 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A multi-specific approach in fish diet studies provides insight into the complexity of trophic interactions in marine communities. The feeding habits of three gurnard species, Aspitrigla cuculus, Chelidonichthys lucerna and Eutrigla gurnardus (Scorpaeniformes: Triglidae), from the north-middle Adriatic Sea were studied to evaluate prey-resource partitioning amongst species and within species, comparing juveniles’ and adults’ diet for each gurnard species. A total of 1818 specimens (390 A. cuculus, 973 C. lucerna, 455 E. gurnardus) were collected by bottom trawling and they were assigned to size classes (juveniles or adults) on the basis of macroscopic evaluation of the gonads. Stomach contents were analysed. A common dietary preference for Crustacea was found in all species and size classes considered. Nevertheless, gurnards showed distinct feeding behaviour: C. lucerna and E. gurnardus were generalist-opportunistic predators, showing a varied diet based on epi-benthic, bentho-pelagic and necto-benthic preys belonging to different taxa such as Teleostei and Mollusca, while A. cuculus may be considered a specialist feeder, feeding almost exclusively on necto-benthic invertebrates. Morisita’s index calculated for critical size classes (juveniles and adults) pointed out differences. At the inter-specific level, possible dietary competition between A. cuculus and E. gurnardus (C > 0.65) was found for all size classes combined, due to the prey abundance of Lophogaster typicus (Crustacea: Mysida). At the intra-specific level, high diet overlap was found between juveniles and adults of C. cuculus (C = 0.98) and between juveniles and adults of E. gurnardus (C > 0.84). In contrast, C. lucerna did not compete with increasing body size (C < 0.20), showing a clear change from crustaceans to fish in its diet preferences. The possibility that A. cuculus and E. gurnardus may compete for the same prey resources while C. lucerna shows food resource partitioning is discussed. Better understanding of the ecology of these coexisting predators should lead to improved conservation and improved fisheries management.
Introduction
Trophic relationships are fundamental to understanding biological interactions in animal communities (Carrassón & Cartes Citation2002). Strategies used by related groups of animals in exploiting resources are of continuing interest in ecological studies. Many of the behaviour patterns and morphological adaptations of fish species have evolved in relation to the capture of food, in addition to the requirements of reproduction and avoidance of predation (Labropoulou & Eleftheriou Citation1997).
Gurnard species (Scorpaeniformes: Triglidae) are demersal fishes that inhabit the continental and insular shelves of tropical and temperate seas to depths of 500 m, and are found on sandy, muddy or rubble substrates (Fischer et al. Citation1987). There are seven different species in the north-middle Adriatic Sea (42–45°N, 13–15°E), showing differences related to biometric features as well as ecological ones such as diet, spawning period and depth distribution (Tsimenides et al. Citation1992; Colloca et al. Citation1994; Vallisneri et al. Citation2010). During their early life cycle they go through a pelagic phase, while during their demersal stage changes in their ecologic behaviour are associated with the onset of sexual maturity (Vallisneri et al. Citation2012), the tendency to migrate to greater depths and a change in diet with a wider trophic spectrum (Colloca et al. Citation1994; Montanini et al. Citation2010). Gurnards use the free radii of their pectoral fins to search for prey on sedimentary beds (Whitehead et al. Citation1989). Aspitrigla cuculus (Linnaeus, 1758), Chelidonichthys lucerna (Linneaus, 1758) and Eutrigla gurnardus (Linneaus, 1758) are included in the list of reference species of the MEDITS project (International Bottom Trawl Surveys in the Mediterranean) and are the subject of a new Memorandum of Understanding (MOU) from the International Council for the Exploration of the North Sea (ICES Citation2006) in order to investigate biological parameters for stock assessment purposes. In fact, subsequent to the decline in traditionally exploited fish stock, Triglidae have been considered new emerging species by trawlers (Boudaya et al. Citation2008 – South Mediterranean), and emergent key predators as is the case with Eutrigla gurnardus in the North Sea, reported by Floeter and Temming (Citation2005) and Weinert et al. (Citation2010).
All previous studies on the diet of the three species reported that crustaceans were the most abundant preys, but these studies were undertaken on separated populations (Valiani Citation1934; Froglia Citation1976; Montanini et al. Citation2010; Stagioni et al. Citation2012). No study has taken into account diet in terms of niche overlap, resource partitioning and ontogenetic diet shift as a possible important contributing factor to the segregation of gurnard species in the studied area. Resource partitioning allows fish species to avoid inter- and intra-specific competition, influencing the number of individuals that can coexist in the same area. Resource partitioning may occur by segregation from one of the three main resource axes: food, space and time, where trophic separation explains most of the mechanisms that allow coexistence among closely related species (Schuckel et al. Citation2012).
The aim of this study was to compare trophic relationships among three gurnard species in the Adriatic Sea, northern Mediterranean. Our specific goal was to investigate whether prey-resource partitioning or potential competition occurs among these species.
Materials and methods
Sampling
The sampling site was in the GFCM-GSA 17 area (Northern and Central Adriatic Sea) and covered a surface area of 59,400 km2 from the Gulf of Trieste (45°40ʹN, 13°37ʹE) to the Tremiti Islands (42°08ʹN, 15°16ʹE) at depths of between 12 and 252 m. Samples were taken on a seasonal basis (summer and autumn–winter) between 2005 and 2009 as part of several national and international oceanographic bottom trawl surveys, namely MEDITS (summer surveys) and GRUND (Assessment of Italian Demersal Resources – autumn/winter surveys). The sampling gear included an experimental bottom trawl featuring four panels and a mesh cod-end size of 20 mm (stretched mesh) for the MEDITS, while a 40 mm cod-end was used for the GRUND surveys (Fiorentini et al. Citation1999).
Data analyses
A total of 1818 gurnards (390 Aspitrigla cuculus, 973 Chelidonichthys lucerna and 455 Eutrigla gurnardus) were collected (). Specimens from each trawl were measured to the nearest mm (total length = TL) and weighed to the nearest 0.1 g. The sex was defined following four categories: male, female, undetermined (impossible to determine by eye) and not determined (the individual was not examined). The gonadal maturity levels were defined according to the code of sexual maturity used in the European MEDITS project (MEDITS Citation2007). Fish lengths were classified into two size groups according to the above maturity scale: juveniles (individuals corresponding to stages 0 and 1) and adults (individuals corresponding to stages from 2a to 4b). Juvenile to adult transition size was marked off at 110 mm for A. cuculus, 180 mm for C. lucerna and 100 mm for E. gurnardus.
Table I. Summary of some biological and ecological parameters relating to Aspitrigla cuculus, Chelidonichthys lucerna and Eutrigla gurnardus from the north-middle Adriatic Sea sampled between 2005 and 2009. For each gurnard, samples were classified into two ontogenetic groups: juvenile and adult. TL = total length; W = weight.
Samples were dissected and stomachs were removed and preserved in 70% ethanol for analysis of stomach contents. Gut contents were analysed with a stereoscopic microscope identifying each prey to the lowest taxonomic level possible by using specialised keys. Prey types were counted (Np) and weighed (Wp) after removal of excess moisture.
Statistical analyses
Depth distribution of the specimens belonging to the three species was analysed by Pearson’s Chi-squared test in order to test the significance of the relationship between size classes and depth. Differences in species niche depth were compared using a Kruskal–Wallis one-way analysis of variance (ANOVA) on ranks and a post hoc all pairwise multiple comparison procedure using Dunn’s method.
Diets were expressed as percentage abundance (Cn%) by prey item for each dietary category, percentage by weight (Cw%) and percentage by frequency of occurrence (F%), being understood as the proportion of non-empty stomachs containing a given prey item or category. According to N’Da (Citation1992), prey items are considered dominant when Cn% > 50%; secondary prey have values of Cn% ranging from 10% – 50%; and accidental prey are defined as Cn% < 10%. The main food items were identified using the index of relative importance (IRI):
These indexes were calculated for each prey category and used in diet comparisons (Pinkas et al. Citation1971; Cortés Citation1997). Data was sorted on the basis of the decreasing value of IRI%.
The ontogenetic shift in the diet was explored using a hierarchical cluster analysis based on numerical abundance Bray–Curtis dissimilarity (Clarke & Warwick Citation1994). Data was transformed by Wisconsin double standardisation (square root transformation) and then analysed by non-metric multidimensional scaling (nMDS) in relation to size class and depth. Regression fit goodness (Stress S) was determined on the basis of the sum of squared differences between ordination-based distances and predicted distances. Low Stress S values indicated goodness of fit.
For the analysis of trophic niche breadth, the normalised version of the Levins (Bi) index (Hurlbert Citation1978) was used:
where pij is the proportion of prey j in the diet of predator i, and n is the number of prey categories. Bi was expressed on a scale from 0 to 1.0 (with a low value indicating a specialist predator with a diet dominated by few prey items, and a high value indicating a generalist predator) and calculated for each size class.
Species diversity in prey number was calculated using the Shannon–Wiener index (H´) and the evenness measure of the Shannon–Wiener function (J´) (Colwell & Futuyma Citation1979). The Shannon–Wiener formula was expressed as:
where pj is the proportion of individuals found in or using resource j. A high H´ value indicates feeding on a wider spectrum of prey-items. As Shannon–Wiener measures may range from 0 to ∞, an evenness measure (J´) was calculated so as to standardise H´ on a 0 to 1 scale:
where n is the total number of possible resource states. A high J´ indicates a specimen feeding on a relatively larger number of a few main prey types (Shuozeng Citation1995).
The simplified Morisita index (CH) proposed by Horn (Citation1966) was used to investigate intra- and inter-specific niche overlap among size classes and species. This index ranges from 0 (no resource used in common) to 1.0 (full overlap), with values > 0.6 being considered to be indicative of a biologically significant overlap (Pusey & Bradshaw Citation1996). Morisita’s measure was expressed as:
where pij is the corresponding proportion of resource i in the total resource used by species j, and pik is the corresponding proportion of resource i in the total resource used by species k.
Multivariate analysis was conducted using the R software version 3.3.2 (R Core Team Citation2016) base and the Vegan package for community ecology.
Results
Biological and ecological parameters
Biological and ecological parameters of Aspitrigla cuculus, Chelidonichthys lucerna and Eutrigla gurnardus are summarised in . Significant differences were found in distribution by depth (Pearson’s Chi-squared test with Yates’ continuity correction: χ-squared = 84.9339, df = 1, p-value < 0.001). More precisely, these significant differences were found by comparing all possible size-class pairs according to the post-hoc Dunn test (p-value < 0.001) in species niche depth (Kruskal–Wallis test; p-value < 0.001) both among species (inter-specific differences) and between juveniles and adults within the same species (intra-specific differences). Aspitrigla cuculus was generally encountered at greater depths, while C. lucerna was more common at shallow depths near the coast. For all three gurnard species, juveniles were found at shallower waters than adults, which consistently migrate to deeper depths.
Diet composition
Dietary analysis of the main taxa preyed by the three species examined is summarised in . Examination of the collected specimens did not reveal any sign of regurgitation. Sixteen food items were identified in the diet of A. cuculus and classified into four main categories: Crustacea, Teleostei, Mollusca and Echinodermata. Almost the entire diet of this species was based on crustaceans (%Cn = 93.88; %Cw = 91.15; %IRI = 98.26), while other taxa were classified as accessory prey. Sixty different preys belonging to five main groups (Crustacea, Teleostei, Mollusca, Anellida and Bryozoa) were identified in the stomachs of C. lucerna. Crustaceans were the most abundant in number and weight with a high IRI value (%Cn = 90.0; %Cw = 58.11; %IRI = 96.7). Teleostei were considered secondary prey in the diet of C. lucerna, with a significant contribution in terms of weight and frequency of occurrence (%Cw = 40.12; %F = 31.35). Molluscs, annelids and bryozoa were recorded as accidental prey. Thirty-four categories of prey items were identified in the stomachs of E. gurnardus and classified into five main groups: Crustacea, Teleostei, Mollusca, Nematoda and Echinodermata. For E. gurnardus also, the diet consisted essentially of crustaceans (%Cn = 93.65; %W = 61.25; %IRI = 98.5).
Table II. Diet analysis of the main taxa preyed by Aspitrigla cuculus, Chelidonichthys lucerna and Eutrigla gurnardus in the north-middle Adriatic Sea.
The diet of both juveniles and adults of all species was mainly composed of crustaceans. Lophogaster typicus (Lophogastrida) was the most abundant and preferred prey for both ontogenetic groups of A. cuculus (%IRI = 97.08 juveniles; %IRI = 97.81 adults) and E. gurnardus, (%IRI = 64.31 juveniles; %IRI = 47.46 adults). Liocarcinus sp. (Decapoda: Brachyura), Solenocera membranacea (Decapoda: Macrura–Natantia) and Acanthomysis longicornis (Mysida) occurred quite frequently in the diet of juveniles of E. gurnardus, but were replaced in the diet of the adults by Goneplax rhomboides (Decapoda: Brachyura). Philocheras sp. (Decapoda: Macrura–Natantia) was the dominant prey (%IRI = 79.99) of juveniles of C. lucerna, while G. rhomboides (%IRI = 80.63) was the dominant prey of adults of C. lucerna.
Teleostei were considered secondary preys (). Seventeen different species of bony fish preys were found in the diet of adults of C. lucerna (%Cn = 16.86); anchovy Engraulis encrasicolus (%Cw = 12.98) and gobid Gobius niger (%Cw = 10.29) contributed well in terms of weight.
Full details of diet, with juveniles and adults considered separately for each species, are given in the Appendix.
Inter-/intra-specific variation in diet
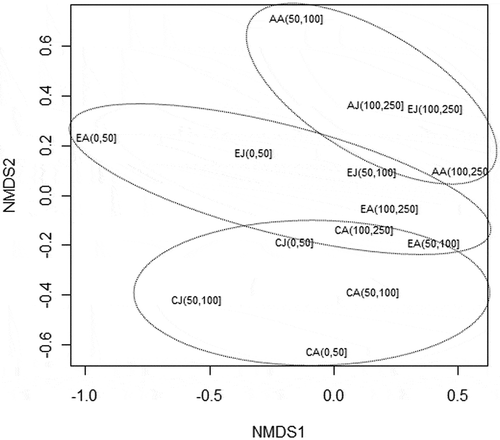
Inter-specific nMDS analysis highlights three different groups, each within the same species: juveniles and adults of each species were grouped together, reflecting specific feeding behaviour (). This trend was further borne out by the nMDS analysis of the size–depth relationship; in fact, same-species clusters were basically found also by this type of analysis ().
Figure 1. Non-metric multidimensional scaling (nMDS) ordination graph based on Bray–Curtis dissimilarity matrix. Stress values: 0.02. Diet overlap was computed on numerical abundance of prey species. Two main size classes (juvenile and adult) were considered for each gurnard (AspJuv = Aspitrigla cuculus juveniles 110 mm; AspAdu = adults > 110 mm; CheJuv = Chelidonichthys lucerna juveniles 180 mm; CheAdu = adults > 180 mm; EutJuv = Eutrigla gurnardus juveniles 100 mm; EutAdu = adults > 100 mm).
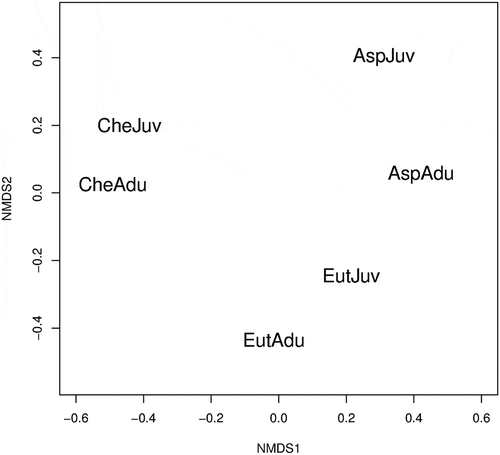
Figure 2. Non-metric multidimensional scaling (nMDS) ordination graph based on Bray–Curtis dissimilarity matrix. Stress values: 0.16. Diet overlap was computed on numerical abundance of prey species. For each species, data was grouped according to size classes (juvenile and adult) and three strata of depth distribution (0–50 m, 50–100 m, 100–250 m). (AJ = Aspitrigla cuculus juveniles 110 mm; AA = adults > 110 mm; CJ = Chelidonichthys lucerna juveniles 180 mm; CA = adults > 180 mm; EJ = Eutrigla gurnardus juveniles 100 mm; EA = adults > 100 mm). Ellipses include 95% confidence interval.
Inter-and intra-specific feeding differences are reported in . Although stomach contents generally showed a high number of different prey items and a relatively wide spectrum, the niche breadth (B’ values) reached low levels in all the size-species groups. In terms of prey species diversity, juveniles of E. gurnardus showed the highest values (H’ = 2.24 and J“ = 0.79), quite similar to those found for the adults of E. gurnardus (H’ = 2.09 and J“ = 0.62) and for adults of C. lucerna (H’ = 2.26 and J“ = 0.56). For the other ontogenetic groups (juveniles and adults of A. cuculus and juveniles of C. lucerna), low species diversity values were recorded (H’ < 1.50; J’ < 0.60).
Table III. Levins index (Bi), Shannon–Wiener index (H´), and evenness measure (J’) for the diet of each ontogenetic group (juvenile and adult) of Aspitrigla cuculus, Chelidonichthys lucerna and Eutrigla gurnardus.
Intra-specific feeding competition was considered between juveniles and adults of A. cuculus and E. gurnardus. In fact, as may be seen in , Morisita index values were high in both species (C = 0.991 for A. cuculus, and C = 0.843 for E. gurnardus). By contrast, very low intra-specific competition levels were recorded for C. lucerna (C = 0.186). Low levels of inter-specific competition show the trophic niche of C. lucerna to be different from that of A. cuculus and E. gurnardus (C range = 0.249–0.589) ().
Table IV. Morisita’s index of dietary overlap for each ontogenetic group (juvenile and adult) of Aspitrigla cuculus, Chelidonichthys lucerna and Eutrigla gurnardus.
Discussion
Inter-/intra-specific variation in depth
The study focuses on the role of fish size and depth distribution in food resource partitioning of gurnards Aspitrigla cuculus, Chelidonichthys lucerna and Eutrigla gurnardus from the Adriatic Sea. Inter-specifically, the narrowest bathymetric distribution, mostly above 100 m, was reported for A. cuculus, while the greatest relative abundance was recorded for C. lucerna at between 0 and 50 m. Intra-specifically, a species-specific distribution pattern associated with nursery-recruitment patterns and fish size was found for each gurnard species, according to Colloca et al. (Citation1994), Manfredi et al. (Citation2013). A spatial separation between juveniles and adults was observed for the three gurnard species, with adults tending to be sampled at greater depths than juveniles; although significant in all cases, juvenile–adult spatial separation was most pronounced for C. lucerna. In fact, C. lucerna shows a consistent nursery area along the coast with younger individuals more frequently found in shallow waters, while adults are dispersed more towards offshore sites, according to the literature (Froglia Citation1976; Papaconstantinou Citation1983; Tsimenides et al. Citation1992; Colloca et al. Citation1994; Serena et al. Citation1998; Boudaya et al. Citation2008).
Inter-specific variation in diet
Our data suggests that the three species show a crustacivorous feeding behaviour; however, they appear to have distinct feeding preferences according to the literature (Colloca et al. Citation1994; Morte et al. Citation1997; Montanini et al. Citation2010; Lopez-Lopez et al. Citation2011; Stagioni et al. Citation2012). Lophogaster typicus (Lophogastrida) represents the most abundant and preferred prey for both A. cuculus and for E. gurnardus, though not for C. lucerna. Four crustacean species were found in the stomachs of gurnard species of all size classes considered: Goneplax rhomboides, Liocarcinus sp., Alpehus glaber and Solenocera membranacea. These crustaceans are in fact among the most common prey items for both fish and other decapod crustaceans in the muddy bottom communities of the lower continental shelf and upper slope of the western Mediterranean Sea (Rufino et al. Citation2006).
Aspitrigla cuculus, which feeds almost exclusively on necto-benthic invertebrates, may be considered a “specialist feeder”, in line with the literature (Colloca et al. Citation1994, Citation2010; Morte et al. Citation1997). In contrast, C. lucerna, which feeds mostly on epibenthic crustacea and necto-benthic teleost fish; and E. gurnardus which feeds on a mixed diet of epibenthic, bentho-pelagic and necto-benthic preys may both be considered “opportunistic predators”, according to the literature (Colloca et al. Citation1994, Citation2010; Morte et al. Citation1997; Montanini et al. Citation2010). These characteristics, allowing them to be able to feed opportunistically, would be typical for fish such as C. lucerna and E. gurnardus that live principally in nearshore waters where the relative abundance of the different prey types varies with temperature and habitat type (Schafer et al. Citation2002).
A possible diet competition was recorded between A. cuculus and E. gurnardus (C > 0.65) only, while C. lucerna showed food resource partitioning. However, our data suggests that prey item distribution, predator size range and depth distribution are the main variables affecting trophic niche breadth and, hence, the relative differences encountered among the gurnards species. Therefore, the three species inhabit overlapping geographic areas, but may occupy different depth ranges and microhabitats within their shared distribution.
Intra-specific variation in diet
At critical sizes of sexual maturity (Vallisneri et al. Citation2012) that mark the change from juveniles to adults (110 mm for A. cuculus; 180 mm for C. lucerna; 100 for E. gurnardus), the gurnard species migrate to greater depths and change dietary habits, increasing the variety of food items eaten, according to the literature for other Mediterranean areas (Moreno-Amich Citation1992; Colloca et al. Citation1994; Boudaya et al. Citation2008). The trophic niches occupied were different in spatial distribution but not in terms of prey-item composition. In fact, the predominant prey of the three species (for juveniles and adults), consisted of Crustacea.
An ontogenetic dietary shift found for all species examined indiated that the number of prey items in the gut increased with increasing predator body size. Ontogenetic dietary shifts are often explained as a reflection of the changing abilities of fish; essentially, as fish grow they become more proficient at handling larger prey, which are more profitable. Increasing prey size usually leads to taxonomic changes in the diet. Migratory patterns associated with reproduction and feeding patterns have also been reported for gurnards, and the occupation of new niches may result in differences in diet (Papaconstantinou Citation1983; Colloca et al. Citation1994). The demersal migration undertaken by adults allows them to feed on a larger variety of prey species (more than twice the number found in the diet of juveniles). The higher number of different items consumed by adult as compared to juvenile individuals does not, however, correspond to a higher diversity index. In fact, this index value is high only in the case of C. lucerna, while in the case of E. gurnardus and A. cuculus diversity values are greater in juveniles, a finding that highlights species-specific differences. This result suggests that individual specialisation may occur within a predator population, lending support to Bolnick et al.’s conclusion (Citation2007) that the more generalized the population, the higher the level of individual specialization, as also reported in the case of the opportunistic feeding behaviour observed for E. gurnardus from the North Sea (Weinert et al. Citation2010). The low niche-breadth index value (B“) showed that juvenile and adult gurnard diet was characterised by a preference for few species. Aspitrigla cuculus displayed low values for both niche-breadth and prey species diversity (H’ and J’), while for adults of C. lucerna and E. gurnardus low niche breadth did not match with prey species diversity. These species may be considered generalist-opportunistic, even if they prey on preferred species.
No high values for intra-specific juvenile–adult difference in the trophic niche overlap index (C) were observed for A. cuculus or E. gurnardus, indicating that both size groups feed on similar preys. In contrast, a low value for the trophic niche overlap index is reported for C. lucerna, showing different trophic niches colonised during growth.
These findings suggest habitat partitioning mainly at the inter-specific level, and trophic segregation/competition at the intra-specific level, in line with studies on Atlantic gurnards (Lopez-Lopez et al. Citation2011). According to Amorim et al. (Citation2004), smaller E. gurnardus compete for food by contest tactics whereas larger specimens predominantly scramble for food, probably because their larger body size affords them an advantage in locating, capturing and handling prey. Food resource availability depends not only on prey abundance, but also on the interaction of other factors, including prey size, micro-distribution, capture success and speed of movement.
Conclusion
In brief, our data suggest, at the inter-specific level, the possibility that A. cuculus and E. gurnardus may compete for the same prey resources while C. lucerna showed food resource partitioning, but it should be considered that A. cuculus is a “specialist predator” in contrast to E. gurnardus and C. lucerna which are “generalist-opportunistic predators”. At the intra-specific level, our data suggest a high dietary overlap between juveniles and adults of A. cuculus and between juveniles and adults of E. gurnardus, while we found resource partitioning between juveniles and adults of C. lucerna that change from preying on crustaceans to a piscivorus diet. However, according to Schafer et al. (Citation2002), various factors including depth and micro-habitat can influence the distribution and composition of prey items; thus, even the most fine-scale habitat partitioning may reduce inter- and intra-specific competition through differential niche utilisation.
In conclusion, the existence of “fish strategies” tending to partition food resources and feeding areas are essential in minimising the effects of possible competition for the most desirable prey and for the co-existence of the species, and therefore they should be considered in developing adequate fishery management practices.
Acknowledgements
We are grateful to Prof. Corrado Piccinetti, working for the Laboratory of Marine Biology and Fisheries of the University of Bologna, who organised haul fish sampling in the study area and made this research possible.
References
- Amorim MCP, Stratoudakis Y, Hawkins AD. 2004. Sound production during competitive feeding in the grey gurnard. Journal of Fish Biology 65:182–194. doi:10.1111/jfb.2004.65.issue-1
- Bolnick DI, Svanback R, Araujo MS, Persson L. 2007. Comparative support for the niche variation hypothesis that more generalized populations also are more heterogeneous. Proceedings of the National Academy of Sciences 104:10075−10079. doi:10.1073/pnas.0703743104
- Boudaya L, Neifar L, Rizzo P, Badalucco C, Bouain A, Fiorentino F. 2008. Growth and reproduction of Chelidonichthys lucerna (Linnaeus) (Pisces: Triglidae) in the Gulf of Gabès, Tunisia. Journal of Applied Ichthyology 24:581−588. doi:10.1111/j.1439-0426.2008.01095.x
- Carrassón M, Cartes JE. 2002. Trophic relationships in a Mediterranean deep-sea fish community: Partition of food resources, dietary overlap and connections within the benthic boundary layer. Marine Ecology Progress Series 241:41–55. doi:10.3354/meps241041
- Clarke KR, Warwick RM. 1994. Change in marine communities: an approach to statistical analysis and interpretation. 1st ed. Plymouth: Plymouth Marine Laboratory. pp. 144.
- Colloca F, Ardizzone GD, Gravina MF. 1994. Trophic ecology of gurnards (Pisces: Triglidae) in the central Mediterranean Sea. Marine Life 4:45–57.
- Colloca F, Carpentieri P, Balestri E, Ardizzone G. 2010. Food resource partitioning in a Mediterranean demersal fish assemblage: The effect of body size and niche width. Marine Biology 157:565−574. doi:10.1007/s00227-009-1342-7
- Colwell RK, Futuyma DJ. 1979. On the measurement of niche breadth and overlap. Ecology 52:567−576.
- Cortés E. 1997. A critical review of methods of studying fish feeding based on analysis of stomach contents: Application to elasmobranch fishes. Canadian Journal of Fisheries and Aquatic Science 54:726−738. doi:10.1139/f96-316
- Fiorentini L, Dremière PY, Leonori I, Sala A, Palumbo V. 1999. Efficiency of the bottom trawl used for the Mediterranean International trawl survey (MEDITS). Aquatic Resources 12:187−205.
- Fischer W, Scheneider M, Bouchot ML. 1987. Fiches FAO d’identification des especes pour les besoins de la peche (Revision I) Mediterranee et Mer Noire. Zone the peche 37. Rome: Food Agricolture Organization. pp.761−1530
- Floeter J, Temming A. 2005. Analysis of prey size preference of North Sea whiting, saithe, and grey gurnard. ICES Journal of Marine Science 62:897−907. doi:10.1016/j.icesjms.2005.03.004
- Froglia C. 1976. Osservazioni sull’alimentazione dei giovani di Trigla lucerna della classe di età nel Medio Adriatico (Pisces, Triglidae). Archive of Oceanography and Limnology 18:365−373.
- Horn HS. 1966. Measurement of “overlap” in comparative ecological studies. American Naturalist 100:419−424. doi:10.1086/282436
- Hurlbert SH. 1978. The measurement of niche overlap and some relatives. Ecology 59:67–77. doi:10.2307/1936632
- ICES. 2006. Report of the working group on the assessment of new MoU Species (WGNEW), 13-15 December 2005, ICES Headquarters. Copenhagen: ICES Advisory Committee on Fishery Management. pp. 234
- Labropoulou M, Eleftheriou A. 1997. The foraging ecology of two pairs of congeneric demersal fish species: Importance of morphological characteristics in prey selection. Journal of Fish Biology 50:324–340. doi:10.1111/jfb.1997.50.issue-2
- Lopez-Lopez L, Preciado I, Velasco F, Olaso I, Gutiérrez-Zabala JL. 2011. Resource partitioning among five coexisting species of gurnards (Scorpaeniforme: Triglidae): role of trophic and habitat segregation. Journal of Sea Research 66:58–68. doi:10.1016/j.seares.2011.04.012
- Manfredi C, Montanini S, Stagioni M, Tommasini S, Vallisneri M. 2013. Note su distribuzione e “aree di nursery” di due specie di triglidi (Scorpaeniformes) nel Nord-Centro Adriatico. Atti Della Società Dei Naturalisti E Matematici Di Modena 144:156.
- MEDITS. 2007. International bottom trawl survey in the Mediterranean (Medits). Instruction manual. Version 5. Ifremer, Nantes. Available: http://archimer.ifremer.fr/doc/00002/11321/. Accessed Nov 2016 14.
- Montanini S, Stagioni M, Vallisneri M. 2010. Diet of the grey gurnard, Eutrigla gurnardus in the Adriatic Sea, north-eastern Mediterranean. Cybium 34:367–372.
- Moreno-Amich R. 1992. Feeding habits of red gurnard Aspitrigla cuculus (Linneaus, 1758) (Scorpaeniformes, Triglidae), along the Catalan coast (northwestern Mediterranean). Hydrobiology 228:175–184. doi:10.1007/BF00006584
- Morte MS, Redon MJ, Sanz-Brau A. 1997. Trophic relationships between two gurnards Trigla lucerna and Aspitrigla cuculus from the western Mediterranean. Journal of Marine Biology Association of UK 77:527–537. doi:10.1017/S0025315400071848
- N’Da K. 1992. Regime alimentaire du rouget de roche Mullus surmuletus (Mullidae) dans le nord du golfe de Gascogne. Cybium 16:159–168.
- Papaconstantinou C. 1983. Observations on the ecology of gurnards (Pisces: Triglidae) in the Greek Seas. Cybium 7:71–88.
- Pinkas LM, Oliphant S, Iverson ILK. 1971. Food habits of albacore, bluefin tuna and bonito in California waters. California Department of Fish and Game 152:1–150.
- Pusey BJ, Bradshaw SD. 1996. Diet and dietary overlap in fishes of temporary waters of southwestern Australia. Ecology of Fresh Water Fish 5:183–194. doi:10.1111/eff.1996.5.issue-4
- R Development Core Team. 2016. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. Available: http://www.R-project.org. Accessed Jan 2017 18.
- Rufino MM, Maynou F, Abelló P, Sardá F. 2006. Spatial and environmental factors affecting the distribution of the main decapod crustacean prey species in the NW Mediterranean. Hydrobiology 1:129–141.
- Schafer LN, Platell ME, Valesini FJ, Potter IC. 2002. Comparisons between the influence of habitat type, season and body size on the dietary compositions of fish species in nearshore marine waters. Journal of Experimental Marine Biology and Ecology 278:67–92. doi:10.1016/S0022-0981(02)00337-4
- Schuckel AF, Sell AF, Kroncke I, Reiss H. 2012. Diet overlap among flatfish species in the southern North Sea. Journal of Fish Biology 80:2571–2594. doi:10.1111/j.1095-8649.2012.03309.x
- Serena F, Voliani A, Auteri R. 1998. Nursery areas and some biological information of tub gurnard (Trigla lucerna Linneaus, 1758) off Tuscany coasts (Italy). Rapport Commission International Pour L’exploration Scientifique De La Mer Mediterranee 35:482–483.
- Shuozeng D. 1995. Food utilization of adult flatfishes co-occurring in the Bohai Sea of China. Netherlands Journal of Sea Research 34:183–193. doi:10.1016/0077-7579(95)90026-8
- Stagioni M, Montanini S, Vallisneri M. 2012. Feeding of tub gurnard Chelidonichthys lucerna (Scorpaeniformes, Triglidae) in the north-east Mediterranean. Journal of the Marine Biological Association 92:605–612. doi:10.1017/S0025315411000671
- Tsimenides N, Machias A, Kallianiotis A. 1992. Distribution patterns of triglids (Pisces: Triglidae) on the Cretan shelf (Greece), and their interspecific associations. Fisheries Research 15:83–103. doi:10.1016/0165-7836(92)90006-F
- Valiani S. 1934. Contributo allo studio dell’alimentazione dei pesci, T. lyra, T. gurnardus. Bollettino Pesca Piscicoltura E Idrobiologia 10:37–46.
- Vallisneri M, Montanini S, Stagioni M. 2010. Length–weight relationships for the family Triglidae in the Adriatic Sea, north-eastern Mediterranean. Journal Applied Ichthyology 26:460–462. doi:10.1111/j.1439-0426.2009.01389.x
- Vallisneri M, Montanini S, Stagioni M. 2012. Size at maturity of triglid fishes in the Adriatic Sea, north-eastern Mediterranean. Journal Applied Ichthyology 28:123–125. doi:10.1111/j.1439-0426.2011.01777.x
- Weinert M, Floeter J, Kröncke I, Sell A. 2010. The role of prey composition for the condition of grey gurnard (Eutrigla gurnardus). Journal of Applied Ichthyology 26:75–84. doi:10.1111/(ISSN)1439-0426
- Whitehead PJP, Bauchot ML, Hureau JC, Nielsen J, Tortonese E. 1989. Fishes of the North-eastern Atlantic and Mediterranean. Paris: UNESCO. Vol. 3, pp. 1473.
