05; Photoperiod: F1,47 = 0.162, P > 0.05; Food: F1,47 = 0.019, P > 0.05; Temperature × Photoperiod: F1,47 = 0.131, P > 0.05; Temperature × Food: F1,47 = 0.408, P > 0.05; Photoperiod × Food: F1,47 = 0.081, P > 0.05; Temperature × Photoperiod × Food: F1,47 = 1.239, P > 0.05). On day 28, body mass was only significantly affected by temperature and food (Temperature: F1,47 = 9.861, P < 0.01; Photoperiod: F1,47 = 0.778, P > 0.05; Food: F1,47 = 55.08, P < 0.01; Temperature × Photoperiod: F1,47 = 0.36, P > 0.05; Temperature × Food: F1,47=0.617, P > 0.05; Photoperiod × Food: F1,47 = 0.021, P > 0.05; Temperature × Photoperiod × Food: F1,47 = 0.375, P > 0.05): body mass in cold-temperature groups was reduced by 9.95% compared with the warm-temperature groups, and in food-restriction groups body mass decreased by 28.13% compared with food ad libitum groups (). Body fat mass was affected by temperature, food, interaction between temperature and food, and interaction among temperature, photoperiod and food on day 28 (Temperature: F1,47 = 67.224, P < 0.01; Photoperiod: F1,47 = 0.676, P > 0.05; Food: F1,47 = 75.552, P < 0.01; Temperature × Photoperiod: F1,47 = 3.662, P > 0.05; Temperature × Food: F1,47 = 5.869, P < 0.05; Photoperiod × Food: F1,47 = 0.011, P > 0.05; Temperature × Photoperiod × Food: F1,47 = 4.452, P < 0.05): body fat mass in cold-temperature groups decreased by 35.02% compared with the warm-temperature groups, and in food restriction groups body fat mass decreased by 39.75% compared with food ad libitum groups ().
Table II. Effects of temperature, photoperiod and food on body mass, body fat mass, serum leptin levels and hypothalamic neuropeptides expressions in Eothenomys miletus.
Serum leptin levels and hypothalamic neuropeptide gene expression
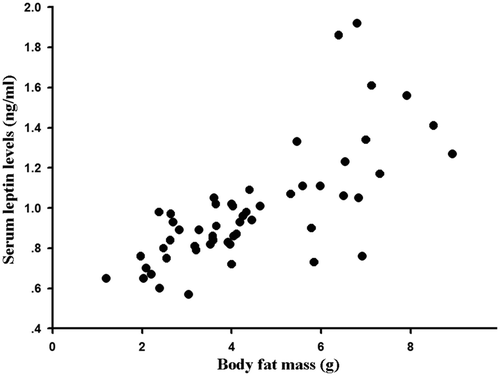
Serum leptin levels were affected by temperature, food, and interaction between temperature and food on day 28 (Temperature: F1,47 = 41.209, P < 0.01; Photoperiod: F1,47 = 3.189, P > 0.05; Food: F1,47 = 78.746, P < 0.01; Temperature × Photoperiod: F1,47 = 0.063, P > 0.05; Temperature × Food: F1,47 = 8.329, P < 0.01; Photoperiod × Food: F1,47 = 0.708, P > 0.05; Temperature × Photoperiod × Food: F1,47 = 0.918, P > 0.05). Serum leptin levels in cold-temperature groups decreased by 22.72% compared with the warm-temperature groups, and in food restriction groups serum leptin levels decreased by 22.97% compared with food ad libitum groups (). Serum leptin levels were positively correlated with body fat mass (r = 0.725, P < 0.01, ).
Figure 5. Correlation of body fat mass with serum leptin levels in Eothenomys miletus under different temperature, photoperiod and food restriction conditions.
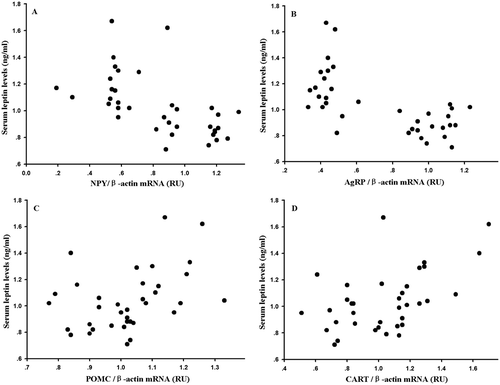
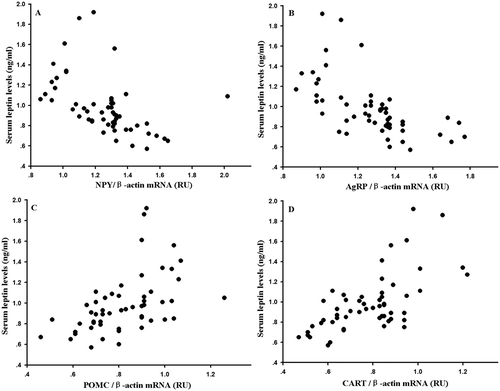
Hypothalamic neuropeptide NPY gene expression was significantly affected by temperature and food (Temperature: F1,47 = 38.699, P < 0.01; Photoperiod: F1,47 = 2.143, P > 0.05; Food: F1,47 = 26.083, P < 0.01; Temperature × Photoperiod: F1,47 = 0.044, P > 0.05; Temperature × Food: F1,47 = 1. 349, P > 0.05; Photoperiod × Food: F1,47 = 0.700, P > 0.05; Temperature × Photoperiod × Food: F1,47 = 0.025, P > 0.05). Hypothalamic neuropeptide NPY gene expression was significantly affected by temperature and food (Temperature: F1,47 = 38.699, P < 0.01; Photoperiod: F1,47 = 2.143, P > 0.05; Food: F1,47 = 26.083, P < 0.01; Temperature × Photoperiod: F1,47 = 0.044, P > 0.05; Temperature × Food: F1,47 = 1. 349, P > 0.05; Photoperiod × Food: F1,47 = 0.700, P > 0.05; Temperature × Photoperiod × Food: F1,47 = 0.025, P > 0.05). Hypothalamic neuropeptide AgRP gene expression was significantly affected by temperature and food (Temperature: F1,47 = 63.680, P < 0.01; Photoperiod: F1,47 = 0.537, P > 0.05; Food: F1,47=39.268, P < 0.01; Temperature × Photoperiod: F1,47 = 0.104, P > 0.05; Temperature × Food: F1,47 = F1,47 = 0.843, P > 0.05; Photoperiod × Food: F1,47 = 0.033, P > 0.05; Temperature × Photoperiod × Food: F1,47 = 0.068, P > 0.05). POMC gene expression was significantly affected by temperature and food (Temperature: F1,47 = 32.815, P < 0.01; Photoperiod: F1,47 = 3.444, P > 0.05; Food: F1,47 = 31.240, P < 0.01; Temperature × Photoperiod: F1,47 = 0.654, P > 0.05; Temperature × Food: F1,47 = 0.056, P > 0.05; Photoperiod × Food: F1,47 = 0.027, P > 0.05; Temperature × Photoperiod × Food: F1,47 = 1.108, P > 0.05). CART gene expression was significantly affected by temperature and food (Temperature: F1,47 = 81.199, P < 0.01; Photoperiod: F1,47 = 3.021, P > 0.05; Food: F1,47 = 35.697, P < 0.01; Temperature × Photoperiod: F1,47 = 0.185, P > 0.05; Temperature × Food: F1,47 = 0.014, P > 0.05; Photoperiod × Food: F1,47 = 0.023, P > 0.05; Temperature × Photoperiod × Food: F1,47 = 0.139, P > 0.05, ). Serum leptin levels were negatively correlated with NPY expression (r = −0.487, P < 0.01, )), and was negatively correlated with AgRP expression (r = −0.617, P < 0.01, )); it was positively correlated with POMC expression (r = 0.508, P < 0.01, )), and positively correlated with CART expression (r = 0.638, P < 0.01, (d)).
Figure 6. Correlation of (A) neuropeptide Y (NPY), (B) agouti-related protein (AgRP), (C) pro-opio-melanocortin (POMC) and (D) cocaine- and amphetamine-regulated transcript (CART) with serum leptin levels in Eothenomys miletus under different temperature, photoperiod and food restriction conditions.
Discussion
Body mass, body fat mass and food intake
Phenotypic plasticity represents the ability of animals to adapt to the environment (Bush et al. Citation2008). Many studies showed that mammals, such as the Djungarian hamster (Steinlechner et al. Citation1983), reduced body mass and body fat mass, and increased food intake and thermogenic capacity in winter (Lovegrove Citation2005). In the present study, body mass in E. miletus showed seasonal changes: it was lower in winter and higher in summer. This may be due to the increased energy consumption in winter: E. miletus needs to reduce body mass to reduce energy expenditure. Body fat mass in E. miletus also showed obvious seasonal variations: it was significantly lower in winter than in summer, which relates to cold temperature and poor quality of food resources in winter (Mercer Citation1998). Food intake in E. miletus increased significantly in winter, which may be related to cold temperatures in winter. A previous study showed that E. miletus in winter significantly increased its resting metabolic rate and non-shivering thermogenesis (Zhu et al. Citation2014b). Eothenomys miletus needs to increase food intake to compensate for the increase in energy consumption in winter.
Many small mammals can reduce body mass and body fat mass in cold temperatures (McNab Citation2002), and the reduction in body mass is a kind of adaptation to the cold stress (Bartness et al. Citation2002). Short photoperiod reduced body mass in Sekeetamys calurus (Haim Citation1996) and Lasiopodomys brandtii (Li & Wang Citation2007). Moreover, food restriction can reduce body mass in rats (Grégoire Citation2008), but it has no effect on mice (Hambly & Speakman Citation2005). In the present study, body mass in cold-temperature groups was 9.95% lower than in warm-temperature groups, while body fat mass was reduced by 35.02% more in cold-temperature groups than in warm-temperature groups, suggesting that mobilization of adipose tissue may be an adaptive strategy in E. miletus to the excessive energy consumption in cold acclimation (Zhang & Wang Citation2007). Food restriction can also reduce body mass and body fat mass in E. miletus: body mass in food-restricted groups reduced by 28.13%, and body fat mass decreased by 39.75%. However, photoperiod had no effect on body mass or body fat mass, which may indicate that in the natural environment, because the light cycle of the Hengduan Mountains is not obvious, the sensitivity of photoperiods to E. miletus is lower, so E. miletus has higher sensitivity to temperature and food. In comparing the effects of temperature and food restriction on body mass and body fat mass, it was found that body mass and body fat mass decreased more under food restriction, which may be because food resources are a source of energy; thus, when the food was restricted, E. miletus experienced a more obvious change in its body mass. And when E. miletus faces cold temperatures in winter, it can decrease its activity or storage of food to keep the body mass balance, so changes of body mass in cold-temperature conditions were smaller than those of food restriction. However, this does not mean that E. miletus was more sensitive to food restriction than to cold stress. Daily temperature change was obvious in the Hengduan Mountains region, but abundance of food was relatively higher in a year, so food restriction stress was less over the year. We speculated that the abnormal weather conditions in the field, which may cause poor food resources, probably caused E. miletus to be more sensitive to food quantity, but if, under the normal climate change, the quantity of food is relatively abundant, E. miletus is more sensitive to temperature.
Serum leptin levels and hypothalamic neuropeptide gene expression
Serum leptin levels can reflect body fat content, which can regulate energy intake and expenditure in small mammals (Coleman Citation1978; Schwartz et al. Citation2000). It has been shown that leptin is positively correlated with body fat mass (Bozinovic et al. Citation2004; Zhang & Wang Citation2006), similar to our results in the present study. Leptin can regulate food intake in small mammals; serum leptin levels and food intake showed seasonal changes in the present study, and changes of leptin were contrary to those of food intake, suggesting that the low concentration of leptin in winter increases food intake. Our results also show that cold temperature and food restriction lead to a decrease of serum leptin levels, but photoperiod had no effect on leptin levels. Changes of leptin levels were closely related to body fat mass: leptin levels in cold groups were 22.72% lower than in warm groups, and decreased by 22.97% in food-restriction groups compared to food ad libitum groups, which indicates that temperature and food were two important ecological factors to affect leptin levels.
The hypothalamus plays an important role in the maintenance of energy homeostasis in small mammals. NPY expression showed seasonal changes in Jaculus orientalis, which influenced body mass and energy metabolism (Lakhdar-Ghazal et al. Citation1995). During seasonal changes, mammals changed AgRP (Kitamura et al. Citation2006), POMC (Endo & Park Citation2004) and CART expression to regulate body mass and energy metabolism (Khorooshi et al. Citation2008). In the present study, the expression of NPY and AgRP in E. miletus showed seasonal variations: they were higher in winter, and lower in summer. This is mainly because higher expression of NPY and AgRP can promote appetite and increase food intake in E. miletus. POMC and CART expression decreased in winter, but they showed no significant differences among the four seasons. Higher POMC and CART expression in winter can stimulate the increase of thermogenic capacity. Moreover, NPY, AgRP, POMC and CART expression showed significant differences in Apodemus chevrieri, which were mainly distributed in the northern areas in China (Zhu et al. Citation2016), and for Tupaia belangeri, only NPY expression showed significant differences, which were mainly distributed in the southern areas in China (Zhu et al. Citation2014a), suggesting that there may be a species-specific pattern in hypothalamic regulation in E. miletus located in the Hengduan Mountains region.
NPY expression increased by 75% compared with that of the control group in rats under cold exposure of 18 hours (McCarthy et al. Citation1993). AgRP expression and food intake increased significantly in Lasiopodomys brandtii under cold acclimation, and returned to the control level after rewarming (Tang et al. Citation2009). POMC expression decreased significantly in lactating Lasiopodomys brandtii under cold exposure (Zhang et al. Citation2011). During cold acclimation, female rats increased CART expression significantly (Sánchez et al. Citation2007). However, cold acclimation had no effect on AgRP and CART expression in Cricetulus barabensis (Zhao et al. Citation2014). Exogenous injection of NPY under different photoperiods could affect body mass and activity changes in mice significantly (Kim & Harrington Citation2008). Short photoperiod significantly increased AgRP expression in Siberian hamsters (Mercer & Tups Citation2003). Different photoperiods induced significant changes in POMC expression in rats (Jamali & Tramu Citation1997). Short photoperiod reduced CART expression significantly in rats (Khorooshi et al. Citation2008). Long-term food restriction increased NPY expression in rats (Grégoire Citation2008). Long-term food restriction also significantly decreased body mass and body fat mass, while it increased AgRP expression significantly, and decreased POMC and CART expression (Henry et al. Citation2001). In addition, fasting decreased CART expression in rats (Tian et al. Citation2004). Hunger reduced CART expression and increased appetite in mice (Yoo et al. Citation2011). In the present study, we showed that hypothalamic neuropeptide gene expression was mainly affected by cold temperature and food restriction, but photoperiod had no significant effect on hypothalamic neuropeptide gene expression. Cold temperature and food restriction can lead to increased expression of NPY and AgRP, and increased POMC and decreased CART expression, so as to promote appetite in E. miletus.
Previous studies showed that intraventricular injection of exogenous leptin reduced NPY expression significantly, which may be because exogenous leptin can inhibit NPY expression, so as to reduce food intake and increase energy consumption (Casanueva & Dieguez Citation1999). Another study showed that AgRP expression increased in obese mice by leptin gene knockout, which indicates that leptin may be involved in the regulation of AgRP expression (Mizuno & Mobbs Citation1999). There is a positive relationship between leptin and POMC expression, which indicates that a higher concentration of leptin combines with its receptor in the hypothalamus to increase POMC expression, thereby inhibiting appetite and decreasing body mass (Mercer et al. Citation2000). Leptin plays an important role in regulating CART expression, and the content of leptin is positively correlated with CART expression (Kristensen et al. Citation1998). In the present study, in both Experiment 1 and Experiment 2, there was a correlation between leptin and the gene expression of four hypothalamic neuropeptides, suggesting that leptin may play a role in regulation of body mass and energy metabolism by acting on hypothalamic neuropeptide NPY/AgRP and CART/CART pathways in E. miletus.
In conclusion, E. miletus regulated body mass and food intake by adjusting its hypothalamic neuropeptide gene expression during seasonal variations. Cold temperature and food restriction increased NPY and AgRP expression significantly, while decreasing POMC and CART expression significantly, but there was no effect of photoperiod on the above index. Changes of hypothalamic neuropeptide expression under seasonal variations and acclimation to different environment factors in E. miletus were, finally, to adapt to the habitat environment of the Hengduan Mountains region: small annual range of temperature, large diurnal temperature range and relatively abundant food resources.
Acknowledgements
This research was financially supported by the National Science Foundation of China (No. 31560126), and Launch scientific research projects of Yunnan Normal University. We wish to thank Prof. Burkart Engesser at Historisches Museum Basel, Switzerland, for correcting the English usage in the draft. We also thank the anonymous reviewers and the editor of the journal for their valuable comments.
Additional information
Funding
References
- Abizaid A, Walker C-D, Woodside B. 1997. Changes in neuropeptide Y immunoreactivity in the arcuate nucleus during and after food restriction in lactating rats. Brain Research 761:306–312. DOI:10.1016/S0006-8993(97)00351-X.
- Bartness TJ, Demas GE, Song CK. 2002. Seasonal changes in adiposity: The roles of the photoperiod, melatonin and other hormones, and sympathetic nervous system. Experimental Biology and Medicine 227:363–376.
- Bing C, Pickavance L, Wang Q, Frankish H, Trayhurn P, Williams G. 1997. Role of hypothalamic neuropeptide Y neurons in the defective thermogenic response to acute cold exposure in fatty zucker rats. Neuroscience 80:277–284. DOI:10.1016/S0306-4522(97)00121-8.
- Boss-Williams KA, Bartness TJ. 1996. NPY stimulation of food intake in siberian hamsters is not photoperiod dependent. Physiology & Behavior 59:157–164. DOI:10.1016/0031-9384(95)02037-3.
- Bozinovic F, Bacigalupe LD, Vásquez RA, Visser GH, Veloso C, Kenagy GJ. 2004. Cost of living in free-ranging degus (Octodon degus): Seasonal dynamics of energy expenditure. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 137:597–604. DOI:10.1016/j.cbpb.2003.11.014.
- Bush NG, Brown M, Downs CT. 2008. Seasonal effects on thermoregulatory responses of the Rock Kestrel, Falco rupicolis. Journal of Thermal Biology 33:404–412. DOI:10.1016/j.jtherbio.2008.06.005.
- Casanueva FF, Dieguez C. 1999. Neuroendocrine regulation and actions of leptin. Frontiers in Neuroendocrinology 20:317–363. DOI:10.1006/frne.1999.0187.
- Coleman DL. 1978. Obese and diabetes: Two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia 14:141–148. DOI:10.1007/BF00429772.
- Cortés PA, Franco M, Sabat P, Quijano SA, Nespolo RF. 2011. Bioenergetics and intestinal phenotypic flexibility in the microbiotherid marsupial (Dromiciops gliroides) from the temperate forest in South America. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 160:117–124. DOI:10.1016/j.cbpa.2011.05.014.
- Duan K, Yu W, Lin Z, Tan S, Bai X, Gao T. 2014. Endotoxemia-induced muscle wasting is associated with the change of hypothalamic neuropeptides in rats. Neuropeptides 48:379–386. DOI:10.1016/j.npep.2014.10.002.
- Endo D, Park MK. 2004. Molecular characterization of the leopard gecko POMC gene and expressional change in the testis by acclimation to low temperature and with a short photoperiod. General and Comparative Endocrinology 138:70–77. DOI:10.1016/j.ygcen.2004.04.009.
- Grégoire E. 2008. Chronic food restriction differentially affects npy mrna level in neurons of the hypothalamus and in neurons that innervate liver. Neuroscience Letters 433:174–177. DOI:10.1016/j.neulet.2008.01.004.
- Haim A. 1996. Food and energy intake, non-shivering thermogenesis and daily rhythm of body temperature in the bushy-tailed gerbil Sekeetamys calurus: The role of photoperiod manipulations. Journal of Thermal Biology 21:37–42. DOI:10.1016/0306-4565(96)81275-7.
- Hambly C, Speakman JR. 2005. Contribution of different mechanisms to compensation for energy restriction in the mouse. Obesity Research 13:1548–1557. DOI:10.1038/oby.2005.190.
- Henry BA, Rao A, Ikenasio BA, Mountjoy KG, Tilbrook AJ, Clarke IJ. 2001. Differential expression of cocaine- and amphetamine-regulated transcript and agouti related-protein in chronically food-restricted sheep. Brain Research 918:40–50. DOI:10.1016/S0006-8993(01)02918-3.
- Huang CM, Zhu WL, Yu TT, Yang SC, Wang ZK. 2013. Amplification and sequence analysis of partial cDNA sequence of NPY, AgRP, POMC and CART genes in Eothenomys miletus. Acta Theriologica Sinica 33:186–192. In Chinese.
- Jamali AK, Tramu G. 1997. Daily cycle of fos expression within hypothalamic POMC neurons of the male rat. Brain Research 771:45–54. DOI:10.1016/S0006-8993(97)00767-1.
- Khorooshi R, Helwig M, Werckenthin A, Steinberg N, Klingenspor M. 2008. Seasonal regulation of cocaine- and amphetamine-regulated transcript in the arcuate nucleus of djungarian hamster (Phodopus sungorus). General & Comparative Endocrinology 157:142–147. DOI:10.1016/j.ygcen.2008.04.007.
- Kim HJ, Harrington ME. 2008. Neuropeptide Y-deficient mice show altered circadian response to simulated natural photoperiod. Brain Research 1246:96–100. DOI:10.1016/j.brainres.2008.09.040.
- Kitamura T, Feng Y, Kitamura YI, Jr CS, Xu AW, Barsh GS. 2006. Forkhead protein foxo1 mediates agrp-dependent effects of leptin on food intake. Nature Medicine 12:534–540. DOI:10.1038/nm1392.
- Kristensen P, Judge ME, Thim L, Ribel U, Christjansen KN, Wulff BS. 1998. Hypothalamic cart is a new anorectic peptide regulated by leptin. Nature 39:72–76. DOI:10.1038/29993.
- Lakhdar-Ghazal N, Oukouchoud R, Pévet P. 1995. Seasonal variation in NPY immunoreactivity in the suprachiasmatic nucleus of the jerboa (Jaculus orientalis), a desert hibernator. Neuroscience Letters 193:49–52. DOI:10.1016/0304-3940(95)11666-K.
- Li X-S, Wang D-H. 2007. Photoperiod and temperature can regulate body mass, serum leptin concentration, and uncoupling protein 1 in Brandt’s Voles (Lasiopodomys brandtii) and Mongolian Gerbils (Meriones unguiculatus). Physiological and Biochemical Zoology 80:326–334. DOI:10.1086/513189.
- Lovegrove BG. 2005. Seasonal thermoregulatory responses in mammals. Journal of Comparative Physiology B 175:231–247. DOI:10.1007/s00360-005-0477-1.
- Marcelin G, Jo YH, Li X, Schwartz GJ, Zhang Y, Dun NJ. 2013. Central action of FGF19 reduces hypothalamic AgRP/NPY neuron activity and improves glucose metabolism. Molecular Metabolism 3:19–28. DOI:10.1016/j.molmet.2013.10.002.
- McCarthy HD, Kilpatrick AP, Trayhurn P, Williams G. 1993. Widespread increases in regional hypothalamic neuropeptide y levels in acute cold-exposed rats. Neuroscience 54:127–132. DOI:10.1016/0306-4522(93)90388-V.
- McNab BK. 2002. The physiological ecology of Vertebrates. 1st ed. Ithaca: Cornell University Press. pp. 224.
- Mercer JG. 1998. Regulation of appetite and body weight in seasonal mammals. Comparative Biochemistry & Physiology 119:295–303.
- Mercer JG, Moar KM, Ross AW, Morgan PJ. 2000. Regulation of leptin receptor, pomc and agrp gene expression by photoperiod and food deprivation in the hypothalamic arcuate nucleus of the male siberian hamster (Phodopus sungorus). Appetite 34:109–111. DOI:10.1006/appe.1999.0301.
- Mercer JG, Tups A. 2003. Neuropeptides and anticipatory changes in behaviour and physiology: Seasonal body weight regulation in the Siberian hamster. European Journal of Pharmacology 480:43–50. DOI:10.1016/j.ejphar.2003.08.091.
- Mizuno TM, Mobbs CV. 1999. Hypothalamic agouti-related protein messenger ribonucleic acid is inhibited by leptin and stimulated by fasting. Endocrinology 140:814–817. DOI:10.1210/endo.140.2.6491.
- Rafael LR, Darren R, Kasey DF. 2013. The evolution and evolutionary consequences of social plasticity in mate preferences. Animal Behavior 85:1041–1047. DOI:10.1016/j.anbehav.2013.01.006.
- Rene Q, Villavicencio CP, Addis E, Wingfield JC, Vasquez RA. 2014. Seasonal variations of basal cortisol and high stress response to captivity in Octodon degus, a mammalian model species. General & Comparative Endocrinology 197:65–72. DOI:10.1016/j.ygcen.2013.12.007.
- Rezai-Zadeh K, Yu SH, Jiang YY. 2014. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Molecular Metabolism 3:681–693. DOI:10.1016/j.molmet.2014.07.008.
- Ribelayga C, Pévet P, Simonneaux V. 1998. Possible involvement of neuropeptide Y in the seasonal control of hydroxyindole-O-methyltransferase activity in the pineal gland of the European hamster (Cricetus cricetus). Brain Research 801:137–142. DOI:10.1016/S0006-8993(98)00556-3.
- Rousseau K, Atcha Z, Loudon ASI. 2003. Leptin and seasonal mammals. Journal of Neuroendocrinology 15:409–414. DOI:10.1046/j.1365-2826.2003.01007.x.
- Ruth BS. 2014. Direct and indirect effects of leptin on adipocyte metabolism. Biochimica Et Biophysica Acta (Bba)-Molecular Basis of Disease 1842:414–423. DOI:10.1016/j.bbadis.2013.05.009.
- Sánchez E, Fekete C, Lechan RM, Joseph-Bravo P. 2007. Cocaine- and amphetamine-regulated transcript (CART) expression is differentially regulated in the hypothalamic paraventricular nucleus of lactating rats exposed to suckling or cold stimulation. Brain Research 1132:120–128. DOI:10.1016/j.brainres.2006.11.020.
- Schwartz MW, Woods SC, Porte D, Seeley RJ, Baskin DG. 2000. Central nervous system control of food intake. Nature 404:661–671.
- Speakman JR. 2008. The physiological costs of reproduction in small mammals. Philosophical Transactions of the Royal Society B: Biological Sciences 363:375–398. DOI:10.1098/rstb.2007.2145.
- Speakman JR, Król E. 2005. Limits to sustained energy intake IX: A review of hypotheses. Journal of Comparative Physiology B 175:375–394. DOI:10.1007/s00360-005-0013-3.
- Steinlechner S, Heldmaier G, Becker H. 1983. The seasonal cycle of body weight in the Djungarian hamster: Photoperiodic control and the influence of starvation and melatonin. Oecologia 60:401–405. DOI:10.1007/BF00376859.
- Tang G-B, Cui J-G, Wang D-H. 2009. Role of hypoleptinemia during cold adaptation in Brandt’s voles (Lasiopodomys brandtii). American Journal of Physiology, Regulatory Integrative and Comparative Physiology 297:1293–1301. DOI:10.1152/ajpregu.00185.2009.
- Tian DR, Li XD, Shi YS, Wan Y, Wang XM, Chang JK. 2004. Changes of hypothalamic alpha-msh and cart peptide expression in diet-induced obese rats. Peptides 25:2147–2153. DOI:10.1016/j.peptides.2004.08.009.
- Trayhurn P, Bing C. 2006. Appetite and energy balance signals from adipocytes. Philosophical Transactions of the Royal Society B: Biological Sciences 361:1237–1249. DOI:10.1098/rstb.2006.1859.
- Villa RF, Ferrari F, Gorini A. 2012. Energy metabolism of rat cerebral cortex, hypothalamus and hypophysis during ageing. Neuroscience 227:55–66. DOI:10.1016/j.neuroscience.2012.09.041.
- Yoo SB, Ryu V, Lee S. 2007. The arcuate expression of NPY, POMC and CART responding to food deprivation was exaggerated by experience of maternal separation in female rats. Appetite 49:341. DOI:10.1016/j.appet.2007.03.219.
- Yoo SB, Ryu V, Park EY. 2011. The arcuate NPY, POMC, and CART expressions responding to food deprivation are exaggerated in young female rats that experienced neonatal maternal separation. Neuropeptides 45:343–349. DOI:10.1016/j.npep.2011.07.005.
- Zhang X-Y, Wang D-H. 2006. Energy metabolism, thermogenesis and body mass regulation in Brandt’s voles (Lasiopodomys brandtii) during cold acclimation and rewarming. Hormones and Behavior 50:61–69. DOI:10.1016/j.yhbeh.2006.01.005.
- Zhang X-Y, Wang D-H. 2007. Thermogenesis, food intake and serum leptin in cold-exposed lactating Brandt’s voles Lasiopodomys brandtii. Journal of Experimental Biology 210:512–521. DOI:10.1242/jeb.02659.
- Zhang X-Y, Zhang Q, Wang D-H. 2011. Pre- and post-weaning cold exposure does not lead to an obese phenotype in adult Brandt’s voles (Lasiopodomys brandtii). Hormones and Behavior 60:210–218. DOI:10.1016/j.yhbeh.2011.05.004.
- Zhao ZJ, Chi QS, Cao J. 2014. Seasonal changes of body mass and energy budget in Striped hamsters: The role of leptin. Physiological and Biochemical Zoology 87:245–256. DOI:10.1086/674974.
- Zhao ZJ, Zhu QX, Chen KX. 2013. Energy budget, behavior and leptin in Striped hamsters subjected to food restriction and refeeding. Plos ONE 8:e54244. DOI:10.1371/journal.pone.0054244.
- Zhu W-L, Cai J-H, Lian X, Wang Z-K. 2010. Adaptive character of metabolism in Eothenomys miletus in Hengduan Mountains region during cold acclimation. Journal of Thermal Biology 35:417–421. DOI:10.1016/j.jtherbio.2010.09.002.
- Zhu W-L, Cai J-H, Xiao L, Wang Z-K. 2011. Effects of photoperiod on energy intake, thermogenesis and body mass in Eothenomys miletus in Hengduan Mountain region. Journal of Thermal Biology 36:380–385. DOI:10.1016/j.jtherbio.2011.06.014.
- Zhu WL, Cai JH, Zhang L, Wang ZK. 2014a. Seasonal changes of body mass, serum leptin levels and hypothalamic neuropeptide express levels in Tupaia belangeri. Journal of Biology 31:33–37.
- Zhu WL, Cai JH, Zhang L, Wang ZK. 2016. Seasonal variations of hypothalamic neuropeptide expression in Chevrier′s field mouse (Apodemus chevrieri). Chinese Journal of Zoology 51:817–825. In Chinese.
- Zhu W-L, Jia T, Lian X, Wang Z-K. 2008. Evaporative water loss and energy metabolic in two small mammals, voles (Eothenomys miletus) and mice (Apodemus chevrieri) in Hengduan mountains region. Journal of Thermal Biology 33:324–331. DOI:10.1016/j.jtherbio.2008.04.002.
- Zhu WL, Mu Y, Zhang H, Gao WR, Zhang L, Wang ZK. 2014b. Effects of random food deprivation on body mass, behavior and serum leptin levels in Eothenomys miletus (Mammalia: Rodentia: Cricetidae). Italian Journal of Zoology 81:227–234. DOI:10.1080/11250003.2014.902511.
- Zhu WL, Yang SC, Wang ZK. 2012a. Adaptive characters of energy metabolism, thermogenesis and body mass in Eothenomys miletus during cold exposure and rewarming. Animal Biology 62:263–276. DOI:10.1163/157075611X618200.
- Zhu W-L, Zhang H, Wang Z-K. 2012b. Seasonal changes in body mass and thermogenesis in tree shrews (Tupaia belangeri): The roles of photoperiod and cold. Journal of Thermal Biology 37:479–484. DOI:10.1016/j.jtherbio.2012.04.007.
- Zhu WL, Zhang H, Zhang L, Wang ZK. 2014c. Thermogenic properties of Yunnan red-backed voles (Eothenomys miletus) from the Hengduan mountain region. Animal Biology 64:59–73. DOI:10.1163/15707563-00002430.